Abstract
Background
Based on previous in vivo studies and human trials, intrathecal cell delivery is a safe and relevant therapeutic tool for improving patient's quality of life with neurological conditions. We aimed to characterise the safety profile of intrathecally delivered Mesenchymal stem cells (MSCs).
Methods
Ovid MEDLINE, Embase, Scopus, Cochrane Library, KCI-Korean Journal Database, and Web of Science. Databases were searched from their inception until April 13, 2023. Randomised Controlled Trials (RCTs) that compared intrathecal delivery of MSCs to controls in adult populations were included. Adverse events (AEs) were pooled and meta-analysed using DerSimonian-Laird random effects models with a correction factor 0.5 added to studies with zero count cells. Pooled AEs were described using Risk ratio (RR) and 95% confidence intervals (95% CI). Then, a random-effects meta-regress model on study-level summary data was performed to explore the relationship between the occurrence of AEs and covariates thought to modify the overall effect estimate. Finally, publication bias was assessed.
Results
303 records were reviewed, and nine RCTs met the inclusion criteria and were included in the quantitative synthesis (n = 540 patients). MSCs delivered intrathecally, as compared to controls, were associated with an increased probability of AEs of musculoskeletal and connective tissue disorders (categorised by Common Terminology Criteria for Adverse Events—CTCAE version 5.0) (RR: 1.61, 95% CI 1.19–2.19, I2 = 0%). The random-effects meta-regress model suggested that fresh MSCs increased the probability of occurrence of AEs compared to cryopreserved MSCs (RR: 1.554; p-value = 0.048; 95% CI 1.004–2.404), and the multiple-dose, decreased the probability of AEs by 36% compared to single doses (RR: 0.644; p-value = 0.048; 95% CI 0.416–0.996); however, univariate random effects meta-regression models revealed a not significant association between the occurrence of AEs from MSCs intrathecal delivery and each covariate.
Conclusions
Intrathecal delivery of MSCs was associated with a slight increase in AEs associated with musculoskeletal and connective tissue disorders, albeit without serious AEs. We conclude that intrathecal MSCs delivery is safe for patients with neurological conditions. However, further high-quality, large-scale RCTs are needed to confirm these findings.
Similar content being viewed by others
Introduction
Neurological conditions constitute a wide range of disorders affecting the central and peripheral nervous systems, with reduced cognitive and sensorimotor functioning, representing a prominent global disease burden and a diminished quality of life [1]. With a mortality count of 9·0 million (95% CI 8.8–9.4) and accounting for 16.5% (95% CI 16.1–17.0) of worldwide fatalities, neurological disorders stand as the second most prevalent contributor to mortality following cardiovascular disease [2]. Most neurological disorders are characterised by widespread neuronal death and meagre regenerative potential of the brain [3]. Moreover, the available treatment options are restrained compared with other conditions [4].
MSCs are distinguished by their unique characteristics, such as the capacity for self-renewal and the ability to differentiate into various types of cells [3]. Previous clinical trials have assessed their safety and efficacy as therapies for neurological conditions, including Alzheimer’s disease, dementia, Parkinson’s disease, spinal cord injury, spinal muscular atrophy, and multiple sclerosis [5,6,7,8,9,10]. MSCs exhibit tropism to injury sites and secrete a diverse range of growth and neurotrophic factors, eliciting immunomodulatory and neuroprotective effects that promote neuronal survival and regeneration [11, 12]. Therefore, MSC therapy could be considered a significant approach for ameliorating neurological dysfunction [13].
MSC therapy can be administered to reach the subarachnoid space through various routes, including intra-arterial, intravenous, intralesional, and intrathecal [7, 14]. Intra-arterial or intravenous routes have been observed to exhibit suboptimal cell delivery into lesion areas, owing to the possible inability of cells to cross the blood–brain barrier (BBB) or potential retention in other organs [10, 15]. Although MSCs have a high potential to cross the BBB, their intrathecal delivery elicits the most significant therapeutic effect, resulting in enhanced cell bioavailability near damaged CNS regions. Furthermore, the apparent absence of significant serious adverse events (SAEs) after intrathecal cell therapy suggests that many cells could be administered without risk [16].
In addition, controlled clinical trials have demonstrated comparable incidences of SAEs between intrathecal injection of MSCs and sham/placebo control groups [15]. Nevertheless, in neurologic MSC therapy studies, the intravenous route remains the most commonly utilised, closely followed by intrathecal injection [17]. This preference may be attributed to ethical considerations arising from the potential risks associated with lumbar puncture and MSCs administration [11]. In this systematic review and meta-analysis, our main objective is to assess the safety profile of intrathecal MSCs administration in adult patients with neurological conditions based on evidence from RCTs that met the inclusion criteria.
Methods
Study design
The present study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses—PRISMA 2020 statement, and the review protocol was registered on Prospero 2023 [CRD42023422142].
Search strategy and selection criteria
We included only published RCTs limited to human studies available in English. The following electronic databases were searched: Ovid MEDLINE, Embase, Scopus, Cochrane Library, KCI-Korean Journal Database, and Web of Science Databases from their inception until April 13, 2023. Additionally, reference lists were examined by cross-checking the bibliographies of articles and the relevant reviews retrieved. The entire search strategy for all databases is presented in the Supplementary Material.
The inclusion criteria were studies conducted in (a) adults with neurological disorders with (b) intrathecal administration of MSCs from any source or differentiated MSCs (NSC—neuronal stem cell), only or combined with intravascular or intramuscular administration, regardless of cell source; only or combined with standard therapy; (c) control group did not receive MSCs-based therapy; a control group received a placebo, nontreatment, standard treatment or sham procedure; (d) that reported safety outcomes (any AEs associated with MSCs or differentiated MSCs (NSC—neuronal cell) treatment; one AEs reported by more than one study; regardless of the efficacy of MSCs therapy for neurological disorders; and were (e) published RCTs. The inclusion criteria of the studies are summarised in Table 1.
Study selection
After deleting duplicated studies using Rayyan software, two independent authors (LEM and JCC) screened the titles and abstracts retrieved through the search strategy and performed the study selection. The full-text versions of all relevant articles were retrieved for the screening process, enabling the assessment of their compliance with the predefined inclusion criteria (LEM and JCC). Disagreements between the two independent reviewers were resolved by discussing or referring to a third reviewer (LLQ) for the final decision. During the data analysis phase, studies lacking information regarding safety outcomes were excluded. Additionally, studies where authors were requested to provide safety-related data via email but failed to do so were excluded from the analysis.
Data extraction
Two independent authors (LEM and JCC) used a predefined data extraction form to collect the data for each included study. Any disagreements between the two independent reviewers were resolved by a third reviewer (LLQ) for the final decision. Any missing information was requested from the study's corresponding author through e-mail.
Safety and feasibility
The primary outcome measures included the safety profile evaluated by assessing the occurrences of AEs: an AE was defined as any untoward medical occurrence in a patient, that does not necessarily have a causal relationship with the intrathecal delivery of MSCs treatment. SAEs were considered if they resulted in death or an immediately threatened life, resulted in hospitalisation or longer than anticipated stay in the hospital, or resulted in persistent or significant disability or incapacity. In the context of this study, AEs included any complications associated with the intrathecal delivery of MSCs or differentiated MSCs (NSC—neuronal stem cell) treatment; one AE reported by more than one study, regardless of the efficacy of MSCs therapy for neurological conditions, or serious AEs (SAEs) associated with treatment. The occurrences of AEs were categorised by CTCAE version 5.0 (Common Terminology Criteria for Adverse Events). We used the CONSORT approach for harm reporting to assess the completeness of AEs documentation [18]. Specifically, we examined whether the expected AEs (types of events, frequency, and follow-up) had been monitored and recorded in the methods section. The definitions of safety outcomes are available in Table 2. All the data were extracted at the final follow-up.
The data extracted from the RCTs included in this study were related to (a) study characteristics as (first author, publication year, location, number of centres, sample size, and follow-up); (b) patient characteristics (age and sex); (c) characteristics of the MSCs treatment and control treatment: dosage, MSCs sources, origin (autologous or unmatched allogeneic), delivery route, MSCs preparation (fresh or cryopreserved), administration frequency (single or multiple doses) (d) information on the co-therapy.
Quality assessment and certainty of the evidence
Two independent reviewers (LEM and FBA) assessed the risk of bias for each included study. Discussions resolved disagreements, or a third reviewer (LLQ) was consulted for the final decision. The revised Cochrane risk-of-bias tool for randomised controlled trials was applied for each study included in the systematic review and meta-analysis [19]. We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to rate the certainty of evidence for the safety and efficacy outcomes extracted [20].
Data analysis
Meta-analyses for each pre-specified AE classification were categorised by CTCAE version 5.0 and performed using Stata SE 18—Multivariate meta-analysis software (Stata Corp, Texas, USA). The data were analysed with a random effects model using the DerSimonian-Laird method with the correction of zero-count cells. The pooled dichotomous outcome was described using Relative Risks (RR) with their corresponding 95% confidence intervals (95% CIs). Inconsistency between RCTs was measured using I2 and a 10% cut-off for significance [21,22,23]. Additionally, we used the L’Abbé plot to explore the potential sources of heterogeneity in the meta-analysis. Although we used a random-effects meta-analysis to incorporate heterogeneity among studies, mainly for unexplained heterogeneity, this was not a substitute for a thorough investigation among studies. We considered random-effects meta-regression as an approach to address the clinical and methodological heterogeneity of effect estimates (occurrence of AEs categorised by CTCAE version 5.0) between studies and to explore whether a linear association exists between explanatory variables (as conditions: drug-resistant symptomatic epilepsy, amyotrophic lateral sclerosis (ALS), spinal cord injury (SCI), multiple sclerosis (MS), traumatic brain injury (TBI); MSCs characteristics: cell type, origin, dosage; MSCs preparation: fresh or cryopreserved, xenogeneic or xeno-free medium) and the comparative occurrence of AEs, along with the direction of that association. Finally, we used the GRADE approach to present the safety and efficacy outcomes in a summarised descriptive table.
Reporting for bias assessment
Publication bias, outcome reporting bias (ORB), and clinical heterogeneity (variability in the participants, interventions, and outcomes) in small studies are also important sources for small-study effects (SSE). Bias analysis was evaluated at the outcome level with a funnel plot and Harbord’s modified test based on regression for small-study effects. A p-value < 0.05 suggested evidence for SSE [24].
Role of funding
The funder provided support through salaries for the authors [LEM, JCC, LLQ, FBA, KH, JAEP]. Nevertheless, it had no additional role in the study design, data collection and analysis, publication decision, or manuscript preparation. The specific roles of these authors are articulated in the “author contributions' section.” Finally, our commercial affiliation did not play a role in this study.
Results
Study selection
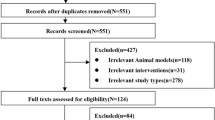
A total of 581 articles were extracted from the literature search. After omitting duplicate studies using an automated tool (Rayyan System, Inc. 2022), 303 articles underwent title and abstract screening. Among these articles, 13 were selected for full-text review. Finally, nine RCTs were included in the study quality assessment and data analysis [7, 9, 10, 12, 17, 25,26,27,28]. The literature search and study inclusion process are presented in Fig. 1.
Study characteristics
Nine RCTs were included (n = 540 patients), and their sample sizes ranged from 10 to 189 patients (60 ± 52.18, mean ± standard deviation); the follow-up periods ranged from one day to 14 months (9.22 ± 3.93, mean ± standard deviation), and the publication dates from 2013 to 2021. The included RCTs were conducted in six different countries: Belarus (two RCTs), the USA (two RCTs), Spain (one RCT), China (two RCTs), Israel (one RCT), and the Republic of Korea (one RCT). Seven studies enrolled patients from a single-centre [7, 10, 12, 17, 25,26,27], and two were multi-centre [9, 28]. The study characteristics and details about the participants, such as age, sex, intervention and control groups, location, follow-up, clinical conditions, and dosage in the nine RCTs included, are listed in Table 3. Among the included studies, three (33.33%) RCTs used Bone Marrow-derived Mesenchymal Stem Cells (BM-MSCs) [10, 12, 27], two (22.22%) used Umbilical Cord Mesenchymal Stem Cells UC-MSCs [7, 25], and four (44.44%) used Bone Marrow-derived Mesenchymal Stem Cells induced to secrete high levels of Neurotrophic Factors (BMMSCs-NTF) [9, 17, 26, 28]. Concerning the dose, two (22.22%) RCTs administered ≤ 106 cells [17, 26], and seven (77.78%) administered between 107 and 108 cells [7, 9, 10, 12, 25, 27, 28]. Seven (77.78%) RCTs used autologous MSCs [9, 10, 12, 17, 26,27,28], and two (22.22%) used unmatched allogeneic MSCs [7, 25]. Six (66.67%) RCTs used fresh cells [9, 12, 17, 25,26,27], and three (33.33%) used cryopreserved MSCs before administration [7, 10, 28]. None of the RCTs included used xenogeneic products, and nine (100%) RCTs cultured the MSCs in a xeno-free medium. Among the 283 patients in the intervention group, 103 received a single dose [7, 9, 17, 26, 27], and 180 received multiple doses [10, 12, 25, 28]. All included RCTs used intrathecal (IT) injection as a treatment delivery route. The most common standard treatments included were Anti-epileptic drugs (AED) such as riluzole. Some important MSCs characteristics, such as the origin (autologous or unmatched allogeneic), MSC preparation (fresh or cryopreserved), xeno-free culture medium, administration frequency (single or multiple doses) and dosage, related to each one of the included studies are detailed in the Supplementary Material (Table S1).
Risk of bias assessment
The risk of bias in the included studies is summarised in Fig. 2. Five studies comprising six safety outcomes had some bias concerns due to outcome measurement (46.2%) [12, 17, 25,26,27]. For the overall bias, five studies comprising six safety outcomes were defined as having some bias concerns (38.5%) [12, 17, 25,26,27], and four studies comprising seven outcomes were described as having a low risk of bias (61.5%) [7, 9, 10, 28].
Publication bias
Publication bias was evaluated with a funnel plot (see Fig. 3), leading to an asymmetrical appearance with a gap on the bottom left-hand side of the graph, which could suggest missing studies. Since this area contains regions of high significance, publication bias is unlikely to be the underlying cause of asymmetry. As a rule, tests for funnel plot asymmetry should only be used when more than ten studies are in the meta-analysis. The power of the test is too low to distinguish chance from real asymmetry, and publication bias cannot be excluded [24, 29]. Therefore, the test results were interpreted in the context of visual inspection of the funnel plot. Despite having only nine RCTs, we performed Harbord’s modified test based on regression for small-study effects, and the test suggested evidence for small-study effects (p = 0.021).
Adverse events (AEs) and serious adverse events (SAEs)
A description and frequency of AEs and SAEs are provided in Tables 2, 4, and 5. The meta-analysis of overall AEs for all RCTs included in this study showed a not significantly higher rate of AEs in the MSCs group compared to the control group (RR: 1.31; 95% CI 0.97–1.77; I2 = 34.92%; Fig. 4) [7, 9, 10, 12, 17, 25,26,27,28]. We assessed heterogeneity by comparing the rate of overall AEs between the MSCs and control groups (Fig. 5); each circle in the L’Abbé plot represents an individual RCT, and larger circles represent RCTs with more AEs; the dotted diagonal line indicates that the rate of overall AEs is equal in the two groups within RCTs. The overall AEs rates exhibited significant variability in the MSCs group (0.23–57.27%) and the control group (0–85.19%). The highest rate of overall AEs in the MSCs group was reported in the RCT by Cudkowicz et al. (57.27%). On the other hand, the lowest rate of overall AEs in the MSCs group was observed in the trial by Hlebokazov et al. (0.231%). Four RCTs reported SAEs; Cudkowicz et al. reported deaths related to disease progression and other causes unrelated to the study intervention; Petrou et al. adjudged SAEs to MS relapses and an upper respiratory infection; Berry et al. reported that all SAEs were related to ALS disease progression; and Oh K-W et al., regarded deaths as due to respiratory failure related to disease progression and sudden cardiac arrest. The meta-analysis of the pooled effect estimates showed non-significant differences in the occurrence of SAEs in the MSCs group compared to the control group (RR: 1.24; 95% CI 0.70–2.20; I2 = 11.78%; Fig. 6) [9, 10, 12, 28]. Table 6 provides a comprehensive summary of the safety outcomes and the quality of the safety reporting findings.
System organ classes (SOC) related AEs
AEs related to general disorders and administration site conditions
Meta-analysis of four RCTs that reported fever (within 24 h after MSCs injection) and pain (injection site pain) showed a not significant, slightly higher rate of AEs with the MSCs group when compared to the control group (RR: 2.07; 95% CI 0.76–64.94; I2 = 0%; Fig. 7) [9, 10, 12, 17].
AEs related to musculoskeletal and connective tissue disorders
Meta-analysis of six RCTs reported the following AEs: (i) pain in extremities—upper or lower (four RCTs), (ii) back pain—nonspecific, short duration back pain (four 4 RCTs), (iii) arthralgia (one RCT), (iv) myalgia (one RCT), (v) rhabdomyolysis (one RCT), and (vi) neck pain (one RCT). The pooled estimate of the individual effect sizes of the primary RCTs revealed a significant rate of AEs was slightly higher with MScs compared to the control group (RR: 1.61; 95% CI 1.19–2.19; I2 = 0%; Fig. 8) [7, 9, 10, 12, 17, 28].
AEs related to nervous system disorders
Nine RCTs reported headache—nonspecific/positional; one RCT reported muscular weakness; two RCTs reported dizziness; and another RCT informed facial nerve disorder—peripheral facial nerve palsy. Meta-analysis of pooled estimates for AEs related to nervous system disorders revealed a slightly higher rate in the MSCs group compared to the placebo group, but the difference was not significant (RR: 1.16; 95% CI 0.87–1.54; I2 = 0%; Fig. 9) [7, 9, 10, 12, 17, 25,26,27,28].
AEs related to infections and infestations
Meta-analysis of three RCTs reported the occurrence of urinary tract infection (two RCTs), viral infection (one RCT), upper respiratory infection (one RCT), sinusitis (one RCT), scabies infection (one RCT), infection in the distal arm (one RCT), and kidney infection (one RCT). The pooled estimate of effect size revealed a not significant, slightly higher rate of AEs in the MSCs group compared to the control group (RR: 1.40; 95% CI 0.46–4.25; I2 = 0%; Fig. 10) [10, 12, 27].
AEs related to respiratory, thoracic, and mediastinal disorders
Two RCTs reported respiratory failure and cough (both related to disease progression). A pooled analysis detected a higher rate of AEs in the MSCs group compared to the control group, but the difference between the two groups was not significant (RR: 1.19; 95% CI 0.16–9.08; I2 = 20.14%; Fig. 11) [9, 12].
AEs related to gastrointestinal disorders
Four RCTs reported AEs such as nausea, vomiting, gastrointestinal dysfunction, and constipation. The results of the meta-analysis showed that the rate of AEs was slightly higher with MSCs compared to the control group, but the difference was not significant (RR: 1.07; 95% CI 0.63–1.83; I2 = 0%; Fig. 12) [7, 9, 27, 28].
AEs related to injury, poisoning, and procedural complications
The pooled estimate of the effect size of the three RCTs revealed a not significantly higher rate of AEs in the MSCs group than in the control group (RR: 1.07; 95% CI 0.85–1.34; I2 = 0%; Fig. 13). The AEs reported in three RCTs were procedural pain, post-procedural complications, post-lumbar puncture syndrome, falls, fractures (leg/hand), and contusions [10, 12, 28].
AEs related to cardiac disorders
One RCT reported cardiac arrest (not considered to be treatment-related) [12].
AEs related to vascular disorders
One RCT reported a Haematoma (unrelated to the procedure or treatment) [10].
AEs related to metabolism and nutrition disorders
One RCT reported hyponatraemia (not considered treatment-related) [12].
Model of probability p of AEs occurrence with intrathecal MSCs therapy
We performed a comprehensive random effects meta-regression model instead of a subgroup analysis because the meta-regression method efficiently allows the evaluation of one or more covariates simultaneously and explores the sources of clinical or methodological heterogeneity of treatment effects across RCTs. The models obtained are described in Table 7. The pooled estimate of AEs associated with musculoskeletal and connective tissue disorders demonstrated a significantly higher rate in the MSCs group compared to the control group. (RR: 1.61; 95% CI 1.19–2.19; Fig. 7), while a Cochran’s Q statistics test (p = 0.78) and an I2 = 0% suggested homogeneity between studies; however, we were able to show differences in the rate of AEs occurrence (treatment effect) based on the MSCs preparation (fresh or cryopreserved) and administration frequency (single or multiple doses) when we conducted the meta-regression. Upon meta-regression, we demonstrated a significant positive association between the probability p of AE occurrence with intrathecal MSCs therapy when fresh MSCs were used compared to cryopreserved cells. (RR: 1.554; 95% CI 1.004–2.404; p = 0.048*; R-squared = 100% ‘best fit’ line; Model 2). On the other hand, in Model 3, the meta-regression suggested that the probability p of AE occurrence resulting from RCTs that delivered multiple doses of MSCs decreased (36%) compared to the RCTs that offered a single dose of cells (RR: 0.644; 95% CI 0.416–0.996; p = 0.048*; R-squared = 100% ‘best fit’ line; Model 3). However, the joint test for all three covariates yielded a p-value (Model 2) of 0.106 and a p-value (Model 3) of 0.106, indicating that no evidence existed for an association of at least one of the three covariates with the size of the treatment effect (AEs occurrence). Additionally, we performed univariate random effects meta-regression models of the probability p of AE occurrence with intrathecal MSCs therapy to control the higher likelihood of false-positive findings when performing meta-regression with multiple covariates. Nevertheless, the results of the meta-regress did not show evidence for an association between the rate of AE occurrence and any covariate included in the analysis (univariate models can be found in Supplementary Material Table S2).
Certainty of the evidence (GRADE assessment)
We used the GRADE approach to rate the quality of evidence for each critical outcome of our study's safety and efficacy profile. We developed and presented summary findings for all comparisons between MSC therapy and control interventions in the included RCTs.
The quality of evidence was downgraded for risk of bias, inconsistency, and imprecision of the results. The certainty of evidence of the proportion of patients with AEs outcomes in the MSCs group and control group was moderate (outcome measures were reported in 100% of RCTs); the certainty of the proportion of patients with SAEs in the MSCs group and control group was moderate (outcome measures were reported in four (44.4%) RCTs); the certainty of the proportion of patients with AEs outcomes at general disorders and administration site conditions (AEs categorised by CTCAE version 5.0) in the MSCs group and control group was moderate (outcome measures were reported in four (44.4%) RCTs); the certainty of the proportion of patients with AEs outcomes at musculoskeletal and connective tissue disorders in the MSCs group and control group was high (outcome measures were reported in four (66.67%) RCTs); the certainty of the proportion of patients with AEs outcomes at nervous system disorders in the MSCs group and control group was moderate (outcome measures were reported in 100% RCTs); the certainty of the proportion of patients with AEs outcomes at infections and infestations in the MSCs group and control group was moderate (outcome measures were reported in three (33.33%) RCTs); the certainty of the proportion of patients with AEs outcomes at respiratory, thoracic and mediastinal disorders in the MSCs group and control group was moderate (outcome measures were reported in two (22.22%) RCTs); the certainty of the proportion of patients with AEs outcomes at gastrointestinal disorders in the MSCs group and control group was moderate (outcome measures were reported in four (44.44%) RCTs); the certainty of the proportion of patients with AEs outcomes at injury, poisoning and procedural complications in the MSCs group and control group was moderate (outcome measures were reported in three (33.33%) RCTs); For all safety outcomes, downgrading of certainty of evidence due to imprecision were (serious, 1 level); except for AEs related to musculoskeletal and connective tissue disorders where was high. The certainty of evidence for the primary efficacy outcomes summarised can be seen in Supplementary Material Table S3.
Current status and ongoing clinical trials that included a control group
We also searched ongoing trials in the following websites and online databases of clinical research studies (last searched July 28, 2023): the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov); the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) and, The European Union Clinical Trials Register (www.clinicaltrialsregister.eu). The unpublished findings are shown in Supplementary Material Table S4, complementing the current information.
Discussion
To the best of our knowledge, this study is the first systematic review and meta-analysis that summarises the safety profile of the intrathecal delivery of MSCs and supports the findings of trials designed to evaluate the safety and efficacy of single or multiple doses of intrathecal MSCs treatment in which the authors suggest that this route of cell administration is a potentially safe treatment for patients with neurological conditions [7, 9, 10, 12, 17, 25,26,27,28]. However, the results of the meta-analysis showed that the pooled estimate of the individual effect sizes of RCTs revealed that a significant rate of AEs related to musculoskeletal and connective tissue disorders was slightly higher with MSCs when compared to the control group; our univariate random effects meta-regression models were not able to demonstrate associations between the rate of AEs occurrence and the covariates included in the analysis. However, it should also be emphasised that these analyses (meta-regression) are exploratory, and the magnitude of the effect and its 95% confidence interval are more relevant than whether a p-value is less than or greater than 0.05. In addition to statistical significance, clinical relevance is essential, especially in systematic reviews and meta-analyses, where only associations, not causality, can be demonstrated [30].
Several studies reported the occurrence of non-SAE, transient AEs concerning the local response to the therapy and the lumbar puncture procedure; these AEs were categorised by CTCAE version 5.0 within general disorders and administration site conditions. Our findings demonstrated that the occurrence of AEs, such as fever and pain at the injection site, was not significant (RR: 2.07; 95% CI 0.76–64.94) compared to the control group that received placebo or conventional therapy. It is important to note that the symptoms related to the occurrence of AEs categorised by CTCAE, such as musculoskeletal and connective tissue disorders, which had a significantly higher rate of AEs (back pain -nonspecific, short-duration back pain; pain in extremities; arthralgia; myalgia; neck pain) give rise to minimal risk to patients undergoing MSCs therapy if they are monitored closely in the early days after intrathecal application.
Our systematic review and meta-analysis were performed using a comprehensive methodology explicitly tailored to studying the safety profile of MSC therapy. We used a wide range of electronic databases to reduce the potential for publication bias and provide a general overview of the literature. While other systematic reviews have also supported the safety of MSC therapy delivered through different routes, our study is notable because it utilises statistical techniques to pool safety data related to the intrathecal delivery of MSCs on a common endpoint for nine RCTs included.
The present study has several limitations that must be acknowledged. First, Harbord’s modified test based on regression for small-study effects suggested evidence supporting such effects (p = 0.021), confirmed following a visual inspection of the funnel plot (see Fig. 3). However, smaller studies may also be biased because they are targeted at high-risk groups (as in our case, patients with neurological conditions), where the MSCs therapy is likely beneficial, resulting in a trend for smaller studies to show higher treatment effects. Second, we included two RCTs [7, 10] that utilised a crossover design. However, this design can be problematic due to the potential for compromised blinding from the treatment effects and for the probability of the patients randomised in the first step not returning to the baseline conditions before the second crossover (carryover effects). In the case of these two RCTs, these concerns were mitigated by authors using a control group such as placebo infusion [7] or placebo sham treatment [10] and using ample time–space (six months) between the first and second cycle of MSC treatment [7, 10].
Conclusions
In summary, the administration of MSCs via intrathecal delivery resulted in a minor increase in AEs linked to musculoskeletal and connective tissue disorders. However, no SAEs were observed, confirming findings from prior clinical trials showing that the intrathecal injection of MSCs may be a safe delivery route for patients with neurological conditions. Limited by the evidence for small-study effects and the crossover design of trials that can be problematic, large sample sizes and well-designed multi-centre RCTs with long follow-up periods are required in future analyses.
Availability of data and materials
The data collected for this systematic review and meta-analysis are secondary and are available in the original published trials. Pooled data through meta-analysis will be available within the manuscript or Supplementary Material. Additional information can be accessed through the corresponding author upon request.
References
Alessandrini M, Preynat-Seauve O, Briun K, Pepper M. Stem cell therapy for neurological disorders. S Afr Med J. 2019;109:70.
Carroll WM. The global burden of neurological disorders. Lancet Neurol. 2019;18(5):418–9.
Zayed MA, Sultan S, Alsaab HO, Yousof SM, Alrefaei GI, Alsubhi NH, et al. Stem-cell-based therapy: the celestial weapon against neurological disorders. Cells. 2022;11(21):3476.
Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal stem cells for neurological disorders. Adv Sci. 2021;8(7):2002944.
Kim HJ, Cho KR, Jang H, Lee NK, Jung YH, Kim JP, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimers Res Ther. 2021;13(1):1–11.
Venkataramana NK, Kumar SKV, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62–70.
Albu S, Kumru H, Coll R, Vives J, Vallés M, Benito-Penalva J, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomised controlled study. Cytotherapy. 2021;23(2):146–56.
Mohseni R, Hamidieh AA, Shoae-Hassani A, Ghahvechi-Akbari M, Majma A, Mohammadi M, et al. An open-label phase 1 clinical trial of the allogeneic side population adipose-derived mesenchymal stem cells in SMA type 1 patients. Neurol Sci. 2022;43(1):399–410.
Berry JD, Cudkowicz ME, Windebank AJ, Staff NP, Owegi M, Nicholson K, et al. NurOwn, phase 2, randomised, clinical trial in patients with ALS. Neurology. 2019;93(24):e2294.
Petrou P, Kassis I, Levin N, Paul F, Backner Y, Benoliel T, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143(12):3574–88.
El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HAMA, et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transpl. 2014;23(6):729–45.
Oh KW, Noh MY, Kwon MS, Kim HY, Oh S, Park J, et al. Repeated intrathecal mesenchymal stem cells for amyotrophic lateral sclerosis. Ann Neurol. 2018;84(3):361–73.
Qu M, Xing F, Xing N. Mesenchymal stem cells for the treatment of cognitive impairment caused by neurological diseases. Biotechnol Lett. 2022;44(8):903–16.
Chen WC, Liu WF, Bai YY, Zhou YY, Zhang Y, Wang CM, et al. Transplantation of mesenchymal stem cells for spinal cord injury: a systematic review and network meta-analysis. J Transl Med. 2021;19(1):1–14.
Amanat M, Majmaa A, Zarrabi M, Nouri M, Akbari MG, Moaiedi AR, et al. Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: a randomised double-blind sham-controlled clinical trial. Stem Cell Res Ther. 2021;12:1–15.
Vaquero J, Zurita M, Rico MA, Aguayo C, Bonilla C, Marin E, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20(6):806–19.
Hlebokazov F, Dakukina T, Potapnev M, Kosmacheva S, Moroz L, Misiuk N, et al. Clinical benefits of single vs repeated courses of mesenchymal stem cell therapy in epilepsy patients. Clin Neurol Neurosurg. 2021;207:106736.
Ioannidis JPA, Evans SJW, Gøtzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomised trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–8.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193.
Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Qstatistics. BMC Med Res Methodol. 2011;11(1):1–12.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine. 2019;98(23):e15987.
Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;26(1532):76–84.
Hlebokazov F, Dakukina T, Ihnatsenko S, Kosmacheva S, Potapnev M, Shakhbazau A, et al. Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: an open label study. Adv Med Sci. 2017;62(2):273–9.
Song H, Suo S, Ning C, Zhang Y, Mu W, Chen S. Bone marrow mesenchymal stem cells transplantation on acute spinal cord injury. J Hard Tissue Biol. 2020;29(2):91–8.
Cudkowicz ME, Lindborg SR, Goyal NA, Miller RG, Burford MJ, Berry JD, et al. A randomised placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve. 2022;65(3):291–302.
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;22(343):d4002.
Baker WL, Michael White C, Cappelleri JC, Kluger J, Coleman CI. From the Health Outcomes and Economics (HOPE) Collaborative Group P. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009;63(10):1426–34.
Acknowledgements
We thank BioXcellerator, BioXscience, and BioXtech for their administrative assistance and critical manuscript review.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualisation: LEM, JCCB, LLQ, JAEP, KH; Study design: LEM, JCCB, LLQ; Literature search: LEM, JCCB; Risk of bias assessment: LEM, FBA, LLQ; Data extraction: LEM, JCCB, LLQ; Data verification: LEM, JCSU; Software: LEM; Formal analysis: LEM, JCSU; Writing—original draft: LEM, JCCB, LLQ; Writing—review and editing: LEM, JCCB, LLQ. All authors had access to all meta-analysed data used in this study and accepted responsibility for the decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interest
BioXcellerator provided salary support for authors [LEM, JCCB, LLQ, FBA, KH, JAEP] but had no additional role in the study design, data collection and analysis, publication decision, or manuscript preparation. The specific roles of these authors are articulated in the “Contributors’ section.” Finally, our commercial affiliation did not play a role in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mesa Bedoya, L.E., Camacho Barbosa, J.C., López Quiceno, L. et al. The safety profile of mesenchymal stem cell therapy administered through intrathecal injections for treating neurological disorders: a systematic review and meta-analysis of randomised controlled trials. Stem Cell Res Ther 15, 146 (2024). https://doi.org/10.1186/s13287-024-03748-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03748-7