Abstract
Background
Transcription factors HAND1 and HAND2 (HAND1/2) play significant roles in cardiac organogenesis. Abnormal expression and deficiency of HAND1/2 result in severe cardiac defects. However, the function and mechanism of HAND1/2 in regulating human early cardiac lineage commitment and differentiation are still unclear.
Methods
With NKX2.5eGFP H9 human embryonic stem cells (hESCs), we established single and double knockout cell lines for HAND1 and HAND2, respectively, whose cardiomyocyte differentiation efficiency could be monitored by assessing NKX2.5-eGFP+ cells with flow cytometry. The expression of specific markers for heart fields and cardiomyocyte subtypes was examined by quantitative PCR, western blot and immunofluorescence staining. Microelectrode array and whole-cell patch clamp were performed to determine the electrophysiological characteristics of differentiated cardiomyocytes. The transcriptomic changes of HAND knockout cells were revealed by RNA sequencing. The HAND1/2 target genes were identified and validated experimentally by integrating with HAND1/2 chromatin immunoprecipitation sequencing data.
Results
Either HAND1 or HAND2 knockout did not affect the cardiomyocyte differentiation kinetics, whereas depletion of HAND1/2 resulted in delayed differentiation onset. HAND1 knockout biased cardiac mesoderm toward second heart field progenitors at the expense of first heart field progenitors, leading to increased expression of atrial and outflow tract cardiomyocyte markers, which was further confirmed by the appearance of atrial-like action potentials. By contrast, HAND2 knockout cardiomyocytes had reduced expression of atrial cardiomyocyte markers and displayed ventricular-like action potentials. HAND1/2-deficient hESCs were more inclined to second heart field lineage and its derived cardiomyocytes with atrial-like action potentials than HAND1 single knockout during differentiation. Further mechanistic investigations suggested TBX5 as one of the downstream targets of HAND1/2, whose overexpression partially restored the abnormal cardiomyocyte differentiation in HAND1/2-deficient hESCs.
Conclusions
HAND1/2 have specific and redundant roles in cardiac lineage commitment and differentiation. These findings not only reveal the essential function of HAND1/2 in cardiac organogenesis, but also provide important information on the pathogenesis of HAND1/2 deficiency-related congenital heart diseases, which could potentially lead to new therapeutic strategies.
Similar content being viewed by others
Background
Normal cardiac morphogenesis relies on the precise regulation of numerous transcription factors (TFs) [1]. Mutations in these TFs are known to cause common cardiac defects [2]. Congenital heart diseases (CHDs) are the most prevalent congenital defects, with a morbidity rate of approximately 1% [3], in which 25% of infants require intervention within the first year after birth [4]. Despite advances in medication and surgical procedures, CHDs continue to be the leading cause of death in individuals with congenital defects [5]. Furthermore, survivors of CHDs often experience cardiac comorbidities, reduced quality of life and increased burden [6]. Due to the characteristic segmental development of the heart, CHDs primarily affect a single chamber or a specific part of the heart [7].
As the chamber-specific TFs, HAND1 and HAND2 (HAND1/2) play crucial roles in cardiac morphogenesis [8]. They are members of the basic helix–loop–helix (bHLH) family, functioning through DNA-binding, protein–protein interaction and reprograming the enhancer/promoter connectome [9,10,11]. Currently, it has been accepted that the cardiac lineage is determined at primitive streak and mesoderm stages during embryo development [12,13,14]. Then, the cardiac mesodermal cells migrate and form cardiac crescent to generate the first heart field (FHF) and second heart field (SHF) progenitors, which produce different subtypes of cardiomyocytes [15]. The FHF progenitors are characterized by the expression of HAND1, TBX5 and HCN4 [16,17,18], and ultimately differentiate into left ventricular cardiomyocytes. The SHF is further classified as anterior SHF (aSHF) and posterior SHF (pSHF). The aSHF progenitors are identified by the markers TBX1, SIX1 and FOXC2 [19, 20], and primarily give rise to right ventricular and outflow tract (OFT) cardiomyocytes while the pSHF progenitors are distinguished by NR2F2, HOXA1, HOXB1 and ALDH1A2 expression [19, 20], and mainly develop into atrial cardiomyocytes. In mouse, the single-cell transcriptome of embryos showed that Hand1 was first detected in nascent mesoderm at E6.5-E6.75 [21]. At E7.75, Hand1 was expressed in cardiac crescent which contributed to a subset of left ventricular cardiomyocytes later [22,23,24]. Hand2 was first detected in nascent mesoderm at E7.0 [21], then, together with Hand1, expressed in cardiac crescent and played vital roles in the patterning of heart fields [15, 25]. Knockout of Hand1 led to left ventricular hypoplasia, interventricular septal defects and cardiac conduction system defects [26, 27]. Hand2 was required for the survival and differentiation of SHF progenitors and its deficiency resulted in right ventricular defects [25, 28,29,30]. Besides, mice with Hand1 and Hand2 double knockout were embryonically lethal, displaying a single ventricle and a common atrium [27].
Due to the lack of human embryo data, the expression profile of human HAND was not as clear as that of the mouse. Currently available early human embryo single-cell sequencing data showed that HAND1 and HAND2 were detected in mesoderm of CS7 (Carnegie stage 7) embryo [31], which is equivalent to mouse E7.0 [32], and expressed in lateral plate mesoderm of human CS10-CS14 embryos [33]. In human heart, HAND1 was specifically expressed in left ventricular cardiomyocytes, which originate from the FHF [34, 35], while HAND2 showed high expression in atrial cardiomyocytes, which are the progeny of the SHF [35, 36]. Concomitantly, during cardiomyocyte differentiation from human pluripotent stem cells (hPSCs), HAND1 was highly expressed in FHF progenitors and ventricular cardiomyocytes, while HAND2 was upregulated in differentiated atrial cardiomyocytes [19, 37]. Furthermore, pathogenic mutations of HAND1 were related to left ventricular hypoplasia and ventricular septum defects [38,39,40], while HAND2 deficiency contributed to ventricular septum defects and double outlet right ventricle [41]. In severe CHDs, such as tetralogy of Fallot, mutations of HAND1/2 have been identified as well [42, 43]. Nevertheless, the roles of HAND in human early cardiac lineage commitment and differentiation are not completely clarified and need further investigation.
The pathogenicity of Hand in mouse is not the same as human HAND1/2 mutation related CHDs. In addition, the embryo development and gene expression between primates and rodents are different [35, 44], which restricts the application of mouse model to investigate HAND function in human heart development and related CHDs. hPSCs have been widely selected as alternative for animal models in studying human diseases in vitro. With the scalability of cardiomyocyte differentiation [45] and the ability to mimic the in vivo heart development process, hPSCs have broad applications such as cardiac disease models, cardiac regeneration and drug screening [46,47,48], especially in cardiac lineage development [49, 50].
Here, we utilized the NKX2.5eGFP H9 human embryonic stem cells (hESCs) along with the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology to establish HAND1/2 single and double knockout cell lines. With an in vitro cardiomyocyte differentiation system, we systematically investigated the effects of HAND1 and HAND2 deficiency on cardiac lineage commitment and differentiation. We characterized the unique and redundant roles of HAND1 and HAND2 and verified TBX5 as one of the key targets in HAND1/2 gene regulatory network during heart development.
Methods
Human embryonic stem cell culture
Human embryonic stem cell H9 (hESC-H9) based NKX2.5 reporter cell line (NKX2.5eGFP H9) was purchased from Shanghai model organism Co., Ltd. NKX2.5eGFP H9 and its derived hESC lines were cultured in Matrigel (Corning, USA) coated cell culture plates with PSCeasy culture medium (Cellapy, China) at 37 °C, 5% CO2. When reaching 80–90% confluence, the cells were dissociated with human multipotent stem cell digestive solution (Cellapy), passaged at 1:3 to 1:6, and cultured in PSCeasy medium with ROCK inhibitor Y-27632 (10 μM) for 24 h. Then, the medium was replenished every day without Y-27632.
Cardiomyocyte differentiation and culture
The cardiomyocyte differentiation protocol was conducted by modulating canonical Wnt signaling with some modifications [51, 52]. When NKX2.5eGFP H9 cells grew to 80–90% confluence, the cells were dissociated with Accutase (Sigma-Aldrich, USA) and resuspended in PSCeasy with Y-27632. Accurate cell counting was necessary to achieve stable differentiation efficiency. 1.5–1.8 × 105 cells (NKX2.5eGFP H9 and HAND single knockout cell lines) were plated in Geltrex (Gibco, USA) coated 12-well plates for the following differentiation. For HAND double knockout cell line, the differentiation started with 1.5–1.6 × 105 cells per 12-well.
On the second day after seeding, the medium was switched to N2B27 (100 mL DMEM/F12, 100 mL Neurobasal with 1 mL N2 and 2 mL B27) [53, 54] with 3 μM CHIR99021 (Selleck, USA) to activate WNT signaling for 48 h (days 0–2). Then, the medium was changed to RPMI1640/B27 minus insulin (RPMI/B27-) for 24 h (day 2–3). At day 3, 2 μM Wnt-C59 (MCE, USA) was added into RPMI/B27- to inhibit WNT signaling for 48 h (days 3–5), then the cells were cultured in RPMI/B27- for 2 days (days 5–7). At day 7, the medium was changed to RPMI/B27 for continuous differentiation. 0.5 mM Vitamin C (Vc) was added to the differentiation medium during days 0–7. The eGFP fluorescence and beating cardiomyocytes were monitored under fluorescence microscope.
To culture hESCs-derived cardiomyocytes, the cells were dissociated with human cardiomyocytes digestive solution I (Cellapy) for 13–15 min and digestive solution II (Cellapy) for 20–25 min at 37 °C. Cells were gently detached and centrifuged at 1200 rpm for 5 min. The cells were resuspended in RPMI/B27 with Y-27632 and replated for the following experiments. Medium was switched to RPMI/B27 after 48 h and then replenished every other day.
Vector construction
Human HAND1 or HAND2 cDNA was cloned into PBCAG transposon under the control of the CAG promotor using homologous recombination to generate PBCAG-HAND1 and PBCAG-HAND2 vectors. Human TBX5 cDNA was cloned into PBTRE transposon under the control of doxycycline (DOX) inducible Tet response element using homologous recombination to generate PBTRE-TBX5 vector.
To construct TBX5 promoter driven luciferase expression vector, the sequence of TBX5 promoter (− 2 kb ~ + 100 bp) was downloaded from the website (https://pubmed.ncbi.nlm.nih.gov/) and amplified from the genome of H9 hESCs with PCR. The purified PCR product was ligated to the restriction enzyme digested pGL3-basic Luciferase vector fragment (Promega, WI, USA) by homologous recombination to construct the pGL3-TBX5-Luciferase vector. The primers used are listed in Additional file 1: Table S1.
Generation of HAND gene knockout (KO) cell line
Single guide RNA (sgRNA) was designed on the website (http://crispor.tefor.net/) for genome editing. HAND1-sgRNA (5′AGCGCGAGGCCGGACCGAAG3′) and HAND2-sgRNA (5′GGACCACTCCCATTACGGGG3′) targeting exon 1 of HAND1 and HAND2, respectively, were used for constructing HAND gene KO cell lines. The sgRNA was cloned into pGL3-U6-PGK-Puromycin vector. 1 × 106 NKX2.5eGFP H9 cells were transfected with 1 μg sgRNA and 2 μg spCas9 by LONZA P3 primary cell 4D-Nucleofector LV KIT according to the manufacturer’s protocol. After transfection, the cells were selected with 0.3 μg/mL puromycin for 3–7 days, then replated as single cells to form clones. During days 7–10, clones were picked and expanded for genotyping. Genomic PCR and Sanger sequencing were used to clarify the details of editing. Primers are listed in Additional file 1: Table S1.
Generation of TBX5 overexpressing cell lines
The HAND1/2-double-KO cell line was electro-transfected with 1 μg PBTRE-TBX5, 1 μg PBEF1α-Tet3G, and 1 μg HyPBase. The cells were selected with 0.3 μg/mL puromycin for 7 days and clones were picked. Genomic PCR was performed to identify positive clones. The expression of TBX5 transgene under 1 μg/mL DOX induction was confirmed by western blot.
In vitro embryoid body (EB) differentiation
EB differentiation was conducted as previously described [55]. 1 × 106 cells in PSCeasy with Y-27632 were plated into 6-cm petri dish on a shaker, at 60 rpm, to form EBs. After two days, when the size of EBs reached about 200 μm in diameter, the medium was switched to DMEM/F12 with 20% knockout serum replacement (KSR, Gibco, USA), then replenished every other day. After 8 days, the EBs were plated into 0.2% gelatin-coated plates for further culture. At day 15, EBs were collected for analysis.
Flow cytometry analysis and fluorescence-activated cell sorting
For flow cytometry analysis of SSEA-4 or MYL2 expression, hESCs or cardiomyocytes were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature (RT). Cells were permeabilized with 0.3% PBSTr (Triton-X100) for 15 min, RT (this step was not needed for SSEA-4). Then cells were blocked with 3% bovine serum albumin (BSA) in PBS for 30 min. After that, the cells were incubated with anti-SSEA-4 antibody (Santa Cruz, sc59368, 1:200) or anti-MYL2 antibody (Abcam, ab79935, 1:100) at 4 °C overnight. The next day, the cells were washed with PBS three times and then incubated with fluorescence-conjugated secondary antibody for 1 h, RT. After washing with PBS three times, the cells were harvested for analysis.
To monitor cardiomyocyte differentiation, at different time points, the cells were dissociated into single cells and washed with PBS twice, then filtered with 40 μm strainer. The percentage of NKX2.5-eGFP+ cells was determined by flow cytometry. FlowJo 10 and NovoExpress software were used for data analysis.
For cell sorting, at differentiation day 7, the cells were dissociated and resuspended in RPMI/B27. After being filtered with 40 μm strainer, the NKX2.5-eGFP+ cells were sorted by Beckman Coulter MoFlo Astrios EQ for subsequent analysis.
Dual-Luciferase assay
Dual-Luciferase assay was performed with HEK 293T cells to verify TBX5 as the target of HAND1/2. The cells were cultured in DMEM with 10% FBS (Gibco, USA) in 6-well plates. When reaching 40–50% confluence, the cells were transfected with 1 μg pGL3-TBX5-Luciferase, 0.1 μg Renilla plasmid and 1 μg PBCAG-HAND1 or PBCAG-HAND2 using Lipofectamine 3000 transfection reagent (Invitrogen, USA). Samples were harvested and analyzed 48 h after transfection, according to the manufacturer’s protocol (Promega).
RNA extraction, reverse transcription and Quantitative PCR (qPCR)
Cells were washed twice with PBS and lysed in Trizol (Takara, Japan). After chloroform extraction, the supernatant was precipitated with isopropanol, washed with 75% ethanol and dissolved with RNase-free water. The RNA concentration was measured with a Spectrophotometer (NanoDrop Technologies, Inc., DE, USA). The reverse transcription was carried out following the instruction of HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, China). qPCR was performed using Taq Pro Universal SYBR qPCR Master Mix with specific primers on ABI QuantStudio™ 6 Flex (Thermofisher). GAPDH expression was used to normalize the gene expression. The gene expression between different groups was compared with ΔΔCT method. The primers used are listed in Additional file 1: Table S1.
Western blot
Cells were dissociated and centrifuged at 1200 rpm for 5 min, then lysed in RIPA buffer with protease inhibitors. After incubating on ice for 30 min, the lysis was centrifuged at 12,000g for 15 min, the supernatant was collected and the protein concentration was measured with BCA methods (Beyotime Biotechnology, China). The samples were boiled for 5 min at 100 °C. NuPAGE 10% Bis–Tris gel was used for electrophoresis under 100 mv for 15 min and then 170 mV for 40 min. The protein was transferred onto PVDF membrane. After blocking with 5% non-fat milk, the membrane was incubated with primary antibodies overnight at 4 °C. The next day, after washing, the membrane was incubated with Alexa Fluor conjugated secondary antibodies for 1 h, RT. The images were captured with the Odyssey system. Primary antibodies used were: anti-GAPDH (Proteintech, 60004-1-Ig, 1:5000), anti-HAND1 (Abclonal, A9855, 1:500), anti-HAND2 (Abcam, ab200040, 1:1000), anti-CX43 (Sigma-Aldrich, C6219, 1:3000), anti-MYL2 (Proteintech, 10906-1-AP, 1:1000), anti-NR2F2 (Cell Signaling Technology, 6434, 1:1000) and anti-SCN5A (Proteintech, 23016-1-AP, 1:1000).
Immunofluorescence staining
Cells were fixed with 4% PFA for 15 min, RT, washed with PBS three times, then permeabilized with 0.3% PBSTr (Triton-X100) for 15 min. Permeabilization is not required for cell surface antigens. After blocking in 3% BSA for 30 min, the cells were incubated with primary antibodies at 4 °C, overnight. The next day, after washing with 0.1% PBST (Tween) three times, the cells were incubated with fluorescence-conjugated secondary antibodies (Abcam, 1:200) for 1 h, RT, then washed with 0.1% PBST three times. The nuclei were stained with DAPI (Sigma-Aldrich, 1:10000). Images were captured with fluorescence microscope (Leica, Germany). The primary antibodies used were: anti-SSEA-4 (Santa Cruz, sc59368, 1:200), anti-OCT4 (Abcam, ab181557, 1:200), anti-TBXT (R&D systems, AF2085, 1:200), anti-CX43 (Sigma-Aldrich, C6219, 1:400), and anti-NR2F2 (Santa Cruz, sc393481, 1:100).
Microelectrode array (MEA) analysis
Cardiomyocytes from differentiation day 30 were dissociated into single cells and counted. 3 × 104 Cells were seeded onto Geltrex coated 24-well CytoView MEA plate. Field potential recording was conducted when the cells began to beat. Local extracellular action potential (LEAP) induction was used to simulate action potential. Corrected action potential duration (APD) was calculated using Bazett’s formula [56]. Data were analyzed by Cardiac Analysis Tool, AxionDataExportTool and Igor.
Whole-cell patch clamp
Whole-cell patch clamp was performed for the electrophysiological characteristics of hESCs-derived cardiomyocytes. 1 × 104 cells were plated into growth-factor-reduced Matrigel (Corning) coated 3.5-cm dish. Patch clamp was conducted between days 2–6 after plating. The tip resistance of Borosilicate glass microelectrodes was 2–3 MΩ. The pipette solution was composed of 140 mM KCl, 10 mM EGTA, 5 mM glucose, 3 mM MgATP and 10 mM HEPES, adjusting pH to 7.2 with KOH. The bath solution was: 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 1.2 mM KH2PO4, 5.5 mM glucose and 5 mM HEPES, adjusting pH to 7.4 with NaOH and the solution was oxygenated for at least 30 min before use. Spontaneous and paced action potential were recorded with EPC-10 amplifier (HEKA, Germany). The data were analyzed with Minianalysis and Clampfit.
RNA-sequencing (RNA-seq) library preparation and sequencing
NKX2.5-eGFP+ cells were sorted at differentiation day 7. Total RNA was extracted using the RNA extraction kit (Sigma-Aldrich). One microgram of total RNA was used in mRNA capture by NEBNext PolyA mRNA Magnetic Isolation Module (New England Biolabs, MA, USA). The RNA-seq libraries were constructed according to the instruction of NEB Next Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs, MA, USA). RNA-seq libraries were then sequenced as 150-bp paired-end reads on an Illumina NovaSeq 6000 platform. The sequencing was performed by Shanghai Genefund Biotech Co., Ltd.
RNA-seq data processing
Raw paired-end RNA-seq reads were trimmed to remove adapters by Trim Galore! (version 0.6.4_dev). The clean reads were aligned to the human genome (assembly GRCh38) using Hisat2 (version 2.2.1) [57]. The counts per gene were quantified to the exon level (-t exon) by featureCounts (version 2.0.1) [58]. Reads were normalized to transcripts per million (TPM) for visualization by a compiled R script (R version 4.0.2). Principal component analysis (PCA) was performed through the irlba (version 2.3.3). Differential expression analysis was performed using DESeq2 (version 1.28.1) [59], and the genes with \(|{\text{fold}}\;{\text{change }}\left( {{\text{FC}}} \right)|\)> 2 and adjusted P value < 0.05 were considered as differentially expressed genes (DEGs). The fuzzy c-means clustering algorithm identified eight distinct gene expression clusters by Mfuzz (version 2.48.0) [60]. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.genome.jp/kegg/) analyses were performed using clusterProfiler (version 3.16.0) [61]. Adjusted P value < 0.05 was considered significant in the enrichment analysis.
Statistical analysis
Data were displayed as mean ± SEM. Statistical analysis was conducted using Prism 8 (GraphPad, Boston, MA). Unpaired 2-tailed Student t test and one-way ANOVA were used for the comparison of groups. Statistical significance was defined as P value < 0.05.
Results
Establishment of HAND1-KO, HAND2-KO and HAND1/2-double-KO (dKO) hESC lines
Previous studies have verified that the introduction of enhanced GFP (eGFP) into NKX2.5 locus could be utilized to isolate and characterize cardiomyocytes differentiated from hESCs [62]. We thus applied NKX2.5eGFP H9 [designated as wild type (WT) hereafter], which was generated by inserting eGFP into the start codon of NKX2.5 (Additional file 1: Fig. S1A), to investigate the function of HAND1 and HAND2 in cardiomyocyte differentiation.
We then employed a modified cardiomyocyte differentiation protocol by modulating canonical Wnt signaling [51]. hESCs were treated with sequential Wnt activator and inhibitor and concomitant use of Vc to promote cardiomyocyte differentiation [52] (Fig. 1A). At day 7, the NKX2.5-eGFP+ cells emerged and the differentiated cardiomyocytes started to beat (Additional file 2: Supplementary Video 1). The differentiation efficiency could reach more than 80% during differentiation days 10–30 (Additional file 1: Fig. S1B), indicating the robustness of this protocol.
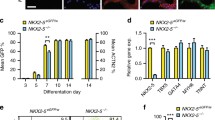
Establishment of H1-KO, H2-KO and H1/H2-dKO hESC lines. A Schematic diagram of cardiomyocyte differentiation protocol. Mesoderm stage was at day 2. Cardiac mesoderm stage was at day 3. Cardiac progenitor stage was at day 5. Cardiomyocytes started beating at day 7. RB-: RPMI/B27-, RB: RPMI/B27, CHIR: CHIR99021, Vc: Vitamin C. B Temporal expression of HAND1 and HAND2 during cardiomyocyte differentiation. Relative to GAPDH expression (n = 3). C Schematic diagram of CRISPR/Cas9 mediated HAND1/2 genome editing. PAM: protospacer adjacent motif. D Expression of OCT4, SOX2 and NANOG in WT and HAND KO hESC lines. Relative to GAPDH expression (n = 3). E Immunofluorescence staining of SSEA-4 and OCT4 in WT and HAND KO hESC lines. Scale bar = 100 μm. F Flow cytometry analysis of SSEA-4+ cells in WT and HAND KO hESC lines. CTRL represented the negative control with secondary antibody incubation only. G qPCR analysis of HAND1 and HAND2 in WT and HAND KO hESC lines-derived day 10 cardiomyocytes. Relative to GAPDH expression (n = 3). One-way ANOVA. H Western blot analysis of HAND1 and HAND2 expression in WT and HAND KO hESC lines-derived differentiation day 10 cardiomyocytes. GAPDH served as loading control. Corresponding uncropped full-length gels and blots are presented in Additional file 8: Fig. S7. **p < 0.01, ***p < 0.001, ****p < 0.0001
We then examined the expression level of HAND1/2 at different time points during the differentiation. HAND1 began to be expressed on day 3, at the cardiac mesoderm stage [63, 64], and continued to increase to day 10, then maintained at a low-level during days 20–30, while HAND2 was first detected on day 5, at the cardiac progenitor stage [63, 64], and expressed at a relatively stable level during the differentiation (Fig. 1B). Expression of HAND1 preceded that of HAND2 during our monolayer cardiomyocyte differentiation, consistent with previous study using EB differentiation protocol [37].
Next, we knocked out HAND1, HAND2 in NKX2.5eGFP H9 cells to establish HAND1-KO, HAND2-KO and HAND1/2-dKO cell lines (designated as H1-KO, H2-KO and H1/H2-dKO hereafter) with CRISPR/Cas9 technology. Exon 1 of HAND1/2 was targeted to generate H1-KO or H2-KO cell lines, respectively (Fig. 1C). For H1/H2-dKO cell lines, the sgRNAs of HAND1 and HAND2 were transfected into NKX2.5eGFP H9 cells with spCas9 simultaneously. Homozygous KO cell lines of HAND1 or/and HAND2 were selected for further analysis. We obtained at least two monoclonal clones with different gene editing for each KO cell line. Two independent clones from each line were selected for subsequent studies (Additional file 1: Fig. S1C). PCR and Sanger sequencing confirmed that the selected cell lines had no predicted off-targets (Additional file 1: Fig. S1D). Besides, the KO cell lines showed normal karyotype with 46, XX (Additional file 1: Fig. S1E).
To compare the stemness of HAND KO cell lines with WT, we determined the expression of pluripotency genes, OCT4, SOX2 and NANOG [65], which was comparable among WT and KO cell lines (Fig. 1D). Also, immunofluorescence staining of pluripotent cell surface marker SSEA-4 [65] and OCT4 showed no difference (Fig. 1E). The SSEA-4+ cells were consistently above 95% in all cell lines (Fig. 1F). The expression of tridermic markers was low, similar to WT, suggesting that HAND KO cell lines did not differentiate significantly toward the three germlayers in stem cell culture (Additional file 1: Fig. S1F). The pluripotency of KO cell lines was further demonstrated by the expression of endoderm (AFP), mesoderm (α-SMA) and ectoderm (TUBB3) [65] markers during spontaneous EB differentiation (Additional file 1: Fig. S1G). These results indicated that HAND KO cell lines exhibited comparable pluripotency to the parental cell line.
We then performed cardiomyocyte differentiation with WT and HAND KO cell lines. After 10 days’ differentiation, we examined the expression of HAND1 or/and HAND2 in H1-KO, H2-KO and H1/H2-dKO cell lines-derived cardiomyocytes, which showed significantly decreased HAND1 or/and HAND2 expressions compared with WT cells (Fig. 1G). Moreover, HAND1 or/and HAND2 protein was not detected by western blot (Fig. 1H). These results demonstrated that we have successfully established HAND1/2 single and double KO cell lines which could be used for functional research.
HAND1 deficiency promoted SHF and its derived cardiomyocyte differentiation
As HAND1 was known to be expressed in the mesoderm lineage in mouse and human embryo development [21, 31], we conducted immunofluorescence analysis to investigate the impact of HAND1 deficiency on mesoderm differentiation. At differentiation day 2, the expression of TBXT, a mesoderm marker [66], was comparable between WT and H1-KO cells (Additional file 1: Fig. S2A). At differentiation day 3, qPCR analysis showed the expression of cardiac mesoderm marker MESP1 was similar between WT and H1-KO cells (Additional file 1: Fig. S2B). These results suggested that HAND1 did not participate in the process of mesoderm and cardiac mesoderm induction from hESCs.
We then analyzed the cardiomyocyte differentiation of H1-KO cells. Similar to WT hESCs, some beating H1-KO cardiomyocytes were first observed at day 7. Furthermore, by flow cytometry, no difference in the percentage of NKX2.5-eGFP+ cells in WT and H1-KO cells was detected during differentiation (Fig. 2A, Additional file 1: Fig. S2C), consistent with the transcriptional level of NKX2.5 (Additional file 1: Fig. S2D). The absence of HAND1 did not influence the pan-cardiomyocyte differentiation efficiency.
HAND1 deficiency promoted SHF and its derived cardiomyocyte differentiation. A The percentage of NKX2.5-eGFP+ cells in WT and H1-KO cells at different time points of cardiomyocyte differentiation (n = 3). B, C Expression of FHF (B) and SHF (C) markers in early cardiomyocyte differentiation of WT and H1-KO cells. Relative to GAPDH expression (n = 3). Unpaired t test. D, E Expression of NKX2.5 (D) and ventricular, atrial and outflow tract cardiomyocyte (VCM, ACM and OFT) markers (E) in WT and H1-KO-derived day 30 cardiomyocytes. Relative to GAPDH expression (n = 3). Unpaired t test. F Western blot analysis of CX43 and MYL2 expression in WT and H1-KO-derived day 30 cardiomyocytes. GAPDH served as loading control. Corresponding uncropped full-length gels and blots are presented in Additional file 8: Fig. S8. G The percentage of MYL2+ cardiomyocytes in WT and H1-KO cells at differentiation day 30 (n = 5). H Immunofluorescence staining of NR2F2 in WT and H1-KO-derived day 30 cardiomyocytes. Scale bar = 100 μm. I The field potential and simulated action potential recorded by MEA in WT and H1-KO-derived day 30 cardiomyocytes. Black lines represented the field potential while red lines represented the simulated action potential. J Comparison of corrected APD90, APD50 and APD90/APD50 ratio in WT and H1-KO-derived day 30 cardiomyocytes (n ≥ 7). Unpaired t test. K The action potential paced by 1Hz recorded by whole-cell patch clamp in WT and H1-KO-derived differentiation day 30 cardiomyocytes. L Comparison of APD90 and APD50 in WT and H1-KO-derived day 30 cardiomyocytes (n ≥ 9). Unpaired t test. M Expression of ion channels of WT and H1-KO-derived differentiation day 30 cardiomyocytes. Relative to GAPDH expression (n = 3). Unpaired t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s: non-significant
The FHF and SHF were determined at cardiac mesoderm stage during differentiation [19, 67], and HAND1 is a marker of FHF [18]. So, we next investigated the function of HAND1 in cardiac lineage commitment during early cardiomyocyte differentiation. By examining the expression of heart-field specific markers from days 3 to 10 (cardiac mesoderm stage to cardiomyocyte stage), we revealed that H1-KO cells exhibited a notable reduction in the expression of FHF markers, TBX5 and HCN4 [16, 17], compared to WT cells from days 5 to 10 (Fig. 2B). Conversely, the expression of SHF marker ISL1 was significantly upregulated from day 5 (Fig. 2C). In particular, as early as day 3, the expression of aSHF and pSHF markers, TBX1, SIX1, FOXC2, and NR2F2, HOXA1, HOXB1, ALDH1A2, started to display higher expression level compared with WT cells at different time points until day 7 or day 10. For example, TBX1 expression increased from differentiation days 3 to 5, then began to decrease until differentiation day 10 while NR2F2 was upregulated from differentiation days 3 to 7, then downregulated during differentiation days 7 to 10 (Fig. 2C, Additional file 1: Fig. S2E, F). These results demonstrated that HAND1 deficiency promoted SHF lineage from cardiac mesoderm stage whereas impaired the FHF progenitors.
To distinguish the effects of abnormal early cardiac lineage specification, we explored the characteristics of H1-KO cardiomyocytes at differentiation day 30. Firstly, the H1-KO-derived cardiomyocytes displayed comparable levels of NKX2.5, same as the flow cytometry analysis of NKX2.5-eGFP+ cells at day 30 (Fig. 2A, D). Then, we focused on comparing the gene expression related to ventricular, atrial and OFT cardiomyocytes, which were primarily differentiated from FHF, pSHF and aSHF, respectively. The H1-KO-derived cardiomyocytes exhibited reduced expression of ventricular genes, including CX43, MYL2, IRX4 and MYH7 [67, 68], and increased atrial genes, including NR2F2, MYH6, CACNA1D and MYL7 [67] (Fig. 2E). The decreased ratio of MYL2/MYL7 and MYH7/MYH6 [69] also reflected the reduced ventricular cardiomyocyte differentiation (Fig. 2E). Meanwhile, some OFT cardiomyocyte markers, LTBP3 and RSPO3 [20, 70], increased as well (Fig. 2E). Western blot analysis confirmed the reduced expression of CX43 and MYL2 at the protein level (Fig. 2F). Immunofluorescence staining also showed the expression of CX43 decreased in H1-KO cardiomyocytes (Additional file 1: Fig. S2G), which could affect intercellular ion movement and impulse conduction by disrupting the function of gap junctions [71]. The percentage of MYL2+ cardiomyocytes decreased in H1-KO cells (Fig. 2G). Besides, immunofluorescence staining and western blot showed that the expression of NR2F2 increased in H1-KO cardiomyocytes (Fig. 2H, Additional file 1: Fig. S2H). Those results reflected that HAND1 deficiency promoted cardiomyocytes to express atrial and OFT but not ventricular cardiomyocyte markers, which was consistent with the effect of HAND1 on early cardiac lineage differentiation.
To further characterize the H1-KO cardiomyocytes, we used LEAP induction to transform field potential into action potential. Figure 2I shows the relationship between field potential and LEAP induction of action potential recorded by the MEA. Compared to WT, the H1-KO cardiomyocytes had a shortened corrected APD90 and APD50 and increased APD90/APD50 ratio, which were similar to the electrophysiological characteristics of atrial-like cardiomyocytes (Fig. 2J). As the MEA recorded the electrophysiological characteristics of bulk cardiomyocytes, we further performed whole-cell patch clamp to compare the electrophysiological properties of single cells. We found that H1-KO cardiomyocytes displayed relatively short APD and comparable action potential amplitude (Fig. 2K, Additional file 1: Fig. S2I). The APD90 and APD50 of H1-KO-derived cardiomyocytes were shorter than WT cardiomyocytes (Fig. 2L), which aligned with the MEA findings. Additionally, to explore the molecular foundations that caused shortened APD in H1-KO cardiomyocytes, we checked the expression of ion channels. In H1-KO cardiomyocytes, the expression of SCN5A which encodes the subunits of sodium channel Nav1.5 and is essential for depolarization of cardiomyocyte [72], reduced (Fig. 2M, Additional file 1: Fig. S2J). Conversely, the expression of KCNH2 and KCNQ1 which mediate the repolarization current of Ikr and Iks, respectively [72], increased (Fig. 2M). Both of these changes contributed to the shortened APD [73, 74]. These results were consistent with the inference that HAND1 deficiency decreased ventricular cardiomyocyte differentiation while promoted atrial cardiomyocyte differentiation.
HAND2 knockout impaired SHF-derived cardiomyocyte differentiation
As HAND2 was also expressed in mesoderm, but later than HAND1 [21, 31], we examined TBXT and MESP1 expression at days 2 and 3, respectively, which showed no difference between WT and H2-KO cells (Additional file 1: Fig. S3A, B). We then differentiated H2-KO hESCs into cardiomyocytes, which exhibited comparable differentiation kinetics to WT cells (Fig. 3A, Additional file 1: Fig. S3C) with cell beating initiated at day 7. Additionally, the expression of NKX2.5 in H2-KO cells was comparable to WT cells during differentiation days 3 to 10 (Additional file 1: Fig. S3D). Thus, HAND2 deficiency had no significant effects on pan-cardiomyocyte differentiation efficiency.
HAND2 knockout impaired SHF-derived cardiomyocyte differentiation. A The percentage of NKX2.5-eGFP+ cells in WT and H2-KO cells at different time points of cardiomyocyte differentiation (n = 3). B, C. Expression of FHF (B) and SHF (C) markers in early cardiomyocyte differentiation of WT and H2-KO cells. Relative to GAPDH expression (n = 3). Unpaired t test. D, E Expression of NKX2.5 (D) and ventricular cardiomyocyte (VCM) markers (E) in WT and H2-KO-derived differentiation day 30 cardiomyocytes. Relative to GAPDH expression (n = 3). Unpaired t test. F Western blot analysis of MYL2 expression in WT and H2-KO-derived differentiation day 30 cardiomyocytes. GAPDH served as loading control. Corresponding uncropped full-length gels and blots are presented in Additional file 8: Fig. S9. G The percentage of MYL2+ cardiomyocytes in WT and H2-KO cells at differentiation day 30 (n ≥ 3). H Expression of atrial and OFT cardiomyocyte (ACM, OFT) markers in WT and H2-KO-derived differentiation day 30 cardiomyocytes. Relative to GAPDH expression (n = 3). Unpaired t test. I Immunofluorescence staining of NR2F2 in WT and H2-KO-derived differentiation day 30 cardiomyocytes. Scale bar = 100 μm. J The field potential and simulated action potential recorded by MEA in WT and H2-KO-derived differentiation day 30 cardiomyocytes. Black lines represented the field potential while red lines represented the simulated action potential. K Comparison of corrected APD90 and APD50 in WT and H2-KO-derived differentiation day 30 cardiomyocytes (n ≥ 8). Unpaired t test. L The action potential paced by 1Hz recorded by whole-cell patch clamp in WT and H2-KO-derived day 30 cardiomyocytes. M Comparison of APD90 and APD50 in WT and H2-KO-derived differentiation day 30 cardiomyocytes (n ≥ 11). Unpaired t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s: non-significant
We next detected the expression of FHF and SHF markers during early cardiac lineage specification in H2-KO cells. qPCR analysis revealed that HAND2 knockout resulted in higher expression of TBX5 and comparable expression of HCN4 compared to WT cells during differentiation days 5 to 7 (Fig. 3B). Meanwhile, the expression of ISL1 increased at differentiation day 5 and the expression of aSHF and pSHF markers was also globally higher than that of WT cells and reduced to the same level as WT cells at differentiation day 10 (Fig. 3C, Additional file 1: Fig. S3E). These results indicated that HAND2 differed from HAND1 in their roles in early cardiac lineage commitment.
We next explored the characteristics of H2-KO cardiomyocytes. At differentiation day 30, H2-KO cardiomyocytes showed comparable expression of NKX2.5 (Fig. 3D). Unlike H1-KO cardiomyocytes, HAND2 knockout did not interrupt the ventricular cardiomyocyte differentiation as indicated by upregulated expression of MYL2 and IRX4 (Fig. 3E). Additionally, the expression of MYL2 protein and the percentage of MYL2+ cardiomyocytes in H2-KO cells increased (Fig. 3F, G), while the expression of CX43 in WT and H2-KO cardiomyocytes was similar (Additional file 1: Fig. S3F). Single-cell analysis of Hand2-null mouse heart revealed that Hand2 did not affect the specification of the right ventricular cardiomyocytes, but it did impact the formation of OFT cardiomyocytes [30]. We therefore determined the expression of OFT cardiomyocyte markers, RSPO3 and SEMA3C [19], which reduced at day 30 (Fig. 3H). The H2-KO cardiomyocytes also exhibited reduced expression of atrial cardiomyocyte markers, including NR2F2, CACNA1D, KCNJ3 and MYL7 (Fig. 3H), indicating that HAND2 affected the formation of SHF-derived cardiomyocytes. Immunofluorescence staining of NR2F2 showed very few H2-KO cardiomyocytes were NR2F2 positive (Fig. 3I), which was further confirmed by western blot (Additional file 1: Fig. S3G).
HAND2 deficiency cells showed elevated expression of aSHF and pSHF markers. However, the expression of SHF-derived atrial and OFT cardiomyocyte markers decreased. We speculated that the differentiation of SHF progenitors into atrial and OFT cardiomyocytes might be impaired in the absence of HAND2. We therefore analyzed the expression of progenitor markers in H2-KO-derived differentiation day 30 cardiomyocytes. Interestingly, the expression of cardiac progenitor cell markers, PDGFRA, FLK1 and ISL1, which expressed in SHF progenitors [75, 76], was still at high level in H2-KO differentiated cells, indicating the impeded SHF differentiation in H2-KO cells (Additional file 1: Fig. S3H).
Finally, we used MEA to characterize the electrophysiological properties of differentiation day 30 H2-KO cardiomyocytes, which displayed comparable action potential pattern to WT cardiomyocytes (Fig. 3J). However, the corrected APD90 and APD50 were found to be slightly shorter compared to WT cardiomyocytes (Fig. 3K). Additionally, whole-cell patch clamp recorded action potentials with more concentrated distribution of APD90 and APD50 compared to WT cardiomyocytes (Fig. 3L, M), although with no significant difference, indicating that H2-KO cardiomyocytes had a tendency toward ventricular cardiomyocytes, consistent with their gene expression profile (Fig. 3E–G). In addition, the action potential amplitude of H2-KO cardiomyocytes was comparable to WT cardiomyocytes as well (Additional file 1: Fig. S3I). Next, we detected the expression of ion channels in H2-KO cardiomyocytes. The expression of SCN5A was moderately decreased in H2-KO cardiomyocytes (Additional file 1: Fig. S3J). Meanwhile, the expression of KCNH2 and KCNQ1 was similar between these two groups (Additional file 1: Fig. S3J), validating the electrophysiological characters of H2-KO cardiomyocytes.
HAND1/2 double knockout impeded the differentiation of cardiomyocytes and impaired their electrophysiological activity
It was generally accepted that HAND1 and HAND2 were partially redundant in heart development [27]. We therefore examined the mRNA and protein expression of HAND1 in H2-KO and HAND2 in H1-KO cell lines, which revealed that HAND2 was significantly upregulated in H1-KO cells during early cardiomyocyte differentiation whereas HAND1 was only slightly upregulated in H2-KO cells before differentiation day 7 (Additional file 1: Fig. S4A-C). These results indicated that HAND1/2 could have some complementary function with HAND1 playing more important roles in our cardiomyocyte differentiation system. To clarify the function of HAND1/2, we generated the H1/H2-dKO cell lines for further analysis.
Firstly, immunofluorescence staining of TBXT at differentiation day 2 revealed comparable fluorescence intensity in H1/H2-dKO cells, suggesting mesoderm differentiation was not impaired (Additional file 1: Fig. S4D). Also, no difference in MESP1 expression was observed at differentiation day 3 (Additional file 1: Fig. S4E). We then applied the same method to induce the differentiation of H1/H2-dKO hESCs into cardiomyocytes. Of note, at day 7, the NKX2.5-eGFP fluorescence was much weaker in H1/H2-dKO cells compared to WT cells (Fig. 4A), consistent with lower percentage of NKX2.5-eGFP+ cells in H1/H2-dKO determined by flow cytometry (Fig. 4B). However, at day 10, the H1/H2-dKO NKX2.5-eGFP+ population reached to comparable level as that of WT cells (Fig. 4B, Additional file 1: Fig. S4F). Additionally, H1/H2-dKO cardiomyocytes exhibited delayed beating onset which was not observed until differentiation days 10–12 (Fig. 4C). The expression of NKX2.5 in H1/H2-dKO cells also lagged behind WT cells (Additional file 1: Fig. S4G). The delayed cardiomyocyte differentiation of H1/H2-dKO hESCs indicated that HAND1 and HAND2 had overlapping functions in the early stage of cardiomyocyte differentiation.
HAND1/2 double knockout impeded the differentiation of cardiomyocytes and impaired their electrophysiological activity. A The phase and fluorescence images of WT and H1/H2-dKO cells at differentiation day 7. B The percentage of NKX2.5-eGFP+ cells in WT and H1/H2-dKO cells at different time points of cardiomyocyte differentiation (n = 3). Unpaired t test. C The beating onset of WT and H1/H2-dKO-derived cardiomyocytes (n = 10). Unpaired t test. D Expression of FHF and SHF markers in early cardiomyocyte differentiation of WT and H1/H2-dKO cells. Relative to GAPDH expression (n = 3). Unpaired t test. E Expression of ventricular, atrial and outflow tract cardiomyocyte (VCM, ACM and OFT) markers in WT and H1/H2-dKO-derived day 30 cardiomyocytes. Relative to GAPDH expression (n = 3). Unpaired t test. F Western blot analysis of CX43 and MYL2 expression in day 30 WT and H1/H2-dKO cardiomyocytes. Corresponding uncropped full-length gels and blots are presented in Additional file 8: Fig. S10. G The percentage of MYL2.+ cardiomyocytes in WT and H1/H2-dKO cells at differentiation day 30 (n = 3). H Immunofluorescence staining of NR2F2 in WT and H1/H2-dKO-derived day 30 cardiomyocytes. Scale bar = 100 μm. I The field potential and simulated action potential recorded by MEA in WT and H1/H2-dKO-derived day 30 cardiomyocytes. Black lines represented the field potential while red lines represented the action potential. J Comparison of corrected APD90, APD50 and APD90/APD50 ratio in WT and H1/H2-dKO-derived differentiation day 30 cardiomyocytes (n ≥ 6). Unpaired t test. K The action potential paced by 1Hz recorded by whole-cell patch clamp in WT and H1/H2-dKO-derived differentiation day 30 cardiomyocytes. L Comparison of APD90, APD50 and action potential amplitude in WT and H1/H2-dKO-derived day 30 cardiomyocytes (n ≥ 10). Unpaired t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
We next determined the expression of FHF and SHF markers in early cardiomyocyte differentiation of H1/H2-dKO cells. Specifically, the expression of FHF markers, TBX5 and HCN4, decreased as that of H1-KO cells, while the expression of SHF markers, including ISL1, TBX1, SIX1, FOXC2, NR2F2, HOXB1, HOXA1 and ALDH1A2, increased with similar trend as that of H1-KO cells, except that the expression of NR2F2 kept in an upward trend from differentiation days 7 to 10 and was significantly higher than that in WT and H1-KO cells at differentiation day 10 (Figs. 2B, C, 4D, Additional file 1: Fig. S2E, F). Besides, the expression of TBX1 and SIX1 was much higher than that of H1-KO cells (Figs. 2C, 4D, Additional file 1: Fig. S2E). These results indicated that H1/H2-dKO hESCs biased toward SHF differentiation.
To assess the effects of H1/H2-dKO on cardiomyocyte subtype differentiation, we profiled differentiation day 30 cells. The H1/H2-dKO cardiomyocytes exhibited comparable level of NKX2.5 (Additional file 1: Fig. S4H). The ventricular cardiomyocyte markers CX43, MYL2, IRX4 and MYH7 were downregulated in H1/H2-dKO cardiomyocytes, while both atrial cardiomyocyte markers, NR2F2, MYH6, CACNA1D and KCNJ3, and OFT cardiomyocyte markers, LTBP3 and RSPO3, were upregulated (Fig. 4E). The ratio of MYL2/MYL7 and MYH7/MYH6 also decreased in H1/H2-dKO cardiomyocytes, resembling the trend observed in H1-KO cardiomyocytes (Figs. 2E, 4E). We also found that the upregulated expression of atrial cardiomyocyte markers NR2F2 and CACNA1D and the OFT cardiomyocyte markers LTBP3 and RSPO3 was significantly higher in H1/H2-dKO cells compared with H1-KO cells (Figs. 2E, 4E), suggesting H1/H2-dKO cells were more prone to SHF-derived cardiomyocyte differentiation than H1-KO cells. Furthermore, western blot analysis confirmed the reduced expression of CX43 and MYL2 (Fig. 4F). Concomitantly, the percentage of MYL2+ cardiomyocytes in H1/H2-dKO cells also decreased (Fig. 4G). Immunofluorescence staining revealed decreased expression of CX43 in H1/H2-dKO cardiomyocytes as well (Additional file 1: Fig. S4I), whereas the expression of NR2F2 significantly increased (Fig. 4H, Additional file 1: Fig. S4J). These results were consistent with the more SHF progenitors generated during early differentiation of H1/H2-dKO hESCs.
To further characterize the H1/H2-dKO cardiomyocyte subtypes, we conducted MEA and whole-cell patch clamp to analyze the electrophysiological properties of these cells. MEA revealed that H1/H2-dKO cardiomyocytes exhibited shortened APD, including corrected APD90 and APD50 (Fig. 4I, J). The ratio of APD90/APD50 increased in H1/H2-dKO cardiomyocytes compared to WT cardiomyocytes (Fig. 4J). Whole-cell patch clamp confirmed that H1/H2-dKO cardiomyocytes displayed shortened APD (Fig. 4K, L), indicating that these cells exhibited electrophysiological characteristics more akin to atrial-like cardiomyocytes. Besides, the amplitude of H1/H2-dKO cardiomyocytes’ action potential was reduced compared to WT cardiomyocytes (Fig. 4L), which was not observed in H1-KO and H2-KO cardiomyocytes (Additional file 1: Figs. S2I, S3I), suggesting greater impairment in conduction speed and electrical activity of cardiomyocytes [77]. The reduced expression of SCN5A and increased expression of KCNH2 and KCNQ1 (Additional file 1: Fig. S4K, L), which were similar to H1-KO cardiomyocytes (Fig. 2M, Additional file 1: Fig. S2J), partially accounted for the changes in electrophysiological characteristics of H1/H2-dKO cardiomyocytes. Overall, these findings demonstrated that H1/H2-dKO hESCs were prone to SHF-derived cardiomyocyte differentiation with severely impaired electrophysiological activity.
Transcriptomic characterization of H1-KO, H2-KO and H1/H2-dKO cardiomyocytes
To provide an integrated view of the implications of HAND1- and HAND2-dependent transcriptional changes, we performed RNA-seq of WT, H1-KO, H2-KO and H1/H2-dKO cardiomyocytes sorted at differentiation day 7 (a time point that NKX2.5-eGFP+ cells emerged and the majority of the differentiating cardiomyocytes diverged) (Figs. 5A, 4A, B, Additional file 1: Fig. S4G). The mapping statistics and PCA analysis showed the high quality of RNA-seq data, with an average of about 25 million mapped reads (Additional file 3: Table S3) and a high correlation between duplicates (Fig. 5B). PCA analysis showed WT and H2-KO cells were transcriptionally similar, while H1-KO and H1/H2-dKO cells were distinct from WT and H2-KO cells. Further pairwise differential expression analysis identified 3786 DEGs (1812 upregulated and 1974 downregulated) between WT and H1-KO cells; 563 DEGs (361 upregulated and 202 downregulated) between WT and H2-KO cells; and 4722 DEGs (2275 upregulated and 2447 downregulated) between WT and H1/H2-dKO cells (Fig. 5C), indicating a significant shift in gene expression profile in H1-KO and H1/H2-dKO cells. Notably, HAND2 expression was elevated in H1-KO cells while HAND1 expression did not change significantly in H2-KO cells (Additional file 1: Fig. S5), similar to the qPCR and western blot data at differentiation day 7 (Additional file 1: Fig. S4A-C). We also observed the downregulated expression of FHF markers (such as TBX5 and HCN4) and upregulated expression of SHF markers (such as ISL1, TBX1, SIX1, FOXC2, NR2F2, HOXA1, HOXB1 and ALDH1A2) in H1-KO and H1/H2-dKO cells (Fig. 5D), consistent with our experimental results (Figs. 2B, C, 4D and Additional file 1: Fig. S2E, F). These findings suggested that HAND1 played a more crucial role in FHF development than HAND2.
Identifying the transcriptomic characteristics of H1-KO, H2-KO and H1/H2-dKO cardiomyocytes. A Schematic diagram of the samples for RNA-seq. WT, H1-KO, H2-KO and H1/H2-dKO cardiomyocytes were collected at differentiation day 7. B PCA analysis of WT, H1-KO, H2-KO and H1/H2-dKO cardiomyocytes. Each group had three duplicates. C The DEGs between different groups. DEGs were defined with |fold change (FC)|> 2 and adjusted P-value < 0.05. D The expression of representative FHF, SHF and cardiomyocyte (CM) genes in RNA-seq. E Gene expression cluster analysis of RNA-seq. Genes included in clustering were at least differentially expressed in one comparison in (C). F GO enrichment analysis of each gene expression cluster. G KEGG pathway enrichment analysis of each gene expression cluster
To figure out the dynamic gene expression changes between WT, H1-KO, H2-KO and H1/H2-dKO cardiomyocytes, we utilized the fuzzy c-means algorithm to cluster gene expression profiles into different groups. Eight distinct clusters were identified (Fig. 5E, Additional file 4: Table S4), representing different expression kinetics in response to H1-KO, H2-KO and H1/H2-dKO. Among them, the changes of the above-mentioned markers were consistent with Fig. 5D, indicating the reliability of the clustering. Clusters 1, 4, 5 and 6 shared similar trends with genes downregulated, and clusters 2, 3, 7 and 8 represented the upregulated genes in H1-KO and H1/H2-dKO cells (Fig. 5E). Furthermore, we applied GO and KEGG enrichments to predict the underlying functions of each gene cluster (Fig. 5F, G). As expected, clusters 1, 4 and 6 (clusters with genes downregulated in H1-KO, H2-KO and H1/H2-dKO cells) were highly enriched in similar GO and KEGG terms associated with cardiomyocyte contraction, development, and heart diseases (hypertrophic cardiomyopathy and dilated cardiomyopathy) (Fig. 5F, G). The other clusters (clusters 2, 3, 5, 7 and 8) were enriched in various cluster-specific terms, such as protein targeting and location (Cluster 2), other organ developments (clusters 5, 7 and 8) (Fig. 5F, G, Additional file 5, 6: Table S5, S6). Taken together, H1-KO, H2-KO and H1/H2-dKO cardiomyocytes manifested corresponding transcriptome changes in accordance with the H1-KO, H2-KO and H1/H2-dKO cardiomyocyte differentiation phenotypes.
HAND1/2 modulated cardiomyocyte differentiation through TBX5
To elucidate the underlying mechanism of HAND1/2 in cardiac lineage differentiation, we downloaded the chromatin immunoprecipitation sequencing (ChIP-seq) database of HAND1 and HAND2 (GSM1505812 and GSM1505811), which was conducted at the mesoderm stage during differentiation from hESCs, equivalent to days 2 to 3 in our differentiation protocol [78]. Comprehensive analysis of RNA-seq and ChIP-seq data unveiled that among the genes downregulated in H1/H2-dKO cardiomyocytes, 649 and 102 genes were regulated by HAND1 and HAND2 alone, respectively. Meanwhile, 492 genes were co-regulated by HAND1 and HAND2, which included 61 TFs activated by HAND1/2 (Fig. 6A, Additional file 1: Table S2). Among these TFs, several were known to play important roles in heart development, such as TBX5 [79], MEF2 family [80, 81], MYOCD [82], ETS2 [83, 84] and NKX2.5 [85, 86] (Fig. 6A, Additional file 1: Table S2). This suggested that the absence of HAND1 and HAND2 could lead to dysregulation of cardiomyocyte differentiation gene network. The reduced expression of these genes in the H1/H2-dKO NKX2.5-eGFP+ cardiomyocytes at differentiation day 7 was validated by qPCR (Fig. 6B). We then focused on TBX5 which is a FHF marker and was significantly downregulated in H1/H2-dKO NKX2.5-eGFP+ cardiomyocytes (Fig. 6B). Previous studies showed that TBX5-deficient hPSCs exhibited decreased cardiomyocyte differentiation efficiency and delayed onset of beating, along with delayed NKX2.5 expression [79]. In addition, single-cell sequencing of heterozygous and homozygous TBX5 deletion hPSC-derived cardiomyocytes revealed a higher proportion of atrial-like cardiomyocytes [79]. Those phenotypes were similar to H1/H2-dKO cells.
HAND1/2 modulated cardiomyocyte differentiation through TBX5. A Venn diagram of HAND1 and HAND2 ChIP-seq and downregulated DEGs in H1/H2-dKO cells compared to WT cells in RNA-seq. B Expression of some TFs in WT and H1/H2-dKO sorted NKX2.5-eGFP+ cells at differentiation day 7. Relative to GAPDH expression (n = 3). Unpaired t test. C The ratio of Luciferase and Renilla activity in HAND1 and HAND2 overexpression 293T cells (n = 6). CTRL represented 293T transfected with pGL3-TBX5-Luciferase and Renilla only. One-way ANOVA. D TBX5 overexpression after DOX induction verified by western blot at differentiation days 5 and 10. GAPDH served as loading control. Corresponding uncropped full-length gels and blots are presented in Additional file 8: Fig. S11. E, F The percentage of NKX2.5-eGFP+ cells in WT and TBX5-OE cells (−DOX and + DOX) at differentiation day 7 (n ≥ 3). One-way ANOVA. G Expression of NKX2.5, MYH6 and TNNT2 in WT and TBX5-OE cells (−DOX and + DOX) at differentiation day 7. Relative to GAPDH expression (n = 3). One-way ANOVA. H Expression of SHF markers in WT and TBX5-OE cells (−DOX and + DOX) at differentiation days 5 and 7. Relative to GAPDH expression (n = 3). One-way ANOVA. I Expression of atrial, OFT and ventricular cardiomyocyte markers in WT and TBX5-OE cells (−DOX and + DOX)-derived differentiation day 30 cardiomyocytes. Relative to GAPDH expression (n = 3). One-way ANOVA. J Comparison of corrected APD90, APD50 and APD90/APD50 ratio recorded by MEA in WT and TBX5-OE cells (−DOX and + DOX)-derived differentiation day 30 cardiomyocytes (n ≥ 8). One-way ANOVA. K Comparison of APD90 and APD50 recorded by whole-cell patch clamp in WT and TBX5-OE cells (−DOX and + DOX)-derived day 30 cardiomyocytes (n ≥ 9). One-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s: non-significant
To confirm the regulation of TBX5 by HAND1/2, we constructed a Luciferase plasmid under the control of TBX5 promoter (Additional file 1: Fig. S6A) and co-transfected with the HAND1 or HAND2 overexpression plasmid (H1-OE, H2-OE) into HEK 293T cells, a commonly used cell line because of its high transfection efficiency. Both HAND1 and HAND2 activated Luciferase expression, indicating HAND1/2 regulated TBX5 promoter activity (Fig. 6C). Subsequently, we constructed TBX5 overexpression H1/H2-dKO cell line with DOX-inducible Tet-On system (referred as TBX5-OE). In the presence of DOX (+ DOX), the expression of TBX5 was activated. We confirmed the completely restored TBX5 protein expression in + DOX group at differentiation days 5 and 10 (Fig. 6D).
With DOX induction, at differentiation day 7, we observed that TBX5 overexpression restored the NKX2.5-eGFP+ population to the level as that of WT cells as evidenced by fluorescence microscope (Additional file 1: Fig. S6B, C) and flow cytometry analysis (Fig. 6E, F). Moreover, the expression of cardiomyocyte markers NKX2.5, MYH6 and TNNT2 in + DOX group was also upregulated compared to −DOX group (Fig. 6G). Thus, with TBX5 overexpression, the delayed cardiomyocyte differentiation was rescued. Intriguingly, TBX5 overexpression did not recover the beating onset of H1/H2-dKO cells as early as WT cells (Additional file 1: Fig. S6D). Next, we explored the effects of TBX5 on H1/H2-dKO cardiac lineage commitment. We observed significant reduction in some SHF markers, including TBX1, SIX1, NR2F2, HOXA1, HOXB1 and ALDH1A2 from differentiation days 5 to 7 and FOXC2 at differentiation day 7 in + DOX group, whereas HCN4 slightly increased at differentiation day 7 (Fig. 6H, Additional file 1: Fig. S6E). At differentiation day 30, TBX5 overexpressing cardiomyocytes exhibited reduced expression of SHF-derived atrial and OFT cardiomyocyte markers, including NR2F2, KCNJ3 and LTBP3, RSPO3 (Fig. 6I), while the ventricular cardiomyocyte marker MYL2 were only slightly upregulated (Fig. 6I).
Finally, we performed MEA and whole-cell patch clamp to analyze the electrophysiology characteristics of H1/H2-dKO cardiomyocytes with and without TBX5 overexpression. Corrected APD90 and APD50 of TBX5 overexpressing cardiomyocytes recorded by MEA were not statistically different from the −DOX group, whereas the ratio of APD90/APD50 decreased in TBX5 overexpressing cardiomyocytes (Fig. 6J). The results of whole-cell patch clamp showed increase of APD90 and APD50 in + DOX cardiomyocytes compared with −DOX group, although the difference was not statistically significant (Fig. 6K). These results indicated that the delayed cardiomyocyte differentiation in H1/H2-dKO cells was caused by reduced TBX5 expression in the absence of HAND1/2. However, the tendency of differentiation into SHF-derived cardiomyocytes was only partially rescued, suggesting that the function of HAND1/2 in cardiac lineage commitment was partially dependent on TBX5.
Discussion
In this study, we investigated the function of HAND1/2 in human cardiac lineage commitment and differentiation by inducing differentiation of HAND depleted NKX2.5eGFP H9 hESCs into cardiomyocytes. We revealed that HAND1 deficiency, as well as HAND1/2 double knockout hESCs, biased to differentiate into SHF lineage and its derived cardiomyocytes at the expense of FHF progenitors, while HAND2 knockout impaired cardiomyocyte differentiation from SHF progenitors. Moreover, HAND1/2 double knockout also delayed the onset of cardiomyocyte differentiation. These results highlighted the difference and redundance of HAND1/2 during the differentiation from cardiac mesoderm to cardiomyocytes.
HAND1 deficiency led to obviously upregulated expression of SHF markers from cardiac mesoderm stage and maintained at higher level than WT throughout early cardiomyocyte differentiation. Conversely, HAND1 knockout downregulated the expression of FHF markers from the cardiac progenitor stage. It was known that HAND1 was initially expressed in mesoderm [19, 22, 31], but its function in human early cardiac lineage commitment was not completely clear. We verified that HAND1 was one of the key factors that determine the FHF and SHF fates from cardiac mesoderm. And with the differentiation of cardiac progenitor cells, HAND1-deficient cardiac mesoderm cells eventually produced more SHF-derived cardiomyocytes instead of FHF-derived cardiomyocytes. Meanwhile, the elevated HAND2 in H1-KO cells could also contribute to the generation of SHF-derived cardiomyocytes, as HAND2 promoted SHF development [29].
In H2-KO cells, the expression of SHF markers slightly increased from cardiac mesoderm stage, while the expression of atrial and OFT cardiomyocyte markers downregulated and the cardiac progenitor markers maintained at relatively high level at differentiation day 30, indicating that HAND2 deficiency impaired SHF differentiation. These results were consistent with the findings in Hand2-null mice which had increased aSHF and pSHF progenitors and impaired OFT differentiation [30]. The elevated MYL2+ cardiomyocyte population and the ventricular-like action potentials of these cardiomyocytes further corroborated the properties of H2-KO cardiomyocytes. Notably, we found that HAND1 was upregulated in H2-KO cells, which was in line with a previous report that Hand1 was robustly expressed in ventricular and lateral mesoderm in Hand2 deficiency embryos [25]. Thus, HAND1 in H2-KO cells could contribute to FHF-derived ventricular cardiomyocytes and restrict SHF differentiation. Considering that HAND2 was primarily expressed in human atrial cardiomyocytes [35] and upregulated in atrial cardiomyocyte differentiation [37], we speculated that HAND2 deficiency could also impair the SHF-derived atrial cardiomyocytes. Unfortunately, the cardiomyocytes generated in our differentiation system were mainly ventricular subtype [67, 87]. The role of HAND2 in atrial cardiomyocyte differentiation from hPSCs warrants further investigation.
During cardiomyocyte differentiation, distinct from H2-KO cells, both H1-KO and H1/H2-dKO cells displayed highly expressed SHF markers and later produced cardiomyocytes with atrial-like action potentials, indicating that H1-KO cells were in proximity to H1/H2-dKO cells, which was confirmed by transcriptome analysis. At the transcriptional level, H1/H2-dKO cells were much closer to H1-KO rather than H2-KO cells, and the SHF markers were upregulated in both H1-KO and H1/H2-dKO cells, although higher in H1/H2-dKO cells. The severer impairment of cardiomyocyte differentiation in H1/H2-dKO cells could be explained by that HAND1 determined the fate of FHF and SHF at cardiac mesoderm stage. On the basis that HAND1 knockout altered the fate of cardiac progenitors, HAND2 deficiency led to further impairment of subsequent cardiomyocyte differentiation and electrophysiological activity, as the case in Hand1/2 deficiency mice which manifested severe heart defects and embryonic death [27]. Intriguingly, the cardiomyocyte differentiation process was delayed in H1/H2-dKO cells, suggesting that to safeguard the timing of cardiac differentiation, either HAND1 or HAND2 was required.
According to the ChIP-seq and RNA-seq analyses, we validated that TBX5 was a key downstream TF activated by HAND1/2. TBX5 deficiency in hPSCs led to delayed cardiac differentiation and biased toward atrial-like cardiomyocytes [79], similar to the phenotypes observed in HAND1/2 defects. Overexpressing TBX5 in H1/H2-dKO cells was able to restore the cardiomyocyte differentiation efficiency to the level of WT cells at differentiation day 7. The onset beating time of H1/H2-dKO cells was not rescued by TBX5, indicating that TBX5 may not be the key regulator for the impaired contractile activity in H1/H2-dKO cardiomyocytes. Although the expression of SHF markers, as well as its derived cardiomyocyte markers, and the ratio of APD90/APD50 decreased with TBX5 overexpression, they did not recover to the level of WT cells. Therefore, for the tendency toward SHF differentiation in H1/H2-dKO cells, it was only partially rescued by TBX5 overexpression which could be attributed to the complex regulatory mechanisms involving HAND1/2 during cardiomyocyte differentiation. Moreover, since the expression of TBX5 was detected from differentiation day 5, later than HAND1 (Figs. 1B, 2B), we hypothesized that the overexpression of TBX5 could not reverse the already determined SHF fate at cardiac mesoderm stage (day 3) due to HAND1 deficiency.
Both HAND1 and HAND2 were capable of activating the TBX5 promoter. However, we found that TBX5 expression was decreased in H1-KO and H1/H2-dKO cells, and slightly increased in H2-KO cells. We speculated that HAND1 exerted stronger regulation over TBX5, and in H1-KO cells, though the elevated expression of HAND2 could not restore TBX5 expression, it could compensate for HAND1 depletion by interacting with TBX5 [88], as evidenced by the onset of cardiomyocyte differentiation which was normal in H1-KO cells, but delayed in H1/H2-dKO cells. In H2-KO cells, the upregulated HAND1 compensated for the loss of HAND2 to activate TBX5 expression. In addition, TBX5 overexpression rescued the delayed cardiomyocyte differentiation in H1/H2-dKO cells, also verifying TBX5 as a downstream target of HAND1/2. Consequently, to timely initiate cardiomyocyte differentiation, either HAND1, HAND2 or sufficient level of TBX5 was required.
The study has several limitations. Firstly, it should be acknowledged that there are vast differences between in vitro and in vivo conditions for cardiomyocyte differentiation. The complex in vivo microenvironment where the human embryonic heart develops is regulated by fine-tuned transcription factors and signaling pathways [1, 89], making it challenging to fully replicate this niche in vitro. Therefore, the findings of the study may be restricted to some extent. For further validation and mechanistic investigation, 3D cardiac organoid models or in vivo experiments on large animals are necessary. Secondly, we found numerous important cardiac developmental TFs such as DPF3, MEF2A and ETS2, which may be modulated by HAND1/2 during cardiac differentiation. In this study, we primarily focused on TBX5, which partially rescued the defects of H1/H2-dKO cells in cardiomyocyte differentiation. Therefore, the extensive mechanisms by which HAND1/2 regulate the cardiac lineage commitment await being elucidated in future.
Conclusion
We revealed the specific and redundant function of HAND1/2 in human heart development by providing comprehensive survey of HAND1/2 on cardiac lineage commitment and differentiation from pluripotent stem cells. Furthermore, TBX5 was verified as one of the key factors in HAND1/2 gene regulatory network during cardiac organogenesis. These findings may potentially facilitate new treatment strategies for CHDs.
Availability of data and materials
The ChIP-seq of HAND1/2 datasets used and analyzed during the current study are available from the GEO/NCBI (GSM1505812 and GSM1505811, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE61475). The raw RNA-seq data of sorted NKX2.5-eGFP+ cells at differentiation day 7 have been deposited into the CNGB Sequence Archive (CNSA) of the China National GeneBank DataBase (CNGBdb) with accession number CNP0005227 (https://db.cngb.org/search/project/CNP0005227/).
Abbreviations
- ACM:
-
Atrial cardiomyocyte
- APD:
-
Action potential duration
- aSHF:
-
Anterior second heart field
- CHD:
-
Congenital heart disease
- CM:
-
Cardiomyocyte
- FHF:
-
First heart field
- hESCs:
-
Human embryonic stem cells
- hPSCs:
-
Human pluripotent stem cells
- LEAP:
-
Local extracellular action potential
- MEA:
-
Microelectrode array
- OFT:
-
Outflow tract
- pSHF:
-
Posterior second heart field
- SHF:
-
Second heart field
- TF:
-
Transcription factor
- VCM:
-
Ventricular cardiomyocyte
References
Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb Perspect Biol. 2013;5:a008292.
McCulley DJ, Black BL. Transcription factor pathways and congenital heart disease. Curr Top Dev Biol. 2012;100:253–77.
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–7.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-322.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle-Colarusso T, Nembhard WN, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–9.
Thomas T, Yamagishi H, Overbeek PA, Olson EN, Srivastava D. The bHLH factors, dHAND and eHAND, specify pulmonary and systemic cardiac ventricles independent of left-right sidedness. Dev Biol. 1998;196:228–36.
George RM, Firulli AB. Hand factors in cardiac development. Anat Rec (Hoboken). 2019;302:101–7.
Liu N, Barbosa AC, Chapman SL, Bezprozvannaya S, Qi X, Richardson JA, et al. DNA binding-dependent and -independent functions of the Hand2 transcription factor during mouse embryogenesis. Development. 2009;136:933–42.
McFadden DG, McAnally J, Richardson JA, Charité J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–88.
Feng Y, Cai L, Hong W, Zhang C, Tan N, Wang M, et al. Rewiring of 3D chromatin topology orchestrates transcriptional reprogramming and the development of human dilated cardiomyopathy. Circulation. 2022;145:1663–83.
Lescroart F, Wang X, Lin X, Swedlund B, Gargouri S, Sànchez-Dànes A, et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science. 2018;359:1177–81.
Ivanovitch K, Soro-Barrio P, Chakravarty P, Jones RA, Bell DM, Mousavy Gharavy SN, et al. Ventricular, atrial, and outflow tract heart progenitors arise from spatially and molecularly distinct regions of the primitive streak. PLoS Biol. 2021;19:e3001200.
Lescroart F, Chabab S, Lin X, Rulands S, Paulissen C, Rodolosse A, et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat Cell Biol. 2014;16:829–40.
Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68.
Protze SI, Lee JH, Keller GM. Human pluripotent stem cell-derived cardiovascular cells: from developmental biology to therapeutic applications. Cell Stem Cell. 2019;25:311–27.
Später D, Abramczuk MK, Buac K, Zangi L, Stachel MW, Clarke J, et al. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat Cell Biol. 2013;15:1098–106.
Barnes RM, Firulli BA, Conway SJ, Vincentz JW, Firulli AB. Analysis of the Hand1 cell lineage reveals novel contributions to cardiovascular, neural crest, extra-embryonic, and lateral mesoderm derivatives. Dev Dyn. 2010;239:3086–97.
Yang D, Gomez-Garcia J, Funakoshi S, Tran T, Fernandes I, Bader GD, et al. Modeling human multi-lineage heart field development with pluripotent stem cells. Cell Stem Cell. 2022;29:1382-401.e8.
Zawada D, Kornherr J, Meier AB, Santamaria G, Dorn T, Nowak-Imialek M, et al. Retinoic acid signaling modulation guides in vitro specification of human heart field-specific progenitor pools. Nat Commun. 2023;14:1722.
Pijuan-Sala B, Griffiths JA, Guibentif C, Hiscock TW, Jawaid W, Calero-Nieto FJ, et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature. 2019;566:490–5.
Zhang Q, Carlin D, Zhu F, Cattaneo P, Ideker T, Evans SM, et al. Unveiling complexity and multipotentiality of early heart fields. Circ Res. 2021;129:474–87.
Tyser RCV, Ibarra-Soria X, McDole K, Arcot Jayaram S, Godwin J, van den Brand TAH, et al. Characterization of a common progenitor pool of the epicardium and myocardium. Science. 2021;371:eabb2986.
Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–70.
Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–60.
Firulli BA, George RM, Harkin J, Toolan KP, Gao H, Liu Y, et al. HAND1 loss-of-function within the embryonic myocardium reveals survivable congenital cardiac defects and adult heart failure. Cardiovasc Res. 2020;116:605–18.
McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201.
Tsuchihashi T, Maeda J, Shin CH, Ivey KN, Black BL, Olson EN, et al. Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol. 2011;351:62–9.
Schindler YL, Garske KM, Wang J, Firulli BA, Firulli AB, Poss KD, et al. Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration. Development. 2014;141:3112–22.
de Soysa TY, Ranade SS, Okawa S, Ravichandran S, Huang Y, Salunga HT, et al. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature. 2019;572:120–4.
Tyser RCV, Mahammadov E, Nakanoh S, Vallier L, Scialdone A, Srinivas S. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature. 2021;600:285–9.
Zhai J, Xiao Z, Wang Y, Wang H. Human embryonic development: from peri-implantation to gastrulation. Trends Cell Biol. 2022;32:18–29.
Zeng B, Liu Z, Lu Y, Zhong S, Qin S, Huang L, et al. The single-cell and spatial transcriptional landscape of human gastrulation and early brain development. Cell Stem Cell. 2023;30:851-66.e7.
Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell. 2004;6:685–98.
Cui Y, Zheng Y, Liu X, Yan L, Fan X, Yong J, et al. Single-cell transcriptome analysis maps the developmental track of the human heart. Cell Rep. 2019;26:1934-50.e5.
Stolfi A, Gainous TB, Young JJ, Mori A, Levine M, Christiaen L. Early chordate origins of the vertebrate second heart field. Science. 2010;329:565–8.
Okubo C, Narita M, Inagaki A, Nishikawa M, Hotta A, Yamanaka S, et al. Expression dynamics of HAND1/2 in in vitro human cardiomyocyte differentiation. Stem Cell Rep. 2021;16:1906–22.
Reamon-Buettner SM, Ciribilli Y, Inga A, Borlak J. A loss-of-function mutation in the binding domain of HAND1 predicts hypoplasia of the human hearts. Hum Mol Genet. 2008;17:1397–405.
Cheng Z, Lib L, Li Z, Liu M, Yan J, Wang B, et al. Two novel HAND1 mutations in Chinese patients with ventricular septal defect. Clin Chim Acta. 2012;413:675–7.
Reamon-Buettner SM, Ciribilli Y, Traverso I, Kuhls B, Inga A, Borlak J. A functional genetic study identifies HAND1 mutations in septation defects of the human heart. Hum Mol Genet. 2009;18:3567–78.
Sun YM, Wang J, Qiu XB, Yuan F, Li RG, Xu YJ, et al. A HAND2 loss-of-function mutation causes familial ventricular septal defect and pulmonary stenosis. G3 (Bethesda). 2016;6:987–92.
Wang J, Hu XQ, Guo YH, Gu JY, Xu JH, Li YJ, et al. HAND1 loss-of-function mutation causes tetralogy of fallot. Pediatr Cardiol. 2017;38:547–57.
Lu CX, Gong HR, Liu XY, Wang J, Zhao CM, Huang RT, et al. A novel HAND2 loss-of-function mutation responsible for tetralogy of Fallot. Int J Mol Med. 2016;37:445–51.
Zhai J, Guo J, Wan H, Qi L, Liu L, Xiao Z, et al. Primate gastrulation and early organogenesis at single-cell resolution. Nature. 2022;612:732–8.
Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–60.
Jiang Y, Lian XL. Heart regeneration with human pluripotent stem cells: prospects and challenges. Bioact Mater. 2020;5:74–81.
Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28.
Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol. 2016;13:333–49.
Andersen P, Tampakakis E, Jimenez DV, Kannan S, Miyamoto M, Shin HK, et al. Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat Commun. 2018;9:3140.
Pezhouman A, Engel JL, Nguyen NB, Skelton RJP, Gilmore WB, Qiao R, et al. Isolation and characterization of human embryonic stem cell-derived heart field-specific cardiomyocytes unravels new insights into their transcriptional and electrophysiological profiles. Cardiovasc Res. 2022;118:828–43.
Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–75.
Cao N, Liu Z, Chen Z, Wang J, Chen T, Zhao X, et al. Ascorbic acid enhances the cardiac differentiation of induced pluripotent stem cells through promoting the proliferation of cardiac progenitor cells. Cell Res. 2012;22:219–36.
Ying QL, Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–41.
Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–9.
Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–41.
Esfandyari D, Idrissou BMG, Hennis K, Avramopoulos P, Dueck A, El-Battrawy I, et al. MicroRNA-365 regulates human cardiac action potential duration. Nat Commun. 2022;13:220.
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–15.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Kumar L, Futschik ME. Mfuzz: a software package for soft clustering of microarray data. Bioinformation. 2007;2:5–7.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7.
Elliott D, Braam S, Koutsis K, Ng E, Jenny R, Lagerqvist E, et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods. 2011;8:1037–40.
Yu Y, Qin N, Lu XA, Li J, Han X, Ni X, et al. Human embryonic stem cell-derived cardiomyocyte therapy in mouse permanent ischemia and ischemia-reperfusion models. Stem Cell Res Ther. 2019;10:167.
Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–58.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Prummel K, Nieuwenhuize S, Mosimann C. The lateral plate mesoderm. Development. 2020. https://doi.org/10.1242/dev.175059.
Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell. 2017;21:179-94.e4.
Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, et al. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat Commun. 2020;11:75.
Small EM, Krieg PA. Molecular regulation of cardiac chamber-specific gene expression. Trends Cardiovasc Med. 2004;14:13–8.
Schmidt C, Deyett A, Ilmer T, Haendeler S, Torres Caballero A, Novatchkova M, et al. Multi-chamber cardioids unravel human heart development and cardiac defects. Cell. 2023;186:5587-605.e27.
Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502.
Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–94.
Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33.
Amin AS, Asghari-Roodsari A, Tan HL. Cardiac sodium channelopathies. Pflugers Arch. 2010;460:223–37.
Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65.
Bax NA, Bleyl SB, Gallini R, Wisse LJ, Hunter J, Van Oorschot AA, et al. Cardiac malformations in Pdgfralpha mutant embryos are associated with increased expression of WT1 and Nkx2.5 in the second heart field. Dev Dyn. 2010;239:2307–17.
Strasburger J, Wacker-Gussmann A. Developmental electrophysiology in the fetus and neonate. In: Polin RA, Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and neonatal physiology. 5th ed. Philadelphia: Elsevier; 2017. p. 522–38.
Tsankov AM, Gu H, Akopian V, Ziller MJ, Donaghey J, Amit I, et al. Transcription factor binding dynamics during human ES cell differentiation. Nature. 2015;518:344–9.
Kathiriya IS, Rao KS, Iacono G, Devine WP, Blair AP, Hota SK, et al. Modeling human TBX5 haploinsufficiency predicts regulatory networks for congenital heart disease. Dev Cell. 2021;56:292-309.e9.
Wales S, Hashemi S, Blais A, McDermott JC. Global MEF2 target gene analysis in cardiac and skeletal muscle reveals novel regulation of DUSP6 by p38MAPK-MEF2 signaling. Nucleic Acids Res. 2014;42:11349–62.
Schlesinger J, Schueler M, Grunert M, Fischer JJ, Zhang Q, Krueger T, et al. The cardiac transcription network modulated by Gata4, Mef2a, Nkx2.5, Srf, histone modifications, and microRNAs. PLoS Genet. 2011;7:e1001313.
Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–44.
Bosman A, Letourneau A, Sartiani L, Del Lungo M, Ronzoni F, Kuziakiv R, et al. Perturbations of heart development and function in cardiomyocytes from human embryonic stem cells with trisomy 21. Stem Cells. 2015;33:1434–46.
Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109:13016–21.
Anderson DJ, Kaplan DI, Bell KM, Koutsis K, Haynes JM, Mills RJ, et al. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat Commun. 2018;9:1373.
Akazawa H, Komuro I. Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacol Ther. 2005;107:252–68.
Galdos FX, Lee C, Lee S, Paige S, Goodyer W, Xu S, et al. Combined lineage tracing and scRNA-seq reveals unexpected first heart field predominance of human iPSC differentiation. Elife. 2023;12:e80075.
Khalil A, Tanos R, El-Hachem N, Kurban M, Bouvagnet P, Bitar F, et al. A HAND to TBX5 explains the link between thalidomide and cardiac diseases. Sci Rep. 2017;7:1416.
MacGrogan D, Münch J, de la Pompa JL. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat Rev Cardiol. 2018;15:685–704.
Acknowledgements
The gene editing tools, including spCas9 and pGL3-U6-PGK-Puromycin vector, were gifts from Xingxu Huang (Gene Editing Center, ShanghaiTech University, China).
Funding
This work was funded by the Grants from the Programs of National Natural Science Foundation of China (82000377, to DS; 81900297, 82070338 and 82222008, to DX; 82070271, to DL; 31871491, to JY; 82088101 and 81930013, to Y-HC), the National Key Research and Development Plan (2019YFA0801501, to Y-HC), Key Research Center Construction Project of Shanghai (2022ZZ01008, to Y-HC), Top-level Clinical Discipline Project of Shanghai Pudong (PWYgf2021-01), Research Unit of Origin and Regulation of Heart Rhythm, Chinese Academy of Medical Sciences (2019RU045), National Key Clinical Specialty and the Fundamental Research Funds for the Central Universities to Y-HC. Prof. Y-HC is a Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, Nanjing Medical University. The funding body played no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
HG proposed the project, designed the experiment, conducted the experiment, analyzed the data and drafted the manuscript. CH designed the experiment, conducted the experiment, analyzed the data and revised the manuscript. BL analyzed the RNA-seq data and ChIP-seq data and revised the manuscript. ZL, HX, MZ, RL and JL conducted the experiments. DS, DX, YL and DL analyzed data and participated in discussions. JY involved in proposing the project, analyzing data, participating in discussions, revising the manuscript, guidance and supervision. Y-HC involved in revising the manuscript, guidance and supervision.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1
. Establishment of H1-KO, H2-KO and H1/H2-dKO hESC lines. Fig. S2. HAND1 deficiency promoted SHF and its derived cardiomyocyte differentiation. Fig. S3. HAND2 knockout impaired SHF-derived cardiomyocyte differentiation. Fig. S4. HAND1/2 double knockout impeded the differentiation of cardiomyocytes and impaired their electrophysiological activity. Fig. S5. Expression of HAND1 in H2-KO cells and HAND2 in H1-KO cells in sorted cardiomyocytes at differentiation day 7 by RNA-seq. Fig. S6. HAND1/2 modulated cardiomyocyte differentiation through TBX5. Table S1. Primers used in PCR and qPCR in the experiments. Table S2. Transcription factors regulated by HAND1 and HAND2 in H1/H2-dKO downregulated genes.
Additional file 2. The video of beating WT cardiomyocytes at differentiation day 7.
Additional file 3:
Table S3. The number of reads mapped per sample of RNA-seq data.
Additional file 4.
Table S4. Genes list of each gene expression cluster in Fig. 5E.
Additional file 5.
Table S5. GO enrichment analysis of each gene expression cluster in Fig. 5F.
Additional file 6.
Table S6. KEGG pathway enrichment analysis of each gene expression cluster in Fig. 5G.
Additional file 7.
Table S7. Expression profiling of the sorted WT and HAND KO cardiomyocytes at differentiation day 7 by RNA-seq and the comparison between samples. The transcriptional levels were shown as TPM.
Additional file 8.
Fig. S7. Full-length blots of Fig. 1H. Western blot analysis of HAND1 and HAND2 expression in KO cell lines-derived day 10 cardiomyocytes. Fig. S8. Full-length blots of Fig. 2F and Fig. S2H, J. Western blot analysis of CX43, MYL2, NR2F2 and SCN5A expression in H1-KO-derived day 30 cardiomyocytes. Fig. S9. Full-length blots of Fig. 3F and Fig. S3G. Western blot analysis of MYL2 and NR2F2 expression in H2-KO-derived day 30 cardiomyocytes. Fig. S10. Full-length blots of Fig. 4F and Fig. S4B, C, J, L. Western blot analysis of CX43, MYL2, NR2F2 and SCN5A expression in H1/H2-dKO-derived day 30 cardiomyocytes. Western blot analysis of HAND1 and HAND2 expression in H2-KO and H1-KO cells, respectively. Fig. S11. Full-length blots of Fig. 6D. Western blot analysis of TBX5 expression in TBX5-OE cell lines (-DOX/+DOX) at differentiation days 5 and 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, H., Hang, C., Lin, B. et al. HAND factors regulate cardiac lineage commitment and differentiation from human pluripotent stem cells. Stem Cell Res Ther 15, 31 (2024). https://doi.org/10.1186/s13287-024-03649-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03649-9