Abstract
Background
Allo-HSCT is a definite approach for the management of a wide variety of lethal and debilitating malignant and non-malignant disorders. However, its two main complications, acute and chronic graft-versus-host disease (GVHD), exert significant morbidities and mortalities. BoS, as a manifestation of chronic lung GVHD, is a gruesome complication of allo-HSCT, and for those with steroid-refractory disease, no approved second-line therapies exist. Mesenchymal stem cells (MSCs) exert anti-inflammatory and growth-promoting effects, and their administration against a wide range of inflammatory and neurologic disorders, as well as GVHD, has been associated with promising outcomes. However, literature on the safety and effectiveness of MSC therapy for BoS and pediatric cGVHD is scarce.
Methods
We designed a single-arm trial to administer adipose tissue (AT)-derived MSCs to pediatric patients with refractory BoS after allo-HSCT. AT-MSCs from obese, otherwise healthy donors were cultured in an ISO class 1 clean room and injected into the antecubital vein of eligible patients with a dose of 1 × 106/kg. The primary endpoints included a complete or partial response to therapy [in terms of increased forced expiratory volume in one second (FEV1) values and steroid dose reduction] and its safety profile.
Results
Four eligible patients with a median age of 6.5 years were enrolled in the study. Steroid-induced osteoporosis and myopathy were present in three cases. A partial response was evident in three cases after a single injection of AT-MSCs. The treatment was safe and tolerable, and no treatment-related adverse events were noted. Two patients developed manageable COVID-19 infections one and 4 months after AT-MSC injection. After a median follow-up duration of 19 months, all cases are still alive and have had no indications for lung transplantation.
Conclusions
AT-MSCs could be safely administered to our pediatric cases with BoS post-allo-HSCT. Considering their advanced stage of disease, their sub-optimal functional capacity due to steroid-induced complications, and COVID-19 infection post-treatment, we believe that AT-MSC therapy can have possible efficacy in the management of pediatric BoS. The conduction of further studies with larger sample sizes and more frequent injections is prudent for further optimization of AT-MSC therapy against BoS.
Trial registration Iranian Registry of Clinical Trials (IRCT), IRCT20201202049568N2. Registered 22 February 2021, https://en.irct.ir/trial/53143.
Similar content being viewed by others
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative strategy against various debilitating malignant and non-malignant disorders [1,2,3], but it is complicated by the development of acute and chronic graft-versus-host disease (aGVHD and cGVHD, respectively) [4, 5]. With improved survival rates of aGVHD, more than 50% of such cases and roughly 30–70% of all allo-HSCT recipients develop cGVHD [6]. Corresponding statistics for pediatric patients are less consistent and, depending on the source and method of performing allo-HSCT, between 6 and 65% of all and 18 and 27% of aGVHD cases are being diagnosed with cGVHD [7, 8]. Despite having lower mortality rates [9], cGVHD poses a significant morbidity and economic burden and is associated with considerable compromise in the quality of life of affected individuals and an increased incidence of infections, respiratory failure, and intensive care unit admissions [9,10,11]. It becomes particularly cumbersome for pediatric cases, as their significantly prolonged survival will be associated with such complications [5]. Lung involvement is among the more difficult-to-treat manifestations of cGVHD and is associated with more profound morbidity and mortality [9].
Bronchiolitis obliterans (BO) is the most common and deleterious form of the late-onset noninfectious pulmonary complication (LONIPC) of allo-HSCT, which is reported to afflict about 3.7–11% and 10–14% of all allo-HSCT and cGVHD cases, respectively [12,13,14]. BO is also associated with increased mortality in allo-HSCT and lung transplant patients [12,13,14]. While its exact pathological underpinnings are not deciphered yet, aberrations in innate and adaptive immune system activation and excessive tissue fibrosis (as the hallmarks of cGVHD and BO) have been documented [6, 15, 16]. The definite diagnosis of BO lies in the pathological assessment of lung biopsy samples. However, owing to the invasiveness nature of lung biopsy, the National Institutes of Health (NIH) has proposed criteria for the clinical diagnosis of bronchiolitis obliterans syndrome (BoS, which denotes the lack of pathological evaluations), which is widely accepted by authors [17, 18]. Current general approaches for the management of BoS comprise a short trial of pulsed corticosteroids with a rapid taper and fluticasone–azithromycin–montelukast [FAM] combination, and, in refractory cases, they include extracorporeal photopheresis (ECP), other immunosuppressives, and lung transplantation (as the last resort) [19,20,21,22,23,24,25,26,27]. However, these approaches are not studied in pediatric cases and are not robustly effective against progressive BoS [20, 28].
Mesenchymal stem/stromal cells (MSCs) are multipotent cells that can be obtained from various tissues (including bone marrow [BM], adipose tissue [AT], umbilical cord, and placenta) [29,30,31], and apart from regenerative capacities, they harbor immunomodulatory and growth-promoting characteristics [32,33,34,35]. Experimental [36, 37] and clinical [38,39,40,41] studies have demonstrated their effectiveness in the treatment of GVHD, and their low and no expression of human leukocyte antigens (HLA) class I and class II, respectively, make them ideal targets for allogeneic transplantation [42]. Compared to other sources, AT-derived MSCs (AT-MSCs) are easy to obtain [43], have less genomic instability and senescence than BM-derived MSCs [44], harbor higher proliferation capacities [37, 45], and are superior in inducing anti-inflammatory effects and surviving after transplantation [37, 45, 46]. Accordingly, it has recently been shown that these features can empower AT-MSCs to demonstrate better protection against aGVHD (compared to BM- and umbilical cord-derived MSCs) [37].
As there are no established second-line therapies for steroid-refractory BoS, we designed a phase I single-arm trial to evaluate the safety and efficacy of AT-MSCs against post-allo-HSCT refractory BOS in pediatrics. Given the beneficial impacts of AT-MSCs and the pathological underpinnings of BoS, we hypothesized that AT-MSCs can effectively resolve or control the progression of BoS after allo-HSCT.
Methods
Study design and patient selection
We designed an open-label, uncontrolled, non-randomized trial to evaluate the safety and efficacy of allogeneic AT-MSCs for the management of BoS in pediatric (< 18 years) patients who underwent allo-HSCT at the stem cell transplantation unit of Children Medical Center Hospital and had diagnosed with BoS between October 2020 and April 2022. The inclusion and exclusion criteria for the enrollment of eligible cases are presented in Table 1. The diagnosis of BoS was made based on the 2015 modified NIH criteria, which was initially released in 2005 [17, 18]. These criteria have been recognized as the gold standard tool for the diagnosis of BoS [23] and are widely accepted by relevant authorities [47].
Two expert pediatrics pulmonologists and two expert pediatrics stem cell transplantation specialists were responsible for the assessment of cases and confirmation of their eligibility. Due to the experimental nature of the study and ethical considerations, patients were allowed to continue receiving other prescribed medications for their allo-HSCT (prednisolone, ECP, and other immunosuppressive agents). We took written informed consent from AT donors and each patient’s parents after the detailed clarification of the experimental nature of the trial and the anonymity of enrolled individuals. This study is undertaken in accordance with the ethical principles and guidelines of the Declaration of Helsinki and is approved by the ethical committee of the Tehran University of Medical Sciences (ethics registration code, IR.TUMS.MEDICINE.REC.1399.406). The findings of this study are reported according to the Consolidated Standards of Reporting Trials (CONSORT) recommendations.
MSC preparation
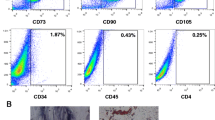
AT was obtained from obese, otherwise healthy, HLA-unrelated, third-party donors who underwent liposuction at university-affiliated centers (their baseline characteristics are demonstrated in Additional file 1: Table S1). The acquisition, expansion, and characterization of MSCs were conducted following the accredited protocols [48] and under good manufacturing practice (GMP) conditions. A detailed explanation of performed procedures is depicted in the Additional file 1: Detailed steps of adipose tissue-derived mesenchymal stem/stromal cells preparation.
Procedure
Patients received a single injection of AT-MSCs with a dose of 1 × 106/kg in their antecubital (median cubital) veins. In addition, if the weight of patients exceeded 35 kg, the total dose was divided into two separate injections at 2-days intervals. Intravenous (IV) injections were conducted with cardiopulmonary monitoring, and patients remained in close monitoring for a minimum of 12 h post-injection. Patients and their parents were instructed to visit the pediatrics HSCT and pulmonology clinics for timely follow-ups (F/Us) and go to the pediatric emergency centers upon the occurrence of adverse events (fever, seizure, anaphylaxis, skin rashes, palpitation, chest pain, dyspnea, etc.).
Outcome measure and endpoints
The endpoints of this study were the safety and efficacy of the application of AT-MSCs to pediatric patients with allo-HSCT-induced BoS. Spirometry was performed before and at 1-, 3-, and 6-months intervals after the injection of AT-MSCs and was interpreted by a pediatrics pulmonologist. High-resolution computed tomography (HRCT) of the chest was taken before and at 1- and 6-months intervals after the injection of AT-MSCs, for a minimum. Changes in the %forced expiratory volume in one second (%FEV1), FEV1/forced vital capacity (FVC), and HRCT features were recorded for each patient. According to the NIH consensus [49], an increase in the %FEV1 of 10% predicted or more was considered a partial response (PR), and an increase of %FEV1 to more than 80% was considered a complete response (CR). A diagnosis of progressive disease was made upon a 10% or more decrease in the absolute to predicted value of %FEV1 [49]. In addition, and according to the descriptions of previous trials [50], we defined a reduction in the steroid dose by at least 50% (without disease progression) as a PR. The grading of adverse events was performed according to the Common Terminology Criteria for Adverse Events (CTCAE), version 6.0.
Results
After evaluating 12 pediatric cases with a diagnosis of BoS post-allo-HSCT, four patients (one female, Table 2) met the eligibility criteria, consented to enroll in the trial, and participated in subsequent follow-up visits. Three cases were diagnosed with acute lymphocytic leukemia (ALL), and one case had thalassemia major. In addition, two had been diagnosed with manageable aGVHD. The characteristics of their allo-HSCT are depicted in Table 2.
The first patient was a 16-year-old boy who was diagnosed with ALL. He received a fully matched allo-HSCT from his brother and, about 6 months later, presented with signs and symptoms in favor of BoS (Table 3). Two months after this diagnosis, and after observing no improvements in response to conventional regimens, he received AT-MSCs with a dose of 1 × 106/kg. One month after the MSC injection, his spirometry parameters were stable (Table 4), and prednisolone and mycophenolate mofetil (MMF) were tapered to 15 mg/d and 250 mg/d, respectively. At the 6-months F/U, he was on prednisolone (10 mg/d), while cyclosporine and MMF were discontinued (Table 3). He also showed a promising response to AT-MSCs in chest CT image sections (Fig. 1 and Table 5).
The second patient, a six-year-old boy with ALL, received one locus mismatched (9/10) allo-HSCT from his father. Approximately 70 days after transplantation, he presented with grade II skin and gastrointestinal aGVHD. The treatment of aGVHD was successful; however, chronic limited skin GVHD persisted. Six months after transplantation, he displayed insidious symptoms suspicious for BoS, and 5 months later, a definite diagnosis of BoS was made by pediatrics pulmonologists (Table 3). Observing minor clinical benefits, he received AT-MSCs 7 months after the diagnosis of BoS. Despite the lack of response to AT-MSCs in early scheduled F/Us, his %FEV1 showed a 10%-increase 8 months after MSC therapy (Table 4). His prednisone was tapered to 5 mg, and with the initiation of ruxolitinib (1 mg/d) and ECP, steroid therapy was discontinued. In the last F/U (20 months), despite a decrease in %FEV1 (23%, Table 4), his only complaint was dyspnea on exertion, and the blood oxygen saturation was 99%.
The third case is a seven-year-old girl with ALL who received a fully matched allo-HSCT from his brother. She developed manageable grade II skin aGVHD. About 6 months after her allo-HSCT, she started to present trivial yet progressive respiratory distress and a decrease in blood oxygen saturation. Treatment with conventional approaches was initiated, and upon their lack of efficacy, AT-MSCs were applied 6 months after the diagnosis of BoS (Table 3). In the 1-month F/U after the injection of AT-MSCs, her FEV1 showed a 10% increase (Table 4), her cyanosis resolved, and her respiratory distress improved partially. Four months after AT-MSC therapy, she developed Coronavirus disease 2019 (COVID-19) infection, and her chest-CT images showed the development and expansion of pneumomediastinum, pneumopericardium, and subcutaneous emphysema (Table 5). However, these complications did not progress in subsequent evaluations (Fig. 2), and on the last F/U (19 months), she had received 19 sessions of ECP and had significant tapering in her immunosuppressive doses (Table 3).
Chest CT images of the third case, who developed COVID-19 infection four months after AT-MSC therapy (panels A and B). Two weeks later, pneumomediastinum, pneumopericardium, and subcutaneous emphysema (orange arrows) progressed (panels C and D); however, these abnormalities did not show a significant progression (blue arrows) in images obtained 12 months later (panels E and F)
The last patient was a known case of thalassemia major who underwent a fully matched allo-HSCT (from his sister) at the age of 4.5 years. About 7 months after allo-HSCT, he exhibited the presentations of BoS. After the failure of conventional therapies in controlling BoS, 2 months after the BoS diagnosis, he received AT-MSCs (Table 3). One month after the administration of AT-MSCs, he developed signs and symptoms of an acute respiratory infection, and upon hospitalization, COVID-19 infection was confirmed. This infection was manageable, and despite the deterioration in chest CT findings (Table 5) at the 2-months F/U after AT-MSC therapy, his spirometry parameters remained stable, and he was weaned from supplementary oxygen therapy. However, his symptoms began to deteriorate, and his spirometry values worsened at the 6-months F/U. As a result, despite an initial taper in prednisolone (7.5 mg/d) and MMF (250 mg, two times per week), he re-maintained on prednisolone (20 mg/d) and ruxolitinib (5 mg/day) 12 months after AT-MSC therapy, and tacrolimus (1 mg/d), and ECP were added to his therapeutic regimen. At the last F/U, tacrolimus was discontinued, and prednisolone was tapered to 12.5 mg/d (Table 3). It should be noted that this patient was diagnosed with steroid-induced myopathy, which might inversely affect the %FEV1.
In summary, the F/U duration for the first case was 13 months, and the other three have been followed for at least 19 months post-AT-MSC therapy. In our evaluations, all treated patients had clinical evidence of improvement in respiratory functions. As such, two cases (#2 and #3) were weaned from supplemental oxygen, and all demonstrated improvements in their daily activities. Moreover, excluding one case (#4), all other patients had at least a 50% decrease in their steroid doses. However, due to the safety concerns and allowance of concomitant administration of other treatments (namely ruxolitinib and ECP), we could not robustly determine whether this steroid-sparing benefit was a direct impact of AT-MSC therapy.
Overall, the treatment was safe and tolerable in all cases. None of them experienced infusion toxicity or adverse drug reactions. Likewise, no treatment-related adverse events occurred after AT-MSC injections. Notably, COVID-19 infections developed 6 and 9 months after the official announcement of COVID-19 spread in the country, when repetitive surges were occurring, and no approved vaccine for pediatrics was available in the country. As a result, we believed that no treatment-related infectious complications developed. Lastly, no relapse of underlying ALL has been observed yet.
Discussion
In this study, we described the preliminary evidence on the safety and efficacy of the first-time AT-MSC therapy for the management of BoS in pediatric patients who had received allo-HSCT. We found that despite sub-optimal objective responses in terms of an increase in %FEV1, eligible cases exhibit possible clinical improvements following this therapy.
As briefly mentioned, MSCs harbor anti-inflammatory and growth-promoting features that are exerted through various distinct paths [51,52,53]. Earlier studies exhibited that MSCs are effective in preventing acute lung injury, inflammation, and fibrosis following exposure to endotoxin [54] and bleomycin [55, 56] and can alleviate collagen deposition in lung tissue [53, 54, 56,57,58].
Most reports on the efficacy of MSC therapies against BoS are limited to lung transplantation in adults. In a phase I single-arm study on ten cases with chronic lung allograft dysfunction and BoS, the IV administration of BM-MSCs (8 × 106/kg, divided into four infusion sessions) was able to meaningfully (but not significantly) diminish the decline in FEV1 values [59]. Another phase Ia study on nine cases with moderate, treatment-refractory BoS after lung transplantation did not observe any benefits from BM-MSC therapy (1 to 4 × 106/kg) in the 1-month F/Us [60]. Nevertheless, in this study’s subsequent phase Ib trial on 13 lung transplant patients with moderate-to-severe BoS, allogenic BM-MSCs (0.5 or 1 × 106/kg) were effective in ceasing the significant reductions in FEV1 and FVC at the 12-months F/U, implicating the long-term efficacy of single-dose MSC therapy in preserving PFT parameters [61].
While the application of MSC therapy in the management of aGVHD is well-discussed in the literature [38,39,40, 62, 63], evidence of its efficacy for cGVHD is scarce. By and all, the outcomes have been promising, even in those with severe steroid-refractory disease [64,65,66,67]. However, some studies have documented conflicting observations on the response of cGVHD (including lung involvement) to autologous BM- [68] and allogeneic umbilical cord-derived MSCs [69]. In fact, in the Stenger et al. [68] study, none of the two adult cases displayed a response in their lung involvement. On the other hand, Shen et al. found that of three cases with lung cGVHD, one obtained a CR, one obtained a PR, and the third case displayed a stable disease [69] at the 3-months F/U.
Concerning allo-HSCT cases with BoS, a phase I/II study [50] enrolled 81 patients (aged between 18 and 59 years) with known BoS to investigate the efficacy of treatment with allogeneic BM-MSCs. Patients were non-randomly allowed to receive either azithromycin and prednisone or their combination, along with MSCs. The initial dose of MSCs was 4 × 106/kg (divided into four infusion sessions), and another 4 × 106/kg was allowed for those who had responded to the initial cycle. This study found that at the 3-months post-enrollment evaluations, 71% in the MSC group versus 44% in the non-MSC groups had a response (defined as an increase in FEV1 or > 50% steroid dose reduction) [50]. However, the differences lost their significance in those with severe BoS. In addition, while the differences in FEV1 stabilization/increase and the estimated 3 years overall survival were not significant between the two groups, MSC therapy was superior in facilitating a steroid dose reduction by at least 50% and mitigating the reduction in FEV1 from baseline [50]. Last but not least, there are inconsistent observations on the considerable enhancements in PFT parameters of allo-HSCT cases with BoS following umbilical cord MSC therapy [70].
Our study reported the efficacy and safety of first-time AT-MSCs administration for the management of pediatric BoS. Regarding the efficacy of this therapy, the objective outcomes were somehow heterogeneous. Two patients had an early (1-month) PR to AT-MSC therapy, while the PR of the third case was observable 8 months later. In addition, the first case exhibited considerable improvements at his 12-months F/U HRCT images.
Of note, despite clinical stability, the second case exhibited deteriorations in PFT parameters at the 12-months F/U. Besides, the last case did not respond to the therapy and exhibited a gradual decrease in %FEV1. However, he had severe steroid-induced osteoporosis (with a Z-score of − 4.1) and documented myopathy with evident gait dysfunction. Nevertheless, as mentioned earlier, all cases showed evidence of considerable improvements in respiratory functions and symptoms. Of note, discrepancies between the clinical picture and spirometry findings might stem from the fact that spirometry cannot be reliably performed in preschool children [71, 72], which was the scenario for three of our included cases. Likewise, it is suggested that FEV1 is not a robust indicator of the respiratory function of children [49]. Added to this, at least three of our cases had evidence of steroid-induced osteoporosis and myopathy, which can profoundly attenuate respiratory muscle functions and, consequently, spirometry performance [73].
This study faces several limitations. First, due to the low incidence of BoS, the refractory disease of the included cases, and the experimental and cell-based nature of the study, we did not add a second or control arm to the study. Second, in concordance with the protocols of previous trials and with consideration of safety measures in pediatric patients, we injected AT-MSCs for one time and at the dose of 1 × 106/kg, which theoretically can result in suboptimal activities of MSCs. Third, the continuation of other treatments was allowed during enrollment. Although none of the included cases were responsive to the conventional treatments for cGVHD, the interaction of these therapies with MSCs cannot be reliably ruled out. Fourth, due to safety concerns and a lack of enough data on MSC therapy in pediatrics, our study was limited to only four cases, which hinders its solid generalizability and the drawing of robust conclusions. Initially, there were twelve identified cases with BoS after allo-HSCT; however, evidence of latent tuberculous infection in the chest HRCT images of three cases and lack of consent to participate in five cases made them ineligible for enrollment in the trial. In addition, one case (#3) refused to perform all scheduled PFTs. Lastly, two patients (#3 and #4) developed COVID-19 infection after the administration of AT-MSCs, and although their infection was manageable, its negative impacts on pulmonary functions might have played a role in the lack of an acceptable clinical response to AT-MSC injection.
Conclusions
In conclusion, this study found that intravenous administration of AT-MSCs is a safe and tolerable approach, with promising subjective and acceptable objective efficacy in controlling BoS and preventing its deterioration following allo-HSCT in pediatrics. In fact, after a median F/U duration of 19 months after the administration of AT-MSCs, all of them have remained alive and are still in no need of lung transplantation. The four enrolled cases in our trial had a definite diagnosis of BoS according to the 2015 modified NIH criteria, and accordingly, all pediatric patients who suffer from BoS and meet these criteria might benefit from allogeneic AT-MSC therapy. Compared to other sources of MSCs, AT-MSCs are easier to retrieve, culture, and preserve and are superior in exerting immunomodulatory and growth-promoting influences. Subsequent studies with larger sample sizes and more frequent injections are required to robustly delineate the efficacy of MSC therapies in different grades of BoS.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1 on the detailed steps of adipose tissue-derived mesenchymal stem/stromal cells preparation and the characteristics of AT-MSC donors.
Abbreviations
- allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- aGVHD:
-
Acute graft-versus-host disease
- cGVHD:
-
Chronic graft-versus-host disease
- JAK:
-
Janus kinase
- BO:
-
Bronchiolitis obliterans
- LONIPC:
-
Late-onset noninfectious pulmonary complication
- NIH:
-
National Institutes of Health
- BoS:
-
Bronchiolitis obliterans syndrome
- FAM:
-
Fluticasone–azithromycin–montelukast
- ECP:
-
Extracorporeal photopheresis
- MSC:
-
Mesenchymal stem/stromal cell
- BM:
-
Bone marrow
- AT:
-
Adipose tissue
- HLA:
-
Human leukocyte antigens
- AT-MSC:
-
Adipose tissue-derived mesenchymal stem cell
- HRCT:
-
High-resolution computed tomography
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- RV:
-
Residual volume
- SVC:
-
Slow vital capacity
- TLC:
-
Total lung capacity
- CONSORT:
-
Consolidated Standards of Reporting Trials
- GMP:
-
Good manufacturing practice
- IV:
-
Intravenous
- Kg:
-
Kilograms
- F/U:
-
Follow-up
- PR:
-
Partial response
- CR:
-
Complete response
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- ALL:
-
Acute lymphocytic leukemia
- ATG:
-
Anti-thymocyte globulin
- BuCy:
-
Busulfan plus cyclophosphamide
- CD:
-
Cluster of differentiation
- CyA/MTX:
-
Cyclosporine and methotrexate
- MNC:
-
Mononuclear cell
- MSD:
-
Matched sibling donor
- PB:
-
Peripheral blood
- MMF:
-
Mycophenolate mofetil
- mg:
-
Milligrams
- COVID-19:
-
Coronavirus disease 2019
- CMV:
-
Cytomegalovirus
- PDN:
-
Prednisolone
- GI:
-
Gastrointestinal
- Hx:
-
History
- N/A:
-
Not available
- PFT:
-
Pulmonary function test
- SpO2:
-
Oxygen saturation
- GGO:
-
Ground glass opacity
- LL:
-
Lower lobe
- LUL:
-
Left upper lobe
- MA:
-
Mosaic attenuation
- PM:
-
Pneumomediastinum
- PP:
-
Pneumopericardium
- PT:
-
Peribronchial thickening
- RLL:
-
Right lower quadrant
- RUL:
-
Right upper quadrant
- SE:
-
Subcutaneous emphysema
- UL:
-
Upper lobe
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-alpha
- IFN-γ:
-
Interferon-gamma
- MIP:
-
Macrophage inflammatory protein
- NK cell:
-
Natural killer cell
- Treg:
-
Regulatory T-cell
- Breg:
-
Regulatory B-cell
References
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26.
Luzzatto L. Diagnosis and clinical management of enzymopathies. Hematol Am Soc Hematol Educ Progr. 2021;2021(1):341–52.
Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022;107(5):1045–53.
Zeiser R, Blazar BR. Acute graft-versus-host disease: biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167–79.
Saleem MS, Aljurf M, Srivastava A, Shamsi T, Lu PH, Hamidieh AA, et al. Challenges in managing graft-versus-host disease in developing countries: a perspective. Bone Marrow Transpl. 2019;54(5):641–7.
Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–79.
Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin North Am. 2010;57(1):297–322.
MacMillan ML, Holtan SG, Rashidi A, DeFor TE, Blazar BR, Weisdorf DJ. Pediatric acute GVHD: clinical phenotype and response to upfront steroids. Bone Marrow Transpl. 2020;55(1):165–71.
DeFilipp Z, Alousi AM, Pidala JA, Carpenter PA, Onstad LE, Arai S, et al. Nonrelapse mortality among patients diagnosed with chronic GVHD: an updated analysis from the Chronic GVHD Consortium. Blood Adv. 2021;5(20):4278–84.
Lueck C, Tzalavras A, Wohlfarth P, Meedt E, Kiehl M, Turki AT, et al. Impact of chronic graft-versus-host-disease on intensive care outcome in allogeneic hematopoietic stem cell recipients. Bone Marrow Transplant. 2022.
Michonneau D, Quignot N, Jiang H, Reichenbach D, Kelly M, Burrell A, et al. Clinical and economic burden associated with graft-versus-host disease following allogeneic hematopoietic cell transplantation in France. Bone Marrow Transpl. 2023.
Bergeron A, Chevret S, Peffault de Latour R, Chagnon K, de Margerie-Mellon C, Rivière F, et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur Respir J. 2018; 51(5).
Vieira AG, Funke VA, Nunes EC, Frare R, Pasquini R. Bronchiolitis obliterans in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transpl. 2014;49(6):812–7.
Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2011;17(7):1072–8.
Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370(19):1820–8.
Soleimani M, Sharif PM, Cheraqpour K, Koganti R, Masoumi A, Baharnoori SM, et al. Ocular graft-versus-host disease (oGVHD): From A to Z. Surv Ophthalmol. 2023.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I: the 2014 diagnosis and staging working group report. Biol Blood Marrow Transpl. 2015;21(3):389-401.e1.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I: diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11(12):945–56.
Williams KM, Pavletic SZ, Lee SJ, Martin PJ, Farthing DE, Hakim FT, et al. Prospective phase II trial of montelukast to treat bronchiolitis obliterans syndrome after hematopoietic cell transplantation and investigation into bronchiolitis obliterans syndrome pathogenesis. Transpl Cell Ther. 2022;28(5):264.e1-e9.
Williams KM, Cheng GS, Pusic I, Jagasia M, Burns L, Ho VT, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2016;22(4):710–6.
Norman BC, Jacobsohn DA, Williams KM, Au BK, Au MA, Lee SJ, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transpl. 2011;46(10):1369–73.
Williams KM. How I treat bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Blood. 2017;129(4):448–55.
Hakim A, Cooke KR, Pavletic SZ, Khalid M, Williams KM, Hashmi SK. Diagnosis and treatment of bronchiolitis obliterans syndrome accessible universally. Bone Marrow Transpl. 2019;54(3):383–92.
Yanik GA, Mineishi S, Levine JE, Kitko CL, White ES, Vander Lugt MT, et al. Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2012;18(7):1044–54.
Schoettler M, Duncan C, Lehmann L, Furutani E, Subramaniam M, Margossian S. Ruxolitinib is an effective steroid sparing agent in children with steroid refractory/dependent bronchiolitis obliterans syndrome after allogenic hematopoietic cell transplantation. Bone Marrow Transpl. 2019;54(7):1158–60.
Uygun V, Karasu G, Daloğlu H, Öztürkmen S, Kılıç S, Yalçın K, et al. Ruxolitinib salvage therapy is effective for steroid-refractory graft-versus-host disease in children: a single-center experience. Pediatr Blood Cancer. 2020;67(4):e28190.
Hamidieh AA, Jafari L, Delkhah M, Asefi N, Karamlou Y, Mohseni R, et al. The outcome of extracorporeal photopheresis using cryopreservation in pediatric patients with acute and chronic gvhd at the largest children’s medical hospital in Iran. Transpl Cell Therapy Offic Pub Am Soc Transpl Cell Therapy. 2023;29(2):S279–80.
Malik MI, Litzow M, Hogan W, Patnaik M, Murad MH, Prokop LJ, et al. Extracorporeal photopheresis for chronic graft-versus-host disease: a systematic review and meta-analysis. Blood Res. 2014;49(2):100–6.
Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–40.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–26.
Wuttisarnwattana P, Eid S, Wilson DL, Cooke KR. Assessment of therapeutic role of mesenchymal stromal cells in mouse models of graft-versus-host disease using cryo-imaging. Sci Rep. 2023;13(1):1698.
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–9.
Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201.
Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13(1):366.
Auletta JJ, Eid SK, Wuttisarnwattana P, Silva I, Metheny L, Keller MD, et al. Human mesenchymal stromal cells attenuate graft-versus-host disease and maintain graft-versus-leukemia activity following experimental allogeneic bone marrow transplantation. Stem Cells. 2015;33(2):601–14.
Wu SCM, Zhu M, Chik SCC, Kwok M, Javed A, Law L, et al. Adipose tissue-derived human mesenchymal stromal cells can better suppress complement lysis, engraft and inhibit acute graft-versus-host disease in mice. Stem Cell Res Ther. 2023;14(1):167.
Resnick IB, Barkats C, Shapira MY, Stepensky P, Bloom AI, Shimoni A, et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res. 2013;3(3):225–38.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86.
Dotoli GM, De Santis GC, Orellana MD, de Lima PK, Caruso SR, Fernandes TR, et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2017;52(6):859–62.
Li Y, Hao J, Hu Z, Yang YG, Zhou Q, Sun L, et al. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review. Stem Cell Res Ther. 2022;13(1):93.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–6.
Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008;449:69–79.
Meza-Zepeda LA, Noer A, Dahl JA, Micci F, Myklebost O, Collas P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12(2):553–63.
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6(1):55.
Kornicka K, Śmieszek A, Węgrzyn AS, Röcken M, Marycz K. Immunomodulatory properties of adipose-derived stem cells treated with 5-azacytydine and resveratrol on peripheral blood mononuclear cells and macrophages in metabolic syndrome animals. J Clin Med. 2018;7(11):383.
Kapila A, Baz MA, Valentine VG, Bhorade SM. Reliability of diagnostic criteria for bronchiolitis obliterans syndrome after lung transplantation: a survey. J Heart Lung Transpl. 2015;34(1):65–74.
Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115–20.
Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV: The 2014 Response Criteria Working Group report. Biol Blood Marrow Transpl. 2015;21(6):984–99.
Chen S, Zhao K, Lin R, Wang S, Fan Z, Huang F, et al. The efficacy of mesenchymal stem cells in bronchiolitis obliterans syndrome after allogeneic HSCT: a multicenter prospective cohort study. EBioMedicine. 2019;49:213–22.
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–33.
Zhao Y, Gillen JR, Harris DA, Kron IL, Murphy MP, Lau CL. Treatment with placenta-derived mesenchymal stem cells mitigates development of bronchiolitis obliterans in a murine model. J Thorac Cardiovasc Surg. 2014;147(5):1668-77.e5.
Guo Z, Zhou X, Li J, Meng Q, Cao H, Kang L, et al. Mesenchymal stem cells reprogram host macrophages to attenuate obliterative bronchiolitis in murine orthotopic tracheal transplantation. Int Immunopharmacol. 2013;15(4):726–34.
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179(3):1855–63.
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100(14):8407–11.
Tashiro J, Elliot SJ, Gerth DJ, Xia X, Pereira-Simon S, Choi R, et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl Res. 2015;166(6):554–67.
Mahdavi Sharif P, Jabbari P, Razi S, Keshavarz-Fathi M, Rezaei N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine. 2020;130:155066.
Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–9.
Chambers DC, Enever D, Lawrence S, Sturm MJ, Herrmann R, Yerkovich S, et al. Mesenchymal stromal cell therapy for chronic lung allograft dysfunction: results of a first-in-man study. Stem Cells Transl Med. 2017;6(4):1152–7.
Keller CA, Gonwa TA, Hodge DO, Hei DJ, Centanni JM, Zubair AC. Feasibility, safety, and tolerance of mesenchymal stem cell therapy for obstructive chronic lung allograft dysfunction. Stem Cells Transl Med. 2018;7(2):161–7.
Erasmus DB, Durand N, Alvarez FA, Narula T, Hodge DO, Zubair AC. Feasibility and safety of low-dose mesenchymal stem cell infusion in lung transplant recipients. Stem Cells Transl Med. 2022;11(9):891–9.
von Bonin M, Stölzel F, Goedecke A, Richter K, Wuschek N, Hölig K, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transpl. 2009;43(3):245–51.
Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–7.
Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transpl. 2010;45(12):1732–40.
Jurado M, De La Mata C, Ruiz-García A, López-Fernández E, Espinosa O, Remigia MJ, et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: a phase I/II study. Cytotherapy. 2017;19(8):927–36.
Boberg E, von Bahr L, Afram G, Lindström C, Ljungman P, Heldring N, et al. Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: a phase II study. Stem Cells Transl Med. 2020;9(10):1190–202.
Macías-Sánchez MDM, Morata-Tarifa C, Cuende N, Cardesa-Gil A, Cuesta-Casas M, Pascual-Cascon MJ, et al. Mesenchymal stromal cells for treating steroid-resistant acute and chronic graft versus host disease: a multicenter compassionate use experience. Stem Cells Transl Med. 2022;11(4):343–55.
Stenger E, Giver CR, Langston A, Kota D, Das PK, Chinnadurai R, et al. Safety of autologous freshly expanded mesenchymal stromal cells for the treatment of graft-versus-host disease. Front Immunol. 2022;13:959658.
Shen MZ, Liu XX, Qiu ZY, Xu LP, Zhang XH, Wang Y, et al. Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation. Ther Adv Hematol. 2022;13:20406207211072840.
Li Z, Wang Y, Li G, Ma N, Li M, Yuan F, et al. Clinical observation on the safety and efficacy of umbilical cord mesenchymal stem cells in the treatment of bronchiolitis obliterans after allogeneic haematopoietic stem cell transplantation. Biotechnol Genet Eng Rev. 2023. https://doi.org/10.1080/02648725.2023.2183611.
Kalhoff H, Breidenbach R, Smith HJ, Marek W. Spirometry in preschool children: time has come for new reference values. J Physiol Pharmacol. 2009;60(Suppl 5):67–70.
Tamburro RF, Cooke KR, Davies SM, Goldfarb S, Hagood JS, Srinivasan A, et al. Pulmonary complications of pediatric hematopoietic cell transplantation: a national institutes of health workshop summary. Ann Am Thorac Soc. 2021;18(3):381–94.
Dekhuijzen PN, Decramer M. Steroid-induced myopathy and its significance to respiratory disease: a known disease rediscovered. Eur Respir J. 1992;5(8):997–1003.
Acknowledgements
None.
Funding
This study is funded by the National Council for Development of Stem Cell Sciences and Technologies.
Author information
Authors and Affiliations
Contributions
RM performed MSC culture and processing and is the head of the cell therapy laboratory. PMS collected and summarized data and drafted the manuscript. MB supervised the F/U visits and the gathering of clinical data, as she is a transplantation specialist in this center. MRM and RS were pediatrics pulmonologists and interpreted the PFT findings. MM, LJ, and FJ contributed to data collection. ZN administered MSC products to patients. AAH designed and supervised the project, as he is the head of this research center and the head of transplantation specialists at this center. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study (Evaluation of Safety and Effectiveness of Allogeneic Adipose-Derived Mesenchymal Stem Cells in Bronchiolitis Obliterans Syndrome (BoS) after Allogeneic Hematopoietic Stem Cell Transplantation) is approved by the ethics committee of the Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1399.406) on 5 September 2020. All of our included cases’ parents and donors of AT-MSCs were asked to complete and sign the written informed consent forms before participation in the study.
Consent for publication
The parents of included cases have consented to the anonymous publication of their children’s data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Detailed steps of adipose tissue-derived mesenchymal stem/stromal cells preparation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohseni, R., Mahdavi Sharif, P., Behfar, M. et al. Evaluation of safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells in pediatric bronchiolitis obliterans syndrome (BoS) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Stem Cell Res Ther 14, 256 (2023). https://doi.org/10.1186/s13287-023-03498-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03498-y