Abstract
Cell therapy is the frontier technology of biotechnology innovation and the most promising method for the treatment of refractory diseases such as tumours. However, cell therapy has disadvantages, such as toxicity and poor therapeutic effects. Plant extracts are natural, widely available, and contain active small molecule ingredients that are widely used in the treatment of various diseases. By studying the effect of plant extracts on cell therapy, active plant extracts that have positive significance in cell therapy can be discovered, and certain contributions to solving the current problems of attenuation and adjuvant therapy in cell therapy can be made. Therefore, this article reviews the currently reported effects of plant extracts in stem cell therapy and immune cell therapy, especially the effects of plant extracts on the proliferation and differentiation of mesenchymal stem cells and nerve stem cells and the potential role of plant extracts in chimeric antigen receptor T-cell immunotherapy (CAR-T) and T-cell receptor modified T-cell immunotherapy (TCR-T), in the hope of encouraging further research and clinical application of plant extracts in cell therapy.
Similar content being viewed by others
Introduction
As the frontline of biotechnological innovation, cell therapy has a significant influence on medical treatment and provides new sights for difficult diseases [1]. Cell therapy mainly includes stem cell therapy and immune cell therapy. Stem cells can be acquired from embryonic tissue, foetal tissue, adult organism, and induced pluripotent stem cells (iPSCs) [2, 3]. According to developmental stage, stem cells include pluripotent stem cells (PSCs) and adult stem cells. Among them, PSCs include embryonic stem cells (ESCs) and iPSCs [3], which are pluripotent. However, ESCs have ethical issues, and the probability of reproductive cloning and tumour formation limited the application of iPSCs [3]. Adult stem cells overcome these problems of PSCs, which contain mesenchymal stem cells (MSCs), neural stem cells (NSCs), and adipose-derived stem cells (ASCs), among others, and have significant therapeutic effects in cardiovascular, neurological, skeletal, and autoimmune diseases [4, 5]. In addition, immune cell therapy, represented by chimeric antigen receptor T-cell immunotherapy (CAR-T), and T-cell receptor modified T-cell immunotherapy (TCR-T), has shown potential efficacy in multiple myeloma and other haematologic malignancies as well as in some solid tumours [6]. Cell therapies have a large latent capacity under the development of modern science and technology. However, cell therapy itself still has deficiencies in practice, such as side effects, inflammatory factor storms caused by excessive immune responses and poor effects in some patients. As a result, combining it with other drugs may be a way to solve this problem. Additionally, protein cytokines and antibodies have been widely used in cell culture and clinical treatment, with disadvantages such as toxicity, and high cost. Therefore, at present, looking for alternative plant extracts that can be used as growth factors and adjuvant therapies in cell therapy is a promising approach [7].
Hormesis effects on stem cells have been observed, mainly in the use of drugs (e.g. metformin, atorvastatin, and isoproterenol), dietary supplements/medicinal plant extracts (e.g. berberine), and endogenous drugs (e.g. estrogen) [8, 9]. Among them, plant extracts are an important source of bioactive small molecules, which are mainly derived from herbal plants, and include flavonoids, alkaloids, polysaccharides, volatile oils, etc. Plant extracts play an important role in disease treatment, especially in cancer and infectious diseases. Herbal therapy is a traditional medical practice that has long been used to treat a variety of diseases. Herbal medicine is safe and affordable [7]. It is a promising alternative approach with a significant effect on alleviating patient disease. Therefore, studying the effects of plant extracts in cell therapy and digging out active plant extracts in herbal medicines can provide guidance for adjuvant therapy combined with cell therapy.
The application of plant extracts in cell therapy has gradually increased with the rapid development of cell therapy. For example, the increased therapeutic effects of stem cells induced by plant extracts have been reported in studies of Alzheimer’s disease [10], chronic kidney disease [11], and stroke [12], and the therapeutic effects of immune cells induced by plant extracts have been reported in diseases such as non-small-cell lung cancer and liver cancer [13, 14]. This review describes the effects of plant extracts, mainly herbal extracts, in cell therapy approaches and clarifies the potential mechanisms of action when possible.
The effect of plant extracts on stem cell therapy
The effect of plant extracts on MSCs
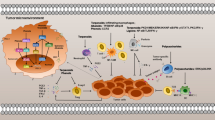
MSCs exist in almost all postnatal human tissues. The major sources of adult MSCs are mainly from bone marrow, adipose tissue, etc. [7, 15]. Compared with ESCs, MSCs are easier to isolate and culture in vitro, and more importantly, there are fewer ethical issues. Furthermore, due to their HLA-DR-negative feature, MSCs do not have immunogenic in therapy [7]. MSCs are characterized by different sources, isolation methods, and epigenetic changes during growth. They can be differentiated into osteocytes, neurons, and angiogenesis, through stimulation with plant extracts (Fig. 1).
Proliferation effect
Many plant extracts, such as Foeniculum vulgare [16], Ferula gummosa [17], amentoflavone (Selaginella tamariscina (P. Beauv.) Spring) [18], gastrodin (Gastrodia elata Bl.) [19], and resveratrol (Polygonum cuspidatum Sieb. et Zucc.) [20], can significantly increase bone marrow-derived human MSCs (BM-hMSCs) proliferation. In addition, ginsenoside Rg1, which is an effective compound in Panax ginseng, Panax notoginseng, and American ginseng, can also promote cell proliferation [21]. Apple ethanol extract promotes proliferation of hASCs and human cord blood-derived MSCs via ERK signalling [22]. Tinospora cordifolia and Withania somnifera are traditional Ayurveda medicinal materials in India that are reported to improve cell proliferation ability and activity, as well as reduce cell apoptosis and postpone aging [23]. ZD-I is a prescription composed of seven traditional Chinese medicines (TCM). It has stimulatory effects on the proliferation of hMSCs [24]. Viscum album induces primitive placenta-derived MSCs (PDSCs) with remarkable proliferative properties through autophagy mechanism. Specifically, Viscum album can regulate the cell cycle to make PDSCs self-renewal and regulate the induction of survival factors, apoptosis and autophagy to reduce cell death [25].
Differentiation effect
Osteogenic effects
Osteogenic effects via transcription factors
Foeniculum vulgare, Ferula gummosa, and amentoflavone can significantly increase the alkaline phosphatase (ALP) activity of hMSCs and promote BM-hMSC differentiation into osteoblasts [16,17,18]. Dipsacus asper and its ingredients hedraganin-3-O-(2-O-acetyl)-α-l-arabinopyranoside enhance osteoblastic differentiation not only by inducing ALP activity but also by inducing bone sialoprotein and osteocalcin expression [26]. Moreover, Fructus Ligustri Lucidi effectively activated ALP, reduced the osteogenic differentiation time of MSCs, and up-regulated the expression of osteogenic related factors such as catenin, BMP2, cyclin D1, membrane matrix metalloproteinase, osteoprotegerin and T-box 3 [27]. Poncirin (Poncirus trifoliata (L.) Raf) [28], Panax notoginseng saponins [29, 30], and naringin (Citrus grandis) [31, 32] decreased peroxisome proliferator-activated receptor γ (PPARγ) 2 mRNA levels, while Panax notoginseng saponins raised the levels of ALP, Cbfa 1, OC, BSP, OPG, β-catenin, and cyclin D1. Harmine (Peganum harmala L.) increased ALP activity and up-regulated osteocalcin expression. Moreover, harmine can up-regulate osterix [33]. The combination of epigallocatechin-3-gallate [34] and bone inducer can up-regulate BMP2 and enhance bone formation. Gastrodin [19] improved ALP, OCN, COL I, and OPN while reducing ROS. Fucoidan enhanced osteogenic specific marker genes, such as ALP, osteopontin, type I collagen, Runx2, and osteocalcin in ASCs [35]. Quercetin (Sophora flavescens Ait.) can increase Osx, Runx2, BMP2, Col1, OPN and OCN, and enhance osteogenic differentiation [36]. BuShenNingXin decoction (BSNXD) up-regulated ALP and collagen type I, osteocalcin, Runx2, and osterix. Furthermore, BSNXD was shown to reduce the quantity of adipocyte and PPARγ mRNA [37]. A summary table of plant extracts that stimulate osteogenesis of MSCs is shown in Additional file 1: Table S1.
Osteogenic effects through Wnt signalling pathways
Flavonoids of epimedii(Epimedium brevicornum Maxim., etc.) were found to increase the rates of osteogenic activity through the BMP or Wnt-signalling pathway [38]. In addition, Angelica sinensis polysaccharide can enhance the osteogenic differentiation of rat BM-MSCs cultured in high-sugar and guide bone regeneration in type 2 diabetes animal model which chained to the Wnt/β-catenin signalling pathway [39]. Ginkgo biloba and its main component ginkgolide B accelerate osteoblast differentiation and the formation of bone via Wnt/β-Catenin signalling [40] (Fig. 2). Berberine (Coptis chinensis Franch.) [41] and salvianolic acid B (Salvia miltiorrhiza Bge.) [42, 43] promote osteogenesis in BM-MSCs through Wnt/β-catenin signalling and strengthen Runx2 expression. Salvianolic acid B influences the ERK signalling pathway and lower PPARγ mRNA, accelerating the osteogenesis of MSCs. The osteogenic-related genes can be strengthened under the induction of naringin, and the expression of Notch1 can be up-regulated at the same time, and activation Wnt signalling activation [44].
Plant extracts that affect MSCs osteogenesis by regulating intracellular signalling pathways. Ginkgolide B, Panax notoginseng saponins, berberine, and salvianolic acid B regulate axin, β-catenin, and TCF in the Wnt signalling pathway; Ginkgo biloba, harmine, silibinin, genistein, and Ligusticum chuanxiong regulate BMP, Runx2 and Smad 1/5/8 in the BMP signalling pathway; Resveratrol, icariin, amentoflavone, quercetin, and fucoidan regulate p38, ERK1/2, and JNK in the MAPK signalling pathway
Osteogenic effects through BMP signalling pathways
Ginkgo biloba has been found to enhanced Runx2 expression and regulated BMP4 in BMP signalling [45]. Moreover, harmine [33], silibinin(Silybum marianum (L.) Gaertn.) [46], and genistein(Genista tinctoria Linn., etc.) [47] activate the BMP and Runx2 pathways. Duhuo Jisheng decoction and its effective component Ligusticum Chuanxiong can activate Smad 1/5/8 and ERK signalling, increase the osteogenic effect of MSCs, and improve BMP-2 and Runx2 [48] (Fig. 2).
Osteogenic effects through MAPK signalling pathways
Most of the plant extracts used in the study of MSCs osteogenesis are TCM monomers. Icariin (Epimedium brevicornum Maxim., etc.), amentoflavone and quercetin promoted osteogenesis via the JNK and p38 MAPK pathways (Fig. 2). Icariin also phosphorylates ERK, and stimulates PI3K-AKT-eNOS-NO-cGMP-PKG pathway in bone marrow stromal cells [18, 49, 50]. Quercetin is a flavonoid that can also activate ERK signalling pathways, decrease the aging and oxidative stress in MSCs, and promote osteogenic differentiation [51, 52]. Fucoidan can induce osteogenic differentiation, activate ERK and JNK mainly through BMP2 Smad 1/5/8 signalling, and regulate osteogenic differentiation markers [53, 54]. One study showed that resveratrol enhanced cell renewing by inhibiting cell aging at a low concentration, while it inhibits cell self-renewal by up-regulating cell senescence, doubling time, and S-phase arrest at a high concentration. In addition, it can stimulate MSCs and promote osteoblast differentiation by acting on ER-dependent mechanisms and activating ERK1/2 [20, 55, 56].
Neurogenic effects
Mucuna gigantea grows natively in Hawai ‘i. It was recorded that it can be used to treat kampavata (excitatory paralysis) [57]. Mucuna gigantea can promote proliferation feature, nestin, and β-III tubulin mRNA expression in MSCs [58].
A study using human umbilical cord Wharton's Jelly-derived MSCs (WS-MSCs) showed that Salvia miltiorrhiza increases the expression of nestin, β-tubulin, neurofilament, GFAP, and neurite outgrowth-promoting protein [59]. Another study in rat BM-MSCs showed that Salvia miltiorrhiza promotes Mash-1 and NGN-1 induced mRNA expression of TUJ-1, NF, and synaptophysin [60].
Ginkgolide B and Astragalus mongholicus can increase NSE-positive neuron-like cells and GFAP-positive astrocyte-like cells to promote MSCs differentiation into nerve cells [61, 62]. Moreover, Astragalus mongholicus also enhances the expression of the Wnt-1 gene and Ngn-1 gene [62]. Ginsenoside Rg1 was found to accelerate the differentiation of neural phenotype in hASCs and upregulate NSE, MAP-2, GAP-43, NCAM, and SYN-1 genes [21]. Another study showed that gisenoside Rg1 can promote neural differentiation in mouse ASCs by miRNA-124 signalling pathway [63]. Similarly, radix Angelicae sinensis can also induce adipose-derived MSCs to differentiate into neuron-like cells [64]. In Ayurvedic medicine, dhanwantharam kashayam is considered a growth stimulant for children, which can promote nerve regeneration [65].
Angiogenesis effects
Treatment of hMSCs with olive leaf extract promoted the differentiation of cells into endothelial cells and development of the tubular construction needed for angiogenesis. At the same time, olive leaf extract can promote VEGF, PCAM, PDGF, and VEGFR-1 [7]. Curcumin is an antioxidant and anti-inflammatory substance in turmeric. Its ethanol extracts can increase the expression of CD34, CD133, and VEGFR2 to cause ASCs to proliferation and differentiation into endothelial progenitor cells [66]. In the animal hindlimb ischaemia model, fucoidan can protect MSCs from oxidative stress and enhance angiogenesis. Another study showed that fucoidan can inhibit the cell death caused by MSCs ischemia, and adjust the levels of apoptosis-related proteins and cellular ROS mainly by MnSOD and Akt pathways [67]. In addition, through ERK-IDO-1 signalling cascade, it increases the proliferation potential and the expression of cell cycle-associated proteins, and enhances the immunoregulation activity of MSCs [11]. Carica papaya leaf extract, rich in papain, was found to enhance the composition of IL-6 and stem cell factors related to platelet production in vitro [68].
Anti-adipogenic effects
Some plant extracts also have anti-adipogenic effects on MSCs. The results of one study confirmed that the antioxidant action of Tithonia diversifolia may influence the expression of HO-1. More importantly, it may regulate carbohydrate and fat metabolism by repressing adipocyte differentiation through activating AMPK [69]. In an experiment using MSCs, after stimulation with aloe-emodin, many indicators were reduced, including resistin, adiponectin, aP(2), lipoprotein lipase, PPARγ, and TNFα, which influence adipogenic pathways [70]. Quzhisu can repress adipogenic differentiation of BM-MSCs by downregulating PPARγ [71]. Similarly, flavonoids of epimedii like Quzhisu downregulate PPARγ, and can also decrease C/EBP-α [72].
Antioxidant stress effects
Undaria pinnatifida, Myrtus community L. and Cirsium setidens showed antioxidant stress effects in MSCs. Undaria pinnatifida, also called Mi-Yoek in Korea, is considered a healthy food. The anti-aging effect of Undaria pinnatifida in BM-MSCs was researched. The results showed that after H2O2 treatment, it had the effect of antioxidant stress, and could decrease aging and improve the differentiation potential of cells by controlling ROS [73]. In addition, icariin protected rabbit BM-MSCs from oxygen, glucose, and apoptosis via inhibition of ERs-mediated autophagy associated with MAPK signalling [74]. Furthermore, residues from the production of Myrtus community L. can counteract the appearance of aging phenotypes in ASCs, reduce oxidative stress and inflammation, and enhance the expression of genes related to pluripotency [75]. The authors also studied the genetic programs responsible for cellular senescence in human ASCs exposed to oxidative stress and found that in the cells stimulated by Myrtle, the SA-β-Gal positive cells and the cell cycle regulation genes were decreased, while TERT and c-Myc genes were increased [76]. Cirsium setidens [77] has a suppressive effect on cell injury by regulating oxidative stress and repressing apoptosis-related signalling pathways.
Adverse
The above studies have shown that certain plant extracts can promote the proliferation and differentiation of MSCs. However, some studies have demonstrated that plant extracts have side effects. Cimicifugae Rhizoma, also called Shengma in China, affects the vita of dental stem cells, and has side effects on the oral cavity at a high content [78]. Additionally, Asiasarum radix is the same as Cimicifugae Rhizoma [79]. As mentioned above, Fructus Ligustri Lucidi has an osteogenesis effect. Nevertheless, Fructus Ligustri Lucidi in a dose of more than 200 μg/mL has cytotoxicity to MSCs [27].
The effect of plant extracts on NSCs
Endogenous NSCs are abundantly in the subventricular region of the hippocampal granular area and the germinal area of the cerebrum. They differentiate cells according to the needs of brain structure and function [80]. NSCs are primarily used to remedy central nervous system injury and degenerative diseases. There are two intervention strategies involving NSCs. One strategy involves using endogenous NSCs to repair the diseased site, but endogenous NSCs are not sufficient and prefer to differentiate into gliocytes instead of neurons; the other strategy is transplantation of exogenous NSCs [61]. However, it is difficult to control the survival, replication, and differentiation of exogenous NSCs into local nerve cells. Sources of NSCs include human ESCs, human iPSCs, human foetal brain-derived neural stem/progenitor cells, and direct reprogramming of astrocytes. According to their functions, they can be divided into pluripotent and multipotent cell types [81]. Plant extracts play an important part in promoting the proliferation and differentiation of NSCs into new neurons. The Notch, Wnt, BMP, and sonic hedgehog signalling pathways have been the most studied [82].
Proliferation effect
Ginsenosides Rg1 advances the incorporation of Bromo-2-deoxyuridine and the expression of nestin and vimentin in NSCs, and promotes the proliferation of NSCs [83]. In addition, ginsenoside Rd can enhance the proliferation of NSCs in vivo and in vitro. It can enhance the size and quantity of neurospheres [84]. After oxygen and glucose deprivation (OGD) /r injury in vitro, resveratrol up-regulated the survival and proliferation of NSCs, and increased patched-1, smoothened (SMO) and Gli-1 [85]. Meanwhile, resveratrol can reduce the damage and raise the proliferation of NSCs by promoting Nrf2, HO-1 and NQO1 [86]. Artesunate is a derivative of artemisinin from Artemisia annua [87]. It can inhibit transcription by inducing Foxo-3a phosphorylation, then downregulating p27kip1, and enhancing the proliferation of NSCs in the infarcted cortex through PI3K/AKT signalling transduction [88].
Differentiation effect
Wnt/β-catenin pathway
The role of ginkgolide B has been mentioned above, it also can enhance the differentiation of NSCs after cerebral ischemia and may improve neural function by increasing the expression of BDNF, EGF, and SOCS2 [12]. Ginkgo biloba extract and Ginkgolide B, was found to accelerate cell cycle exit and neuronal differentiation in NSCs. Furthermore, ginkgolide B up-regulated the nuclear level of β-catenin and activated the classical Wnt to promote neuronal differentiation [89]. Curcumin (Curcuma aromatica Salisb.) has some problems with pharmacokinetics and pharmacodynamics. Thereby Tiwari SK et al. prepared curcumin nanoparticles and found that they can activate the classic Wnt/β-catenin pathway to lead to human neurogenesis [90]. Icariin is an important biologically active ingredient extracted from Epimedium and has neuroprotective properties. Icariin treatment enhanced NSCs neurosphere formation and promoted the expression of nestin, β-III-tubulin and GFAP. Icariin-regulated genes participate in pathways including the Wnt and bFGF signalling [91], ERK/MAPK signalling [92], and BDNF-TrkB-ERK/Akt signalling pathway [93].
PI3K/AKT signalling pathway
One study evaluated the function of salvianolic acid B on the differentiation, proliferation, and neurite growth of mouse NSCs. The proper dose of salvianolic acid B promoted the quality of NSCs and neurospheres, and accelerates the growth of neurites of NSCs and their differentiation into neurons [94]. Zhuang P et al. selected 45 kinds of ingredients from TCM widely applied in the clinical treatment of stroke in China and examined their proliferation-inducing activity on NSCs. Finally, it was found that salvianolic acid B maintains NSCs self-renewal and promotes proliferation through the PI3K/Akt signalling pathway [95]. Salidroside is an ingredient extracted from the plant Rhodiola rosea L. It can inhibit hypoxic NSCs injury by increasing miR-210, thereby repressing BTG3 and influencing PI3K/AKT/mTOR signalling pathway [96]. The protective effect of berberine on OGD-treated cells via inhibiting the cell cycle. It can decrease cyclin D1, p53 and caspase 3, increase the phosphorylation level of p-Bad/tBad, and upregulate PI3K and Akt [97].
BMP signalling pathway
( +)-Cholesten-3-one(Serratula) induced NSCs differentiation into dopaminergic neurons and promoted tyrosine hydroxylase, dopamine transporter, dopa decarboxylase, dopamine secretion, and evidently increased BMPR IB. The p-Smad1/5/8 expression indicates that ( +)-Cholesten-3-one may influence the BMP signalling [98].
Notch signalling pathway
Astragaloside IV is an ingredient in Astragalus membranaceus. Astragaloside IV leads NSCs to β-tubulin III ( +) and GFAP ( +) cells through the Notch signalling pathway [10]. Moreover, in an in vivo study, astragaloside IV can promote proliferative cells(BrdU+), premature neurons (DCX+), early proliferative cells (BrdU+/DCX+), proliferative radial Glia-like cells (BrdU+/GFAP+), and regulate the homeostasis of the CXCL1/CXCR2 signalling pathway [99].
Others
Panax notoginseng saponins notably increased NSCs proliferation and the expression of nestin/BrdU, Tuj-1, and vimentin mRNA in hippocampal NSCs. And the results indicate that Panax notoginseng saponins may promote the proliferation and differentiation of NCSs after OGD in vitro by increasing the area density, optical density and the number of nestin/BrdU, nestin/vimentin, and nestin/tuj-1 positive cells [100]. One study investigated the effects of tetramethylpyrazine, an active element of Ligusticum Chuanxiong, which promotes the differentiation of NSCs into neurons, increases the phosphorylation of ERK1/2, and reduces the phosphorylation of p38 [101]. Baicalin could increase MAP-2 positive cells and decrease the number of GFAP stained cells. Meanwhile, p-STAT3 and Hes1 were downregulated, and NeuroD1 and Mash1 were upregulated. These results suggested that baicalin can promote neural differentiation but inhibit the formation of glial cells. Its role in promoting neurogenesis is related to STAT3 and bHLH genes [102, 103]. Earlier research on NSCs showed that Buyanghuanwu decoction can promote cell growth and differentiation, increase neurofilament (NF) positive cells and GFAP positive cells, and promote intracellular Ca2+ concentrations [104, 105]. Jiaweisinisan has antidepressant effects, promotes hippocampal neurogenesis after stress damage, and significantly increases nestin, β-tubulin-III, and GFAP [106].
The effect of plant extracts on ESCs
ESCs can be obtained from the inner cell mass of a blastocyst. It has the characteristics of in vitro culture capacity, immortal cell proliferation, self-renewal, and multidirectional differentiation [107]. Using ESCs to differentiate into different cell models is a promising drug discovery method and technology [108]. Kami-Shoyo-San is a TCM that can protect neuronal apoptosis in ESCs by promoting brain-derived neurotrophic factor/tropomyosin receptor kinase B signalling pathway [109].
The effect of plant extracts on iPSCs
iPSCs have characteristics similar to those of ESCs in terms of unlimited self-renewal and differentiation capabilities. Plant extracts induce iPSCs production and apoptosis. The Sagunja-tang herbal formula can efficiently produce iPSCs from human foreskin fibroblasts via transcription factors [110]. Prunellae Spica and Magnoliae cortex-mediated apoptosis of undifferentiated iPSCs was found to be p53-dependent, and to have potent anti-teratoma activity, with no genotoxicity toward differentiated cells. Therefore, these compounds can be used for iPSC-based cell therapy to induce apoptosis of possible undifferentiated iPSCs and prevent the occurrence of teratomas [111, 112].
Plant extracts can induce differentiation of iPSCs into nerve cells. Salvia miltiorrhiza can significantly increase the expression of nestin and microtubule-associated protein 2 (MAP2) genes and proteins, and induce the differentiation of iPSCs into neurons [113]. Plant extracts also have an improved effect on the nerve cell model differentiated from iPSCs. N-Butylidenephthalide (n-BP) is derived from Angelica Sinensis. N-BP can reduce Aβ40 deposits, total tau protein, and its hyperphosphorylated form in iPSCs-derived neurons induced by Down syndrome [114]. Graptopetalum paraguayense can improve AD-related phenotypes, such as reducing Aβ 40, Aβ 42, and tau protein phosphorylation [115].
iPSCs are differentiated into cardiomyocytes, which are used in the research of related diseases. One study found that Salvia miltiorrhiza and Crataegus pentagyna have anti-arrhythmic effects. Salvia miltiorrhiza has an antioxidant effect, regulates calcium treatment on myocardial cells during I/R and decreases arrhythmia and apoptosis [116]. Crataegus pentagyna extract has an anti-arrhythmic effect on cardiomyocytes derived from human arrhythmia-specific iPSCs [117]. In addition, Yixinshu capsule has a protective effect on human iPSCs-derived cardiomyocytes by reducing endothelin 1 (ET-1) induced contractile dysfunction, increasing brain natriuretic peptide (BNP) content, and inducing morphological changes [118]. However, some plant extracts are toxic to cardiomyocytes, such as liensinine and neferine [119], mitragynine [120], and Erythrina senegalensis DC [121].
The effect of plant extracts on other stem cells
Ultraviolet-B (UVB) irradiation can damage the epidermis. Andrographis paniculata [122] promotes the proliferation of epidermal stem cells (EpSCs) and anti-aging via increasing integrin β1 and VEGF expression. Morin [123] and Vanillin [124] significantly inhibited UVB-induced damage to human keratinocyte stem cells, and effectively enriched the p53- specific ligasing ability of the mouse double minute 2 homologue in UVB irradiation-induced p53 activation. Likewise, zingerone (Zingiber officinale Rosc.) [125] can protect the epidermis by restraining the UV damage mediated by p42/44 MAPK and p38 MAPK. In other stem cells, Ginkgo biloba[126, 127] activates telomerase through PI3k/Akt signalling pathway to delay the aging of endothelial progenitor cells. Additionally, starting from telomerase, TSY-1 [128] increases telomerase activity in CD34+ haematopoietic stem cells.
Immune cell therapies
Adoptive cell therapy (ACT) is a kind of immunotherapy that is genetically modified T-cells to deliver a CAR or TCR. To a certain extent, mutated cancer cells provide many peptides that are not found in natural cells, which brings a potential target for constructing a new antigen screening system, and promotes the development of ACT, making CAR-T and TCR-T treatment become the most prospective way to treat cancer. However, ACT has great differences in the treatment of various tumours types, and there are still some shortcomings that need improvement [129].
CAR-T
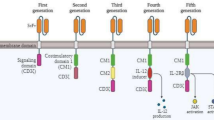
CAR is a kind of engineering, which can enable lymphocytes to identify and eliminate cells delivering homologous target ligands. It has antigen binding domain, hinge, transmembrane domain and intracellular signal domain modules. By changing each component, its function and anti-tumour effect can be adjusted. At present, various types of CARs are being developed and designed to improve the safety and effectiveness in cancer treatment [130]. Clinically, treatment with CAR-T-cells first requires T-cells, which can be obtained from the patient’s peripheral blood, or allogeneic CAR-T-cells obtained from a donor [131]. T-cells are stimulated and expanded in vitro and transduced with specific CAR genes through viral vectors, and then, the CAR-T-cells are infused back into the patient to perform the set tumour-killing effect in the patient's body. This type of therapy is also called CAR-T-cell therapy (Fig. 3).
CAR-T-cell therapy and the four generations of improvements. The first-generation CARs were fused with a single-chain variable fragment (scFv) to a transmembrane domain and an intracellular signalling unit: the CD3 zeta chain. Then, the second-generation CARs improved the costimulatory molecule receptor-like CD28, which is the most commonly used. The second-generation CARs increased the production of cytokines and enhanced durability. The third-generation of CARs design in-corporated an additional costimulatory domain to enhance CAR function and included the scFv, the initial CD3ζ-chain, and the CD28 and 4-1BB or OX40 costimulatory domains [132]. At present, fourth-generation CAR-T therapy has been extended. In this type of CAR-T-cell therapy, cytokine genes have been added to the structure, which can stimulate high cytokines expression that enhances the activity of T-cells after CAR-T-cells are activated, thereby improving the antitumour activity of CAR-T-cells [133]
In 2017, the FDA approved two types anti-CD19 CAR-T-cell products to treat both B-cell ALL and diffuse large B-cell lymphoma, and these products have transformed the field of anticancer immunotherapy [134]. However, there are still some limitations in CAR-T-cell therapy. Mechanisms hampering CAR-T-cell efficiency include limited T-cell persistence and therapy-related toxicity. Furthermore, severe toxicities, restricted trafficking to, infiltration into and activation within tumours, antigen escape and heterogeneity; manufacturing issues; physical properties; and the immunosuppressive capacities of solid tumours have prevented the success of CAR-T-cells in these entities [135, 136]. Additionally, it may not be possible to obtain a sufficient number of T-cells from the patient because the patient is usually not considered for CAR-T-cell therapy, usually due to a reduction in the number of original lymphocytes caused by previous cytotoxic treatment [134]. In application, almost all CAR-T-cell products are derived from CD4+ T-cells and CD8+ T-cells, both cell populations likely contribute to treatment effect [134]. Some plant extracts have beneficial effects on CD4+ T-cells and CD8+ T-cells. For example, Fuzheng Qingjie [137, 138], Fuzheng Fangai [139], Xiaoji [13], Cistanche deserticola [140], Epimedium koreanum Nakai [141], Glycyrrhiza uralensis [142, 143], Aidi [144], and Scolopendra subspinipes [145,146,147] can increase in CD4+ cells and the CD4/CD8 ratio, and produce FN-g, IL-2, IL-4, IL-6, and IL-7; Xiao Ai Ping [148], Lycium barbarum [149,150,151], Dangguibuxue tang [152], Oldenlandia diffusa [153,154,155], Carthamus tinctorius [156], lectin-55 [157], and Tricosanthes kirilow [158] have an effect on increasing in CD8+ cells, and tumour infiltration and increasing IFN-g and IL-10. Shenqi Fuzheng [159], Lycium barbarum [160], Ganoderma lucidum [161], Yunzhi-Danshen [162] can upregulate CD3+, CD4+, CD4 + /CD8 + and NK+ cells. Moreover, gastrodin was found to ameliorated the CD8+ T-cell-mediated immune response and significantly improved protection in tumour-challenged animals. This finding indicates that gastrodin is a potential adjuvant contributing to anticancer immunomodulation.
On the other hand, the tumour microenvironment is a complex pathological system composed of tumour cells, blood/lymphatic vessels, tumour stroma, and tumour-infiltrating myeloid precursors, providing a living environment for tumour cells and promoting tumour metastasis. In the tumour microenvironment, tumour-infiltrating myeloid precursors mainly include tumour-associated macrophages, tumour-associated dendritic cells, and myeloid-derived suppressor cells, which inhibit T-cells or other immune cells and play an important role in its antitumour activity. Therefore, improving the tumour microenvironment by targeting these cells is an effective way to assist CAR-T-cell therapy. Liu J et al. reviewed Chinese herbal medicine and its components that induce tumour cell apoptosis and directly inhibit tumour growth and invasion, providing new research ideas for cell therapy [163].
TCR-T
Due to the limitations of CAR in the application, it only recognizes cell surface protein antigens, while TCR can distinguish intracellular proteins expressed as peptides on MHC class I molecules. Therefore, TCR-T therapy has superiority in the field of solid tumour treatment. The TCR can be produced in two ways. One method is to identify and clone T- cells from patients with antitumour reactions. Their TCRs are inserted into retroviruses or lentiviruses to infect target T-cells. Another method is to isolate TCRs from humanized mice that recognize tumour antigens. TCRs can be immunized with appropriate tumour antigens because they can express human MHC class I or II. After T-cells were isolated, the TCR gene was cloned into a recombinant vector for genetic engineering transformation of patients' autologous T-cells [164].
Although effective responses have been observed in TCR-T-cell therapy, adverse reactions have become a thorny issue in many trials. Most of the reasons are that TCR-T- cells, in addition to their killing effect on tumour cells, severely destroy normal cells with the same antigen [129]. Since TCR-T-cells have only emerged in recent years, there are almost no plant extracts currently used in TCR-T-cell research. Parvifoline AA is an ent-kaurane diterpenoid and can significantly stimulate the level of NKG2D ligands on hepatocellular carcinoma cells, evidently enhancing their recognition and lysis by NK cells [14]. Perhaps improving the efficacy of TCR-T-cells in the immunosuppressive microenvironment and determining that the expression is mainly (if not completely) limited to cancer cell targets may be a future research direction for plant extracts.
Conclusion
Plant extracts are relatively easy to obtain and have significant activity in the treatment of many diseases. The above review shows that plant extracts have an effect on stem cell proliferation or directed differentiation and play an important role in solving the problem of insufficient endogenous stem cells and directed differentiation of stem cells; In immune cell therapy, the effect of plant extracts on stem cells are reflected in the beneficial effects on CD4+ T-cells and CD8+ T-cells and the improvement of the tumour microenvironment. Moreover, plant extracts, such as astragaloside [165], paeoniflorin [166], and licorice [167], have a good immunoregulatory and anti-inflammatory activities and may provide a better treatment plan for the cytokine storm caused by cell therapy [168]. At present, cell therapy is promising. However, to understand the long-term effects, more in-depth research on the dose and side effects of plant extract applications is still needed. Although plant extracts are recognized as excellent alternatives to synthetic interventions, clinical application is challenging due to the variability and complexity of the bioactive components present in the extracts, as well as the effects of solvents during extraction. Therefore, the effects of plant extracts on cell therapy need to be better and more deeply researched to supplement the current deficiencies in cell therapy.
Availability of data and materials
Not applicable.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- NSCs:
-
Nerve stem cells
- CAR-T:
-
Chimeric antigen receptor T-cell immunotherapy
- TCR-T:
-
T-cell receptor modified T-cell immunotherapy
- ASCs:
-
Adipose-derived stem cells
- iPSCs:
-
Induced pluripotent stem cells
- ESCs:
-
Embryonic stem cells
- HLA-DR:
-
Human leukocyte antigen-antigen D related
- BM-hMSCs:
-
Bone marrow-derived human MSCs
- ERK:
-
Extracellular signal-regulated kinase
- Runx2:
-
Runt-related transcription factor 2
- HGPS:
-
Hutchinson–Gilford progeria syndrome
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelium growth factor receptor
- TCM:
-
Traditional Chinese medicine
- ALP:
-
Alkaline phosphatase
- PPARγ:
-
Peroxisome proliferator activated receptor γ
- BSNXD:
-
BuShenNingXin decoction
- WS-MSCs:
-
Wharton's jelly-derived MSCs
- C/EBP-α:
-
CCAAT enhancer-binding protein-α
- Smo:
-
Smoothened
- OGD:
-
Oxygen and glucose deprivation
- bFGF:
-
Basic fibroblast growth factor
- NSPC:
-
Neural stem/progenitor cells
- GFAP:
-
Glial fibrillary acidic protein
- n-BP:
-
Butylidenephthalide
- ET-1:
-
Endothelin 1
- BNP:
-
Brain natriuretic peptide
- UVB:
-
Ultraviolet-B
- EpSCs:
-
Epidermal stem cells
- ACT:
-
Adoptive cell therapy
References
Facklam AL, Volpatti LR, Anderson DG. Biomaterials for personalized cell therapy. Adv Mater. 2020;32: e1902005. https://doi.org/10.1002/adma.201902005.
Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111–26. https://doi.org/10.1016/j.biotechadv.2018.03.011.
Hashemzadeh MR, Taghavizadeh Yazdi ME, Amiri MS, Mousavi SH. Stem cell therapy in the heart: biomaterials as a key route. Tissue Cell. 2021;71: 101504. https://doi.org/10.1016/j.tice.2021.101504.
Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, et al. Cell-based therapy approaches: the hope for incurable diseases. Regen Med. 2014;9:649–72. https://doi.org/10.2217/rme.14.35.
Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A, et al. Stem cell therapy: a new therapeutic option for cardiovascular diseases. J Cell Biochem. 2018;119:95–104. https://doi.org/10.1002/jcb.26169.
Wu W, Wang Y, Tang Z, Gao Y, Huo Y. Regulatory oversight of cell therapy in China: government’s efforts in patient access and therapeutic innovation. Pharmacol Res. 2020;158: 104889. https://doi.org/10.1016/j.phrs.2020.104889.
Udalamaththa VL. Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res Ther. 2016. https://doi.org/10.1186/s13287-016-0366-4.
Jiang L-L, Liu L. Effect of metformin on stem cells: Molecular mechanism and clinical prospect. World J Stem Cells. 2020;12:1455–73. https://doi.org/10.4252/wjsc.v12.i12.1455.
Calabrese EJ. Hormesis and adult adipose-derived stem cells. Pharmacol Res. 2021;172:105803. https://doi.org/10.1016/j.phrs.2021.105803.
Haiyan H, Rensong Y, Guoqin J, Xueli Z, Huaying X, Yanwu X. Effect of astragaloside IV on neural stem cell transplantation in Alzheimer’s disease rat models. Evid Based Complement Alternat Med. 2016;2016:3106980. https://doi.org/10.1155/2016/3106980.
Lee JH, Ryu JM, Han Y-S, Zia MF, Kwon HY, Noh H, et al. Fucoidan improves bioactivity and vasculogenic potential of mesenchymal stem cells in murine hind limb ischemia associated with chronic kidney disease. J Mol Cell Cardiol. 2016;97:169–79. https://doi.org/10.1016/j.yjmcc.2016.05.011.
Zheng P-D, Mungur R, Zhou H-J, Hassan M, Jiang S-N, Zheng J-S. Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both in vivo and in vitro. Neural Regen Res. 2018;13:1204–11. https://doi.org/10.4103/1673-5374.232476.
Li L, Liu J. Effect of Chinese medicine XIAOJI decoction combined with platinum-based chemotherapy and transfusion of cytokine-induced killer cells in patients with stage III B/IV non-small cell lung cancer. J Drug Metab Toxicol. 2015. https://doi.org/10.4172/2157-7609.S10-001.
Zhu H, Wang B, Kong L, An T, Li G, Zhou H, et al. Parvifoline AA promotes susceptibility of hepatocarcinoma to natural killer cell-mediated cytolysis by targeting peroxiredoxin. Cell Chem Biol. 2019;26:1122-1132.e6. https://doi.org/10.1016/j.chembiol.2019.04.003.
Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738–55. https://doi.org/10.1002/term.2914.
Mahmoudi Z, Soleimani M, Saidi A, Khamisipour G, Azizsoltani A. Effects of Foeniculum vulgare ethanol extract on osteogenesis in human mecenchymal stem cells. Avicenna J Phytomed. 2013;3:135–42.
Mahmoudi Z, Soleimani M, Saidi A, Iranshahi M, Azizsoltanli A. Effect of Ferula gummosa ethanolic extract on osteogenesis in human mesenchymal stem cells. J Med Plants. 2013;12:Pe50–9.
Zha X, Xu Z, Liu Y, Xu L, Huang H, Zhang J, et al. Amentoflavone enhances osteogenesis of human mesenchymal stem cells through JNK and p38 MAPK pathways. J Nat Med. 2016;70:634–44. https://doi.org/10.1007/s11418-016-0993-1.
Huang Q, Shi J, Gao B, Zhang H-Y, Fan J, Li X-J, et al. Gastrodin: an ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone. 2015;73:132–44. https://doi.org/10.1016/j.bone.2014.12.059.
Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14:806–14. https://doi.org/10.1016/j.phymed.2007.04.003.
Xu F-T, Li H-M, Yin Q-S, Cui S-E, Liu D-L, Nan H, et al. Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro. Can J Physiol Pharmacol. 2014;92:467–75. https://doi.org/10.1139/cjpp-2013-0377.
Lee J, Shin MS, Kim MO, Jang S, Oh SW, Kang M, et al. Apple ethanol extract promotes proliferation of human adult stem cells, which involves the regenerative potential of stem cells. Nutr Res. 2016;36:925–36. https://doi.org/10.1016/j.nutres.2016.06.010.
Sanap A, Chandravanshi B, Shah T, Tillu G, Dhanushkodi A, Bhonde R, et al. Herbal pre-conditioning induces proliferation and delays senescence in Wharton’s Jelly Mesenchymal stem cells. Biomed Pharmacother. 2017;93:772–8. https://doi.org/10.1016/j.biopha.2017.06.107.
Chen M, Feng W, Cao H, Zou L, Chen C, Baatrup A, et al. A traditional Chinese medicine formula extracts stimulate proliferation and inhibit mineralization of human mesenchymal stem cells in vitro. J Ethnopharmacol. 2009;125:75–82. https://doi.org/10.1016/j.jep.2009.06.013.
Choi JH, Lyu SY, Lee HJ, Jung J, Park WB, Kim GJ. Korean mistletoe lectin regulates self-renewal of placenta-derived mesenchymal stem cells via autophagic mechanisms. Cell Prolif. 2012;45:420–9. https://doi.org/10.1111/j.1365-2184.2012.00839.x.
Kim B-S, Kim Y-C, Zadeh H, Park Y-J, Pi S-H, Shin H-S, et al. Effects of the dichloromethane fraction of Dipsaci Radix on the osteoblastic differentiation of human alveolar bone marrow-derived mesenchymal stem cells. Biosci Biotechnol Biochem. 2011;75:13–9. https://doi.org/10.1271/bbb.100379.
Li G, Zhang X, Zhang J, Chan C, Yew DTW, He M, et al. Ethanol extract of Fructus Ligustri Lucidi promotes osteogenesis of mesenchymal stem cells. Phytother Res. 2010;24:571–6. https://doi.org/10.1002/ptr.2987.
Yoon H-Y, Yun S-I, Kim B-Y, Jin Q, Woo E-R, Jeong S-Y, et al. Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells. Eur J Pharmacol. 2011;664:54–9. https://doi.org/10.1016/j.ejphar.2011.04.047.
Li X-D, Wang J-S, Chang B, Chen B, Guo C, Hou G-Q, et al. Panax notoginseng saponins promotes proliferation and osteogenic differentiation of rat bone marrow stromal cells. J Ethnopharmacol. 2011;134:268–74. https://doi.org/10.1016/j.jep.2010.11.075.
Chen B, Li X, Liu D, Wang H, Xie P, Liu Z, et al. Canonical Wnt signaling is required for Panax notoginseng saponin-mediated attenuation of the RANKL/OPG ratio in bone marrow stromal cells during osteogenic differentiation. Phytomedicine. 2012;19:1029–34. https://doi.org/10.1016/j.phymed.2012.06.002.
Yu G-Y, Zheng G-Z, Chang B, Hu Q-X, Lin F-X, Liu D-Z, et al. Naringin stimulates osteogenic differentiation of rat bone marrow stromal cells via activation of the notch signaling pathway. Stem Cells Int. 2016;2016:7130653. https://doi.org/10.1155/2016/7130653.
Zhang P, Peng-Zhang null, Dai K, Yan S, Yan W, Zhang C, et al. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009;607:1–5. https://doi.org/10.1016/j.ejphar.2009.01.035
Yonezawa T, Lee J-W, Hibino A, Asai M, Hojo H, Cha B-Y, et al. Harmine promotes osteoblast differentiation through bone morphogenetic protein signaling. Biochem Biophys Res Commun. 2011;409:260–5. https://doi.org/10.1016/j.bbrc.2011.05.001.
Jin P, Li M, Xu G, Zhang K, Zheng LI, Zhao J. Role of (-)-epigallocatechin-3-gallate in the osteogenic differentiation of human bone marrow mesenchymal stem cells: an enhancer or an inducer? Exp Ther Med. 2015;10:828–34. https://doi.org/10.3892/etm.2015.2579.
Park S-J, Lee KW, Lim D-S, Lee S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 2012;21:2204–11. https://doi.org/10.1089/scd.2011.0521.
Zhou C, Lin Y. Osteogenic differentiation of adipose-derived stem cells promoted by quercetin. Cell Prolif. 2014;47:124–32. https://doi.org/10.1111/cpr.12097.
Qiu X-M, Wang L, Gui Y-Y, Xu Y-P, Li D-J. BSNXD modulates mesenchymal stem cell differentiation into osteoblasts in a postmenopausal osteoporotic mouse model. Int J Clin Exp Pathol. 2015;8:4408–17.
Zhang J, Li G, Chan C, Meng C, Lin MC, Chen Y, et al. Flavonoids of herba epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/beta-catenin signaling pathway. Mol Cell Endocrinol. 2010;314:70–4. https://doi.org/10.1016/j.mce.2009.08.012.
Liao F, Liu Y, Liu H-H, Hu J, Zhao S, Yang S-M. Effect of Angelica sinensis polysaccharide on the osteogenic differentiation of bone marrow mesenchymal stem cells of rats with high glucose levels. Hua Xi Kou Qiang Yi Xue Za Zhi. 2019;37:193–9. https://doi.org/10.7518/hxkq.2019.02.012.
Gu Q, Chen C, Zhang Z, Wu Z, Fan X, Zhang Z, et al. Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/β-catenin signaling. Pharmacol Res. 2015;97:70–8. https://doi.org/10.1016/j.phrs.2015.04.004.
Tao K, Xiao D, Weng J, Xiong A, Kang B, Zeng H. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicol Lett. 2016;240:68–80. https://doi.org/10.1016/j.toxlet.2015.10.007.
Xu D, Xu L, Zhou C, Lee WYW, Wu T, Cui L, et al. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol. 2014;51:1–9. https://doi.org/10.1016/j.biocel.2014.03.005.
Cui L, Li T, Liu Y, Zhou L, Li P, Xu B, et al. Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS ONE. 2012;7: e34647. https://doi.org/10.1371/journal.pone.0034647.
Wang L, Zhang Y-G, Wang X-M, Ma L-F, Zhang Y-M. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem Biol Interact. 2015;242:255–61. https://doi.org/10.1016/j.cbi.2015.10.010.
Zhu B, Xue F, Zhang C, Li G. Ginkgolide B promotes osteoblast differentiation via activation of canonical Wnt signalling and alleviates osteoporosis through a bone anabolic way. J Cell Mol Med. 2019;23:5782–93. https://doi.org/10.1111/jcmm.14503.
Ying X, Sun L, Chen X, Xu H, Guo X, Chen H, et al. Silibinin promotes osteoblast differentiation of human bone marrow stromal cells via bone morphogenetic protein signaling. Eur J Pharmacol. 2013;721:225–30. https://doi.org/10.1016/j.ejphar.2013.09.031.
Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 2013;9:1089–98. https://doi.org/10.7150/ijbs.7367.
Wang J-Y, Chen W-M, Wen C-S, Hung S-C, Chen P-W, Chiu J-H. Du-Huo-Ji-Sheng-Tang and its active component Ligusticum chuanxiong promote osteogenic differentiation and decrease the aging process of human mesenchymal stem cells. J Ethnopharmacol. 2017;198:64–72. https://doi.org/10.1016/j.jep.2016.12.011.
Wu Y-Q, Xia L-G, Zhou Y-N, Xu Y-J, Jiang X-Q. Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner. Cell Prolif. 2015. https://doi.org/10.1111/cpr.12185.
Zhai Y-K, Guo X-Y, Ge B-F, Zhen P, Ma X-N, Zhou J, et al. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone. 2014;66:189–98. https://doi.org/10.1016/j.bone.2014.06.016.
Zhou Y, Wu Y, Jiang X, Zhang X, Xia L, Lin K, et al. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS ONE. 2015;10: e0129605. https://doi.org/10.1371/journal.pone.0129605.
Geng L, Liu Z, Zhang W, Li W, Wu Z, Wang W, et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell. 2019;10:417–35. https://doi.org/10.1007/s13238-018-0567-y.
Kim BS, Kang H-J, Park J-Y, Lee J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp Mol Med. 2015;47: e128. https://doi.org/10.1038/emm.2014.95.
Ryu BM, Li YX, Kang KH, Kim SK, Kim DG. Floridoside from Laurencia undulata promotes osteogenic differentiation in murine bone marrow mesenchymal cells. J Funct Foods. 2015;19:505–11.
Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, et al. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS ONE. 2012;7: e35712. https://doi.org/10.1371/journal.pone.0035712.
Peltz L, Gomez J, Marquez M, Alencastro F, Atashpanjeh N, Quang T, et al. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS ONE. 2012;7: e37162. https://doi.org/10.1371/journal.pone.0037162.
Tse GG, Kim BB, McMurtray AM, Nakamoto BK. Case of levodopa toxicity from ingestion of Mucuna gigantea. Hawaii J Med Public Health. 2013;72:157–60.
Kongros K. The effects of seed extract of Mucuna gigantea on the expression of neural markers in mesenchymal stem cells. J Med Plants Res. 2012. https://doi.org/10.5897/JMPR11.1406.
Ma L, Feng X, Cui B, Law F, Jiang X, Yang L-Y, et al. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl). 2005;118:1987–93.
Hu L, Yu J, Li F, Chen B, Li L, Liu G. Effects of Salvia miltorrhiza in neural differentiation of rat mesenchymal stem cells with optimized protocol. J Ethnopharmacol. 2011;136:334–40. https://doi.org/10.1016/j.jep.2011.04.043.
Si Y-C, Li Q, Xie C-E, Niu X, Xia X-H, Yu C-Y. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin Med. 2014;9:13. https://doi.org/10.1186/1749-8546-9-13.
Dong L, Wang Y, Lu C, Wang F. Effect of Astragalus mongholicus on inducing differentiations of rat bone marrow-derived mesenchymal stem cells into neurocyte-like cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:417–20.
Dong J, Zhu G, Wang T-C, Shi F-S. Ginsenoside Rg1 promotes neural differentiation of mouse adipose-derived stem cells via the miRNA-124 signaling pathway. J Zhejiang Univ Sci B. 2017;18:445–8. https://doi.org/10.1631/jzus.B1600355.
Wang Q, Zhou L, Guo Y, Liu G, Cheng J, Yu H. Differentiation of human adipose-derived stem cells into neuron-like cells by Radix Angelicae Sinensis. Neural Regen Res. 2013;8:3353–8. https://doi.org/10.3969/j.issn.1673-5374.2013.35.010.
Warrier SR, Haridas N, Balasubramanian S, Jalisatgi A, Bhonde R, Dharmarajan A. A synthetic formulation, Dhanwantharam kashaya, delays senescence in stem cells. Cell Prolif. 2013;46:283–90. https://doi.org/10.1111/cpr.12026.
Widowati W, Sardjono CT, Wijaya L, Laksmitawati DR, Sandra F. Extract of Curcuma longa L. and (-)-Epigallo Catechin-3-gallate enhanced proliferation of adipose tissue–derived mesenchymal stem cells (AD-MSCs) and differentiation of AD-MSCs into endothelial progenitor cells. J US China Med Sci. 2012. https://doi.org/10.17265/1548-6648/2012.01.003.
Han Y-S, Lee JH, Jung JS, Noh H, Baek MJ, Ryu JM, et al. Fucoidan protects mesenchymal stem cells against oxidative stress and enhances vascular regeneration in a murine hindlimb ischemia model. Int J Cardiol. 2015;198:187–95. https://doi.org/10.1016/j.ijcard.2015.06.070.
Aziz J, Abu Kassim NL, Abu Kasim NH, Haque N, Rahman MT. Carica papaya induces in vitro thrombopoietic cytokines secretion by mesenchymal stem cells and haematopoietic cells. BMC Complement Altern Med. 2015;15:215. https://doi.org/10.1186/s12906-015-0749-6.
Di Giacomo C, Vanella L, Sorrenti V, Santangelo R, Barbagallo I, Calabrese G, Genovese C, Mastrojeni S, Ragusa S, Acquaviva R. Effects of Tithonia diversifolia (Hemsl.) A. Gray extract on adipocyte differentiation of human mesenchymal stem cells. PLOS ONE. 2015;10(4):e0122320. https://doi.org/10.1371/journal.pone.0122320.
Subash-Babu P, Alshatwi AA. Aloe-emodin inhibits adipocyte differentiation and maturation during in vitro human mesenchymal stem cell adipogenesis. J Biochem Mol Toxicol. 2012;26:291–300. https://doi.org/10.1002/jbt.21415.
Yan JS, Wei ZJ, Wang B, Song ZL. Interventional effect of Quzhisu on adipogenic differentiation of mesenchymal stem cells derived from aplastic anemia patients. J Clin Rehabilit Tissue Eng Res. 2008;12:4873–6.
Xu Y, Wu C, Wu Y, Tong P, Jin H, Yu N, et al. Epimedium-derived flavonoids modulate the balance between osteogenic differentiation and adipogenic differentiation in bone marrow stromal cells of ovariectomized rats via Wnt/β-catenin signal pathway activation. Chin J Integr Med. 2012;18:909–17. https://doi.org/10.1007/s11655-012-1294-2.
Jeong S-G, Oh YS, Joe I-S, Jeong SY, Cho HM, Lee JS, et al. Functional restoration of replicative senescent mesenchymal stem cells by the brown alga Undaria pinnatifida. Anim Cells Syst (Seoul). 2017;21:108–14. https://doi.org/10.1080/19768354.2017.1292951.
Liu D, Tang W, Zhang H, Huang H, Zhang Z, Tang D, et al. Icariin protects rabbit BMSCs against OGD-induced apoptosis by inhibiting ERs-mediated autophagy via MAPK signaling pathway. Life Sci. 2020;253: 117730. https://doi.org/10.1016/j.lfs.2020.117730.
Cruciani S, Santaniello S, Fadda A, Sale L, Sarais G, Sanna D, et al. Extracts from myrtle liqueur processing waste modulate stem cells pluripotency under stressing conditions. Biomed Res Int. 2019;2019:5641034. https://doi.org/10.1155/2019/5641034.
Cruciani S, Garroni G, Ginesu GC, Fadda A, Ventura C, Maioli M. Unravelling cellular mechanisms of stem cell senescence: an aid from natural bioactive molecules. Biology (Basel). 2020;9:E57. https://doi.org/10.3390/biology9030057.
Lee JH, Jung HK, Han Y-S, Yoon YM, Yun CW, Sun HY, et al. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep. 2016;14:3777–84. https://doi.org/10.3892/mmr.2016.5706.
Jeong S-H, Lee J-E, Kim B-B, Ko Y, Park J-B. Evaluation of the effects of Cimicifugae Rhizoma on the morphology and viability of mesenchymal stem cells. Exp Ther Med. 2015;10:629–34. https://doi.org/10.3892/etm.2015.2578.
Jeong S-H, Lee J-E, Jin S-H, Ko Y, Park J-B. Effects of Asiasari radix on the morphology and viability of mesenchymal stem cells derived from the gingiva. Mol Med Rep. 2014;10:3315–9. https://doi.org/10.3892/mmr.2014.2607.
Wang J, Hu J, Chen X, Lei X, Feng H, Wan F, et al. Traditional Chinese medicine monomers: novel strategy for endogenous neural stem cells activation after stroke. Front Cell Neurosci. 2021;15: 628115. https://doi.org/10.3389/fncel.2021.628115.
Grochowski C, Radzikowska E, Maciejewski R. Neural stem cell therapy-brief review. Clin Neurol Neurosurg. 2018;173:8–14. https://doi.org/10.1016/j.clineuro.2018.07.013.
Qin W, Chen S, Yang S, Qian X, Chuanshan X, Cai J. The effect of traditional Chinese medicine on neural stem cell proliferation and differentiation. Aging Dis. 2017;8(6):792. https://doi.org/10.14336/AD.2017.0428.
Gao J, Wan F, Tian M, Li Y, Li Y, Li Q, et al. Effects of ginsenoside-Rg1 on the proliferation and glial-like directed differentiation of embryonic rat cortical neural stem cells in vitro. Mol Med Rep. 2017;16:8875–81. https://doi.org/10.3892/mmr.2017.7737.
Lin T, Liu Y, Shi M, Liu X, Li L, Liu Y, et al. Promotive effect of ginsenoside Rd on proliferation of neural stem cells in vivo and in vitro. J Ethnopharmacol. 2012;142:754–61. https://doi.org/10.1016/j.jep.2012.05.057.
Cheng W, Yu P, Wang L, Shen C, Song X, Chen J, et al. Sonic hedgehog signaling mediates resveratrol to increase proliferation of neural stem cells after oxygen-glucose deprivation/reoxygenation injury in vitro. Cell Physiol Biochem. 2015;35:2019–32. https://doi.org/10.1159/000374009.
Shen C, Cheng W, Yu P, Wang L, Zhou L, Zeng L, et al. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol Med Rep. 2016;14:3646–54. https://doi.org/10.3892/mmr.2016.5670.
Gugliandolo E, D’Amico R, Cordaro M, Fusco R, Siracusa R, Crupi R, et al. Neuroprotective effect of artesunate in experimental model of traumatic brain injury. Front Neurol. 2018;9:590. https://doi.org/10.3389/fneur.2018.00590.
Zhang K, Yang Y, Hongfei Ge J, Wang XC, Lei X, Zhong J, Zhang C, Xian J, Yongling L, Tan L, Feng H. Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates Ischemia-reperfusion Injury through PI3K/Akt/FOXO-3a/p27kip1 signaling pathway. Aging. 2020;12(9):8029–48. https://doi.org/10.18632/aging.103121.
Li M-Y, Chang C-T, Han Y-T, Liao C-P, Yu J-Y, Wang T-W. Ginkgolide B promotes neuronal differentiation through the Wnt/β-catenin pathway in neural stem cells of the postnatal mammalian subventricular zone. Sci Rep. 2018;8:14947. https://doi.org/10.1038/s41598-018-32960-8.
Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8:76–103. https://doi.org/10.1021/nn405077y.
Yang P, Guan Y-Q, Li Y-L, Zhang L, Zhang L, Li L. Icariin promotes cell proliferation and regulates gene expression in human neural stem cells in vitro. Mol Med Rep. 2016;14:1316–22. https://doi.org/10.3892/mmr.2016.5377.
Huang J, Cai W, Zhang X, Shen Z. Icariin promotes self-renewal of neural stem cells: an involvement of extracellular regulated kinase signaling pathway. Chin J Integr Med. 2014;20:107–15. https://doi.org/10.1007/s11655-013-1583-7.
Lu Q, Zhu H, Liu X, Tang C. Icariin sustains the proliferation and differentiation of Aβ25-35-treated hippocampal neural stem cells via the BDNF-TrkB-ERK/Akt signaling pathway. Neurol Res. 2020;42:936–45. https://doi.org/10.1080/01616412.2020.1792701.
Guo G, Li B, Wang Y, Shan A, Shen W, Yuan L, et al. Effects of salvianolic acid B on proliferation, neurite outgrowth and differentiation of neural stem cells derived from the cerebral cortex of embryonic mice. Sci China Life Sci. 2010;53:653–62. https://doi.org/10.1007/s11427-010-3106-5.
Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, et al. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS ONE. 2012;7: e35636. https://doi.org/10.1371/journal.pone.0035636.
Yan R, Xu H, Fu X. Salidroside protects hypoxia-induced injury by up-regulation of miR-210 in rat neural stem cells. Biomed Pharmacother. 2018;103:1490–7. https://doi.org/10.1016/j.biopha.2018.04.184.
Chai Y-S, Hu J, Lei F, Wang Y-G, Yuan Z-Y, Lu X, et al. Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur J Pharmacol. 2013;708:44–55. https://doi.org/10.1016/j.ejphar.2013.02.041.
Chen D-F, Meng L-J, Du S-H, Zhang H-L, Li H, Zhou J-H, et al. (+)-Cholesten-3-one induces differentiation of neural stem cells into dopaminergic neurons through BMP signaling. Neurosci Res. 2010;68:176–84. https://doi.org/10.1016/j.neures.2010.07.2043.
Huang F, Lan Y, Qin L, Dong H, Shi H, Wu H, et al. Astragaloside IV promotes adult neurogenesis in hippocampal dentate gyrus of mouse through CXCL1/CXCR2 signaling. Molecules. 2018;23:E2178. https://doi.org/10.3390/molecules23092178.
Si Y-C, Zhang J-P, Xie C-E, Zhang L-J, Jiang X-N. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med. 2011;39:999–1013. https://doi.org/10.1142/S0192415X11009366.
Tian Y, Liu Y, Chen X, Zhang H, Shi Q, Zhang J, et al. Tetramethylpyrazine promotes proliferation and differentiation of neural stem cells from rat brain in hypoxic condition via mitogen-activated protein kinases pathway in vitro. Neurosci Lett. 2010;474:26–31. https://doi.org/10.1016/j.neulet.2010.02.066.
Zhuang P-W, Cui G-Z, Zhang Y-J, Zhang M-X, Guo H, Zhang J-B, et al. Baicalin regulates neuronal fate decision in neural stem/progenitor cells and stimulates hippocampal neurogenesis in adult rats. CNS Neurosci Ther. 2013;19:154–62. https://doi.org/10.1111/cns.12050.
Li Y, Zhuang P, Shen B, Zhang Y, Shen J. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 2012;1429:36–42. https://doi.org/10.1016/j.brainres.2011.10.030.
Sun J, Bi Y, Guo L, Qi X, Zhang J, Li G, et al. Buyang Huanwu Decoction promotes growth and differentiation of neural progenitor cells: using a serum pharmacological method. J Ethnopharmacol. 2007;113:199–203. https://doi.org/10.1016/j.jep.2007.05.018.
Sun J-H, Gao Y-M, Yang L, Wang X, Bao L-H, Liu W-J, et al. Effects of Buyang Huanwu Decoction on neurite outgrowth and differentiation of neuroepithelial stem cells. Chin J Physiol. 2007;50:151–6.
Wu L, Ran C, Liu S, Liao L, Chen Y, Guo H, et al. Jiaweisinisan facilitates neurogenesis in the hippocampus after stress damage. Neural Regen Res. 2013;8:1091–102. https://doi.org/10.3969/j.issn.1673-5374.2013.12.004.
Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23:49–69. https://doi.org/10.1038/cr.2012.175.
Pouton CW, Haynes JM. Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov. 2007;6:605–16. https://doi.org/10.1038/nrd2194.
Li Y, Liu Z, Li J, Wang M. Traditional Chinese medicine, Kami-Shoyo-San protects ketamine-induced neurotoxicity in human embryonic stem cell-differentiated neurons through activation of brain-derived neurotrophic factor. NeuroReport. 2019;30:1102–9.
Kim KM, Heo DR, Lee JY, Seo C-S, Chung S-K. High-efficiency generation of induced pluripotent stem cells from human foreskin fibroblast cells using the Sagunja-tang herbal formula. BMC Complement Altern Med. 2017;17:529. https://doi.org/10.1097/WNR.0000000000001328.
Kim A, Lee S-Y, Seo C-S, Chung S-K. Prunellae spica extract suppresses teratoma formation of pluripotent stem cells through p53-mediated apoptosis. Nutrients. 2020;12:E721. https://doi.org/10.3390/nu12030721.
Kim A, Lee S-Y, Seo C-S, Chung S-K. Ethanol extract of Magnoliae cortex (EEMC) limits teratoma formation of pluripotent stem cells by selective elimination of undifferentiated cells through the p53-dependent mitochondrial apoptotic pathway. Phytomedicine. 2020;69: 153198. https://doi.org/10.1016/j.phymed.2020.153198.
Shu T, Pang M, Rong L, Zhou W, Wang J, Liu C, et al. Effects of salvia miltiorrhiza on neural differentiation of induced pluripotent stem cells. J Ethnopharmacol. 2014;153:233–41. https://doi.org/10.1016/j.jep.2014.02.028.
Chang C-Y, Chen S-M, Lu H-E, Lai S-M, Lai P-S, Shen P-W, et al. N-butylidenephthalide attenuates Alzheimer’s disease-like cytopathy in down syndrome induced pluripotent stem cell-derived neurons. Sci Rep. 2015;5:8744. https://doi.org/10.1038/srep08744.
Pei-Chun W, Ming-Ji Fann T, Tran T, Chen SC, Devina T, Cheng IHJ, Lien CC, Kao LS, Wang SJ, Fuh JL, Tzeng TT, Huang CY, Shiao YJ, Wong YH. Assessing the therapeutic potential of Graptopetalum paraguayense on Alzheimer’s disease using patient iPSC-derived neurons. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-55614-9.
Wei W, Liu Y, Zhang Q, Wang Y, Zhang X, Zhang H. Danshen-enhanced cardioprotective effect of cardioplegia on ischemia reperfusion injury in a human-induced pluripotent stem cell-derived cardiomyocytes model. Artif Organs. 2017;41:452–60. https://doi.org/10.1111/aor.12801.
Pahlavan S, Tousi MS, Ayyari M, Alirezalu A, Ansari H, Saric T, et al. Effects of hawthorn (Crataegus pentagyna) leaf extract on electrophysiologic properties of cardiomyocytes derived from human cardiac arrhythmia-specific induced pluripotent stem cells. FASEB J. 2018;32:1440–51. https://doi.org/10.1096/fj.201700494RR.
Zhang M, Wu H, Guo F, Yu Y, Wei J, Geng Y, et al. Identification of active components in Yixinshu Capsule with protective effects against myocardial dysfunction on human induced pluripotent stem cell-derived cardiomyocytes by an integrative approach. Mol Biosyst. 2017;13:1469–80. https://doi.org/10.1039/c6mb00813e.
Yu Y, Sun S, Wang S, Zhang Q, Li M, Lan F, et al. Liensinine- and neferine-induced cardiotoxicity in primary neonatal rat cardiomyocytes and human-induced pluripotent stem cell-derived cardiomyocytes. Int J Mol Sci. 2016;17:E186. https://doi.org/10.3390/ijms17020186.
Lu J, Wei H, Wu J, Jamil MFA, Tan ML, Adenan MI, et al. Evaluation of the cardiotoxicity of mitragynine and its analogues using human induced pluripotent stem cell-derived cardiomyocytes. PLoS ONE. 2014;9: e115648. https://doi.org/10.1371/journal.pone.0115648.
Nembo EN, Atsamo AD, Nguelefack TB, Kamanyi A, Hescheler J, Nguemo F. In vitro chronotropic effects of Erythrina senegalensis DC (Fabaceae) aqueous extract on mouse heart slice and pluripotent stem cell-derived cardiomyocytes. J Ethnopharmacol. 2015;165:163–72. https://doi.org/10.1016/j.jep.2015.02.002.
You J, Roh K-B, Li Z, Liu G, Tang J, Shin S, et al. The antiaging properties of andrographis paniculata by activation epidermal cell stemness. Molecules. 2015;20:17557–69. https://doi.org/10.3390/molecules200917557.
Lee J, Shin Y-K, Song J-Y, Lee K-W. Protective mechanism of morin against ultraviolet B-induced cellular senescence in human keratinocyte stem cells. Int J Radiat Biol. 2014;90:20–8. https://doi.org/10.3109/09553002.2013.835502.
Lee J, Cho JY, Lee SY, Lee K-W, Lee J, Song J-Y. Vanillin protects human keratinocyte stem cells against ultraviolet B irradiation. Food Chem Toxicol. 2014;63:30–7. https://doi.org/10.1016/j.fct.2013.10.031.
Lee J, Oh SW, Shin SW, Lee K-W, Cho J-Y, Lee J. Zingerone protects keratinocyte stem cells from UVB-induced damage. Chem Biol Interact. 2018;279:27–33. https://doi.org/10.1016/j.cbi.2017.11.004.
Chen J, Wang X, Zhu J, Shang Y, Guo X, Sun J. Effects of Ginkgo biloba extract on number and activity of endothelial progenitor cells from peripheral blood. J Cardiovasc Pharmacol. 2004;43:347–52. https://doi.org/10.1097/00005344-200403000-00004.
Dong XX, Hui ZJ, Xiang WX, Rong ZF, Jian S, Zhu CJ. Ginkgo biloba extract reduces endothelial progenitor-cell senescence through augmentation of telomerase activity. J Cardiovasc Pharmacol. 2007;49:111–5. https://doi.org/10.1097/FJC.0b013e31802ef519.
Weibo Y, Qin X, Jin Y, Li Y, Santiskulvong C, Victor V, Zeng G, Zhang Z, Chow M, Rao J. Tianshengyuan-1 (TSY-1) regulates cellular Telomerase activity by methylation of TERT promoter. Oncotarget. 2016;8(5):7977–88. https://doi.org/10.18632/oncotarget.13939.
Wang Z, Cao YJ. Adoptive cell therapy targeting neoantigens: a frontier for cancer research. Front Immunol. 2020;11:176. https://doi.org/10.3389/fimmu.2020.00176.
Chen N, Xu Y, Mou J, Rao Q, Xing H, Tian Z, et al. Targeting of IL-10R on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood Cancer J. 2021;11:144. https://doi.org/10.1038/s41408-021-00536-x.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99. https://doi.org/10.1038/s41573-019-0051-2.
Wilkins O, Keeler AM, Flotte TR. CAR T-cell therapy: progress and prospects. Hum Gene Ther Methods. 2017;28:61–6. https://doi.org/10.1089/hgtb.2016.153.
Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–61. https://doi.org/10.1038/s41568-020-00323-z.
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–85. https://doi.org/10.1038/s41571-019-0184-6.
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147–67. https://doi.org/10.1038/s41571-019-0297-y.
Lesch S, Benmebarek M-R, Cadilha BL, Stoiber S, Subklewe M, Endres S, et al. Determinants of response and resistance to CAR T cell therapy. Semin Cancer Biol. 2020;65:80–90. https://doi.org/10.1016/j.semcancer.2019.11.004.
Chen X, Cao Z, Zhang Y, Li J, Wang S, Du J, et al. Fuzheng Qingjie Granules inhibit growth of hepatoma cells via inducing mitochondria-mediated apoptosis and enhancing immune function. Integr Cancer Ther. 2017;16:329–38. https://doi.org/10.1177/1534735416654761.
Chen X-Z, Cao Z-Y, Zhang Y-Q, Li J-N, Liao L-M, Du J. Fuzheng Qingjie granules potentiate the anticancer effect of cyclophosphamide by regulating cellular immune function and inducing apoptosis in Hepatoma 22 tumor-bearing mice. Oncol Lett. 2017;13:3261–8. https://doi.org/10.3892/ol.2017.5849.
Liu S, Wang X-M, Yang G-W. Action mechanism of fuzheng fangai pill combined with cyclophosphamide on tumor metastasis and growth. Evid Based Complement Alternat Med. 2014;2014: 494528. https://doi.org/10.1155/2014/494528.
Zhang A, Yang X, Li Q, Yang Y, Zhao G, Wang B, et al. Immunostimulatory activity of water-extractable polysaccharides from Cistanche deserticola as a plant adjuvant in vitro and in vivo. PLoS ONE. 2018;13: e0191356. https://doi.org/10.1371/journal.pone.0191356.
Wang C, Feng L, Jiayan S, Cui L, Liu D, Yan J, Ding C, Tan X, Jia X. Polysaccharides from Epimedium koreanum Nakai with immunomodulatory activity and inhibitory effect on tumor growth in LLC-bearing mice. J Ethnopharmacol. 2017;207:8–18. https://doi.org/10.1016/j.jep.2017.06.014.
Ayeka PA, Bian YH, Githaiga PM, Zhao Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complementary Altern Med. 2017. https://doi.org/10.1186/s12906-017-2030-7.
Guo A, He D, Xu H-B, Geng C-A, Zhao J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci Rep. 2015;5:14046. https://doi.org/10.1038/srep14046.
Wang Q, Chen D-Y. Effect of Aidi injection on peripheral blood expression of Th1/Th2 transcription factors and cytokines in patients with esophageal squamous cell carcinoma during radiotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:394–7.
Zhao H, Li Y, Wang Y, Zhang J, Ouyang X, Peng R, et al. Antitumor and immunostimulatory activity of a polysaccharide-protein complex from Scolopendra subspinipes mutilans L. Koch in tumor-bearing mice. Food Chem Toxicol. 2012;50:2648–55. https://doi.org/10.1016/j.fct.2012.05.018.
Ma W, Liu R, Qi J, Zhang Y. Extracts of centipede Scolopendra subspinipes mutilans induce cell cycle arrest and apoptosis in A375 human melanoma cells. Oncol Lett. 2014;8:414–20. https://doi.org/10.3892/ol.2014.2139.
Ding D, Guo Y-R, Wu R-L, Qi W-Y, Xu H-M. Two new isoquinoline alkaloids from Scolopendra subspinipes mutilans induce cell cycle arrest and apoptosis in human glioma cancer U87 cells. Fitoterapia. 2016;110:103–9. https://doi.org/10.1016/j.fitote.2016.03.004.
Li W, Yang Y, Ouyang Z, Zhang Q, Wang L, Tao F, et al. Xiao-Ai-Ping, a TCM Injection, enhances the Antigrowth effects of cisplatin on lewis lung cancer cells through promoting the infiltration and function of CD8(+) T lymphocytes. Evid Based Complement Alternat Med. 2013;2013: 879512. https://doi.org/10.1155/2013/879512.
Deng X, Luo S, Luo X, Hu M, Ma F, Wang Y, et al. Polysaccharides from Chinese Herbal Lycium barbarum Induced systemic and local immune responses in H22 tumor-bearing mice. J Immunol Res. 2018;2018:3431782. https://doi.org/10.1155/2018/3431782.
Wang W, Liu M, Wang Y, Yang T, Li D, Ding F, et al. Lycium barbarum polysaccharide promotes maturation of dendritic cell via notch signaling and strengthens dendritic cell mediated T lymphocyte cytotoxicity on colon cancer cell CT26-WT. Evid Based Complement Alternat Med. 2018;2018:2305683. https://doi.org/10.1155/2018/2305683.
Bo R, Sun Y, Zhou S, Ou N, Gu P, Liu Z, et al. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int J Nanomedicine. 2017;12:6289–301. https://doi.org/10.2147/IJN.S136820.
Hsieh C-C, Lin W-C, Lee M-R, Hsu S-L, Liu H-S, Kao S-T, et al. Dang-Gui-Bu-Xai-Tang modulated the immunity of tumor bearing mice. Immunopharmacol Immunotoxicol. 2003;25:259–71. https://doi.org/10.1081/iph-120020474.
Gupta S, Zhang D, Yi J, Shao J. Anticancer activities of Oldenlandia diffusa. J Herb Pharmacother. 2004;4:21–33.
Yadav SK, Lee SC. Evidence for Oldenlandia diffusa-evoked cancer cell apoptosis through superoxide burst and caspase activation. Zhong Xi Yi Jie He Xue Bao. 2006;4:485–9. https://doi.org/10.3736/jcim20060509.
Willimott S, Barker J, Jones LA, Opara EI. Apoptotic effect of Oldenlandia diffusa on the leukaemic cell line HL60 and human lymphocytes. J Ethnopharmacol. 2007;114:290–9. https://doi.org/10.1016/j.jep.2007.08.030.
Chang J-M, Hung L-M, Chyan Y-J, Cheng C-M, Wu R-Y. Carthamus tinctorius enhances the antitumor activity of dendritic cell vaccines via polarization toward Th1 cytokines and increase of cytotoxic T lymphocytes. Evid Based Complement Alternat Med. 2011;2011: 274858. https://doi.org/10.1093/ecam/nen068.
Ma Y-H, Cheng W-Z, Gong F, Ma A-L, Yu Q-W, Zhang J-Y, et al. Active Chinese mistletoe lectin-55 enhances colon cancer surveillance through regulating innate and adaptive immune responses. World J Gastroenterol. 2008;14:5274–81. https://doi.org/10.3748/wjg.14.5274.
Cai Y, Xiong S, Zheng Y, Luo F, Jiang P, Chu Y. Trichosanthin enhances anti-tumor immune response in a murine Lewis lung cancer model by boosting the interaction between TSLC1 and CRTAM. Cell Mol Immunol. 2011;8:359–67. https://doi.org/10.1038/cmi.2011.12.
Xu R, Lin L, Li Y, Li Y. ShenQi FuZheng Injection combined with chemotherapy in the treatment of colorectal cancer: a meta-analysis. PLoS ONE. 2017;12: e0185254. https://doi.org/10.1371/journal.pone.0185254.
Cao GW, Yang WG, Du P. Observation of the effects of LAK/IL-2 therapy combining with Lycium barbarum polysaccharides in the treatment of 75 cancer patients. Zhonghua Zhong Liu Za Zhi. 1994;16:428–31.
Saleh MH, Rashedi I, Keating A. Immunomodulatory Properties of Coriolus versicolor: The role of polysaccharopeptide. Front Immunol. 2017;8:1087. https://doi.org/10.3389/fimmu.2017.01087.
Bao YX, Wong CK, Leung SF, Chan ATC, Li PW, Wong ELY, et al. Clinical studies of immunomodulatory activities of Yunzhi-Danshen in patients with nasopharyngeal carcinoma. J Altern Complement Med. 2006;12:771–6. https://doi.org/10.1089/acm.2006.12.771.
Liu J, Wang Y, Qiu Z, Lv G, Huang X, Lin H, et al. Impact of TCM on tumor-infiltrating myeloid precursors in the tumor microenvironment. Front Cell Dev Biol. 2021;9: 635122. https://doi.org/10.3389/fcell.2021.635122.
Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. https://doi.org/10.1038/nri3191.
Qi Y, Gao F, Hou L, Wan C. Anti-inflammatory and immunostimulatory activities of Astragalosides. Am J Chin Med. 2017;45:1157–67. https://doi.org/10.1142/S0192415X1750063X.
Zhang L, Wei W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol Ther. 2020;207: 107452. https://doi.org/10.1016/j.pharmthera.2019.107452.
Yang R, Yuan B-C, Ma Y-S, Zhou S, Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol. 2017;55:5–18. https://doi.org/10.1080/13880209.2016.1225775.
Dai Y, Qiang W, Gui Y, Tan X, Pei T, Lin K, et al. A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci Bull (Beijing). 2021;66:884–8. https://doi.org/10.1016/j.scib.2021.01.005.
Acknowledgements
We thank Lei Tong for guidance in drawing from University of science and technology Beijing.
Funding
This study was supported by fundamental Research Funds for the Central public welfare research institutes of China (ZZ13-YQ-082-C1; JBGS2021001), the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A00610).
Author information
Authors and Affiliations
Contributions
H.J Yang, X.Y. Li and P. Chen designed the idea of this review; C.F. Li, Zh. Cui, S.W. Deng and P. Chen co-wrote the paper with input from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Plant Extracts for the Osteogenesis of MSCs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, C., Cui, Z., Deng, S. et al. The potential of plant extracts in cell therapy. Stem Cell Res Ther 13, 472 (2022). https://doi.org/10.1186/s13287-022-03152-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03152-z