Abstract
The multipotency property of mesenchymal stem cells (MSCs) has attained worldwide consideration because of their immense potential for immunomodulation and their therapeutic function in tissue regeneration. MSCs can migrate to tissue injury areas to contribute to immune modulation, secrete anti-inflammatory cytokines and hide themselves from the immune system. Certainly, various investigations have revealed anti-inflammatory, anti-aging, reconstruction, and wound healing potentials of MSCs in many in vitro and in vivo models. Moreover, current progresses in the field of MSCs biology have facilitated the progress of particular guidelines and quality control approaches, which eventually lead to clinical application of MSCs. In this literature, we provided a brief overview of immunoregulatory characteristics and immunosuppressive activities of MSCs. In addition, we discussed the enhancement, utilization, and therapeutic responses of MSCs in neural, liver, kidney, bone, heart diseases, and wound healing.
Similar content being viewed by others
Introduction
In the last decade, stem cells are increasingly applied as a therapeutic method for numerous disorders. Stem cell therapy, traditionally applied for hematopoietic disorders, nonetheless, is now established for the treatment of non-hematologic disorders [1, 2].
Accumulating evidence has shown that mesenchymal stem cells (MSCs) offer an encouraging option for cell treatment and reconstruction of human tissues because of their differentiation multipotency, self‐renewal capacity, long‐term ex vivo proliferation, paracrine potentials, and immunoregulatory effect [3]. Furthermore, MSCs have the capability to support the progression and differentiation of other stem cells. They can release bioactive molecules, which is a key benefit in tissue regeneration [4, 5]. These properties result in progression of treatments for a wide range of diseases, such as diseases affecting the bone, neuron, lung, liver, heart, kidney, etc. [4]. Due to these features, it is obvious that MSCs will hold a major therapeutic role in clinical trials. Because of these properties, we provided a general overview of the latest trials that studied the effectiveness of MSCs in several diseases such as neural, liver, kidney, bone, heart diseases, and wound healing.
Stem cells in regenerative medicine
In the last years, numerous studies have demonstrated that cellular therapy has exhibited great development in both in vitro and in vivo researches. Stem cells have the capability to self-renew, and also to differentiate into all cell types and are involved in physiological regeneration [6]. There are multiple stem cell sources of adult and pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) for tissue regeneration. PSCs have a high potential for pluripotency and self-renewal, which makes these cells an important option for treatment of diseases. However, there are ethical issues when using these cells, in which ESCs are separated from blastocyst-stage embryos, requiring destruction of the embryo [7,8,9]. The results of studies have revealed the regenerative ability of iPSCs in preclinical setting and conducted the first clinical study for treatment of age-associated with macular deterioration [10, 11]. Nonetheless, the tumorigenicity risk remains unsolved. Because of these limitations, researchers began to investigate adult stem cells, the multipotent stem cells found in tissues and organs of adults. Various investigations have reported that stem cell therapy can regenerate and repair injured organs in vivo, including bone repair, cutaneous wound, pulpitis, and ischemic cardiac tissue through stem cell differentiation and production of new particular cells [12,13,14,15]. Moreover, some investigations have demonstrated that cultured adult stem cells release many molecular factors with anti-apoptotic, immunoregulatory, angiogenic, and chemoattractant features that stimulate regeneration [16,17,18]. Hematopoietic stem cells (HSCs) and MSCs are part of adult stem cells, which are the most widely used, generally because they can be isolated from individuals in diseased conditions.
Mesenchymal stem cell
In the late 1960s, Friedenstein and colleagues discovered MSCs as multipotent stem cells for the first time [19]. MSCs are non-hematopoietic cells and have the capability to differentiate into various lineage including mesodermal (adipocytes, osteocytes, and chondrocytes), ectodermal (neurocytes), and endodermal lineage (hepatocytes) [20, 21]. At the beginning, it was thought that MSCs are “stromal” cells instead of stem cells [22]. Several investigators tried to alter the name of MSCs to medicinal signaling cells due to their function in secretion of some metabolites molecules in the sites of diseases, injuries, and inflammations [23, 24]. After that, some studies have stated that MSCs can release prostaglandin E2 (PGE2), which plays a major role in the self-renewal ability, immunomodulation of MSCs, and generating a cascade of events, that demonstrates the stemness of MSCs [25]. Therefore, the term mesenchymal stem cells is justified.
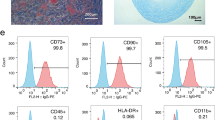
MSCs chiefly found in the bone marrow (BM) possess the ability of self-renewal and also display multilineage differentiation [8, 26, 27]. They were obtained from various tissues and organs including BM, adipose tissue, Wharton’s jelly, peripheral blood, umbilical cord, placenta, amniotic fluid, and dental pulp [3, 28,29,30]. MSCs can express a wide range of surface markers and cytokine profiles according to the origin of isolation [31]. Nevertheless, the common characterization markers of MSCs are CD73, CD105, CD90 and lacking expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR [32,33,34]. During the last decades, MSCs have shown various biological roles such as multilineage differentiation, immunomodulation, angiogenesis, anti-apoptotic and anti-fibrotic activity, chemo-attraction, and tissue repair development [35,36,37]. The MSCs have broad properties that make them a suitable source for cell therapy, such as stemness potency, easily isolation from different sources, they can be rapidly expanded in a large scale for clinical use, have less ethical issues as compared to ESCs, unlike iPSCs, MSCs transport a lower risk of teratoma formation, and they are beneficial for a wide scale of therapeutic applications due to their capability to migrate to injured tissue through chemo-attraction [38,39,40]. In addition, MSCs can release a variety of bioactive components including proteins, growth factors chemokines, microRNAs (miRNAs), and cytokines which can suggest their acceptable application [41].
The biological roles of MSCs
MSCs have the ability to inhibit the immune response in inflammatory cytokine-rich situations, including infections, wounds, or immune-mediated disorders. These immunomodulatory properties were discovered in preclinical and clinical trials, where MSCs effectively suppressed T cell activation and proliferation along with stimulation of macrophages shift from M1 to M2 [42,43,44]. This specific performance of MSCs in the presence and absence of inflammatory mediators is termed MSC polarization. MSCs have the ability to migrate to damaged areas after systemic infusion and consequently exert a beneficial effect by various mechanisms, chiefly immunoregulation, and angiogenesis [45, 46]. Although the related mechanism-mediated MSC immunosuppression has not been entirely clear, it appears that cellular interaction, accompanied by many factors, performs the principal function in this process. In the presence of high levels of inflammatory cytokines, e.g., TNF-α and IFN-γ, MSCs release several cytokines including TGF-β and hepatocyte growth factor (HGF) and produce soluble factors including indoleamine 2,3-dioxygenase (IDO), PGE2, and nitric oxide (NO). These mediators suppress T effector cells and enhance the expression of FOXP3, CTLA4, and GITR in regulatory T cells (Tregs) to increase their immunomodulation effects [47,48,49]. Moreover, cell-to-cell communication facilitates the stimulation of Tregs by cytokine-primed MSCs [50]. Overexpression of inducible co-stimulator ligands (ICOSL) induces the stimulation of efficient Tregs [51].
In addition, MSCs can enhance the generation of Treg cells indirectly. According to the literature, MSCs stimulate M2 macrophage and alter the phenotype through secretion of extracellular vesicles in an in vitro study [52]. Also, M2 cells that are activated by MSCs express CCL-18 and induce Treg cells [53]. Moreover, MSCs increase the expression of cyclooxygenase 2 (COX2) and IDO, resulting in expression of CD206 and CD163 in M2 cells, as well as enhance the expression of IL-6 and IL-10 in the microenvironment [54]. The overexpression of IL-10 that is produced by dendritic cells (DCs) and M2 cells upon MSCs co-culture leads to further immunomodulation via inhibition of effector T cells [55, 56]. Furthermore, the secretion of IDO from MSCs can induce the proliferation, activation, and IgG releasing of B cells, thereby suppressing T effector cells [57, 58].
One of the typical properties of MSCs is their multipotency capacity in which these stem cells are able to differentiate into a number of tissues in vitro [59]. Chondrogenic differentiation of MSCs in vitro occurs commonly via culturing them in the existence of TGF-β1 or TGF-β3, IGF-1, FGF-2, or BMP-2 [60,61,62,63]. MSC differentiation into chondroblasts is characterized by the increasing of various genes such as collagen type II, IX, aggrecan, and proliferation of chondroblast cell morphology. During the process of chondrogenesis, FGF-2 promotes the MSCs induced with TGF-β1 or TGF-β3 and/ or IGF-1 [64]. According to the literature works, several molecular pathways such as hedgehog, Wnt/β-catenin, TGF-βs, BMPs, and FGFs can regulate chondrogenesis [65]. In addition, MSCs can exert the osteogenesis function by inducing MSCs with ascorbic acid, β-glycerophosphate, vitamin D3, and/or BMP-2, BMP-4, BMP-6, and BMP-7 [66].
One of the major abilities of MSCs is anti-fibrotic activity. These cells can differentiate into various cell lineages such as hepatocytes, both in vivo and in vitro [67]. MSCs contain multiple trophic factors which induce cells and matrix remodeling to stimulate progenitor cells and the recovery of damaged cells. MSCs can decrease myofibroblasts and reverse the fibrotic activity of injured tissues [68]. Furthermore, these cells release pro-angiogenic factors including VEGF, IGF-1, and anti-inflammatory factors that participate in the recovery of tissue function. For instance, MSCs can increase neovascularization of ischemic myocardium through VEGF in a mice model of heart disease [69]; also, IGF-1 exerts an advantageous effect on the survival and proliferation of cardiomyocytes [70].
Bone marrow mesenchymal stem cell-based regenerative medicine
So far, increasing data have lately studied the effects of MSCs in the treatment or regeneration of various disorders (Table 1). In this section, we reviewed the latest clinical studies that investigate the potential contribution of MSCs in the regenerative medicine, as shown in Fig. 1.
Neural regeneration
The application of BMSCs has demonstrated promising therapeutic results in the treatment of neurological diseases. Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease, is a neurodegenerative disorder that leads to degeneration of the motor neurons that causes paralysis and muscle weakness [138, 139]. Syková et al. [71] carried out a study that intrathecally injected 15 ± 4.5 × 106 autologous BMSCs into 26 patients with ALS. After mesenchymal stem cells transplantation (MSCT), ALS functional rating scale (ALSFRS) significantly reduced, forced vital capacity (FVC) remained stable or above 70%, and weakness scales (WSs) were stable in 75% of patients. They have shown that the intrathecal BMSCs intervention in ALS patients is a safe method and it can slow down the development of the disease. There were no significant adverse events related to the trial during and after transplantation of BMSCs. Barczewska and colleagues indicated that three intrathecal injections of 30 × 106 Wharton’s jelly-MSCs (WJ-MSCs) improved ALSFRS [77]. They showed that WJ-MSCs are safe and effective in individuals that suffer from ALS. However, one other group found that intrathecal injection of autologous adipose MSCs does not improve clinical symptoms of ALS patients [76]. Their results indicated that the levels of CSF protein and nucleated cells were increased and ALSFRS-R showed development of disease in all treated patients. In the trial by OH et al., autologous BMSCs were injected to treat seven participants that suffer from ALS [75]. The participants were injected twice with autologous BMSCs (one million cells per kg) and followed up for 12 months. No serious adverse events were reported during the follow-up period. Furthermore, during the 12-month follow-up, there was no acceleration in the decrease in the ALSFRS-Revised (ALSFRS-R) score, Appel ALS score, and FVC. Moreover, CSF analysis showed that the levels of TGF-β and IL-10 were evaluated, while MCP-1, which is chemokine-related and exacerbates the motor neuron damage in ALS, was decreased. Their results exhibited that two repeated MSC infusions have safety and feasibility for at least 1 year in seven individuals; nevertheless, the study has some limitations such as low number of participants and short-time follow-up. In another study [73], 15 ALS patients were transplanted with autologous BMSCs. These 15 patients were divided into two groups (group 1: patients who had ALS with an inherently slow course, group 2: individuals who had ALS with an inherently rapid course) and received three intrathecal infusions of MSCs. There were no significant adverse events in the course of multiple intrathecal injections of MSCs. In group 1, there were no major changes in the rate of disease development and in group 2 ameliorating of the disease was indicated following MSCs therapy. According to their observation, the response of patients with ALS to treatment with MSCs was variable. Also, the authors indicated that due to the small number of patients, less subgroups were available for statistical analysis, limiting their ability to draw conclusions from the data.
Spinal cord injury (SCI) is usually related to devastating results. The damage to the spinal cord leads to injury to the motor, sensory, and autonomic roles of the spinal cord that affects patients’ well-being such as their physical and psychological state [140, 141]. In a phase I, nonrandomized, uncontrolled study by Mendonça et al. [84], 15 SCI patients were administered 1 × 107 cells/ml MSCs. The results of the investigation revealed that SCI symptoms were meaningfully decreased by MSCT, all participants showed variable improvements in tactile sensitivity, and eight participants improved lower limb motor functional gains, chiefly in the hip flexors. Seven patients revealed sacral sparing and developed American Spinal Injury Association impairment scale (AIS) grades B or C – partial damage. Nine participants had developments in urologic function and one patient showed alterations in somatosensory evoked potentials (SSEP) 3 and 6 months after MSCT. These results stated that treatment with MSCs ameliorated the organ malfunction in people with SCI and has clinical safety, because no serious adverse effects were reported. The authors indicated that their results should be confirmed in larger and controlled clinical trials. Albu and colleagues have been demonstrated that intrathecal administration of WJ-MSCs considerably improved the pinprick sensation in the dermatomes below the level of damage [88]. Further results showed that bladder maximum capacity was elevated and bladder neurogenic hyperactivity and external sphincter dyssynergy were reduced. In another study [85], ten SCI subjects received four subarachnoid injections of 30 × 106 autologous BMSCs, maintained in autologous plasma, at weeks 1, 16, 28, and 40 of the trial and followed up for 12 months. There were no adverse events and all participants tolerated the therapy. Vaquero et al. [86] demonstrated that MSCT is safe and improves sensitivity, motor power, spasms, spasticity, neuropathic pain, sexual function, or sphincter dysfunction in the SCI patients. The results of their study have shown that 55.5% of patients improved in SSEP and 44.4% of patients ameliorated in voluntary muscle contraction together with intralesional active muscle reinnervation. Hur et al. carried out a study in which 14 patients with SCI were administered intrathecally 9 × 107 adipose MSCs [87]. Their observations showed mild progresses in neurological function. No serious adverse events were observed. In a phase 2 study, 13 patients with SCI were intravenously administered a single dose of autologous MSCs cultured in auto-serum [82]. The results of this trial revealed that SCI symptoms were considerably declined by MSC therapy, ASI, International Standards for Neurological and Functional Classification of Spinal Cord (ISCSCI-92), and Spinal Cord Independence Measure (SCIM-III) demonstrated functional improvements after MSC injection. No severe adverse effects were related to MSC administration.
Parkinson’s disease (PD) is a neurological disorder principally characterized by the deterioration of motor activities due to the impairment of the dopaminergic nigrostriatal system [142, 143]. It has been indicated that MSCs improved the symptoms of PD. In a phase I controlled, randomized clinical study, patients that suffer from progressive supranuclear palsy were administered autologous BMSCs via intra-arterial injection [78]. The results of the study exhibited that autologous BMSCs are safe and reduce disease progression. Canesi et al. [79] have demonstrated that injection of MSCs into cerebral arteries of PD patients led to positive results in 17 PD participants: all treated participants were alive and motor function rating scales remained stable for at least 6 months during the 12-month follow-up period. One patient died 9 months after the injection for reasons not associated with cell infusion or to disease development.
In a study conducted by Jaillard and colleagues in 2019 [89], 31 individuals with subacute stroke were administered the intravenous injections of autologous BMSCs. The results of the trial exhibited significant improvements in motor-National Institute of the Health Stroke Scale (NIHSS) score, motor-Fugl-Meyer scores, and task-related functional MRI activity in motor cortex-4a. However, there was no remarkable progress in Barthel Index, NIHSS, and modified Rankin scores. In general, their results suggested that BMSCs improved motor recovery via sensorimotor neuroplasticity. In another study, 17 patients with subacute middle cerebral artery infarct received two million cells/kg autologous BMSCs [92]. During the follow-up process, NIHSS score, modified Rankin Scale or Barthel Index did not improve after the transplantation. Nonetheless, there was a significant improvement in absolute change in median infarct volume, but no treatment-related adverse effects were observed.
In sum, these outcomes suppose that BMSCs can safely and efficiently treat neural diseases, inhibit disease development, and considerably ameliorate the quality of life and clinical manifestations of patients. Consequently, BMSCs can become a new option for the clinical treatment of neural diseases.
Liver regeneration
The potential of BMSCs to differentiate into the endodermal lineage, such as hepatocyte‐like cells, makes them an attractive alternative for the treatment of liver diseases [144]. Some clinical studies have demonstrated the efficacy and feasibility of BMSC therapy in patients with liver diseases. The effect of BMSCs has been studied in individuals suffering from liver cirrhosis by Suk et al. [98]. Seventy-two patients were enrolled in this trial and randomly classified into three groups: one control group and two autologous BMSC groups that received one-time or two-time hepatic arterial administrations of fifty million autologous BMSCs 30 days after BM aspiration. Fibrosis quantification exhibited that in one-time and two-time BMSC groups there are a reduction of 25% and 37% in the proportion of collagen, respectively. In addition, the Child–Pugh (CP) scores of both test groups were meaningfully improved following BMSC administration in comparison with the control group. No serious adverse events were associated with MSC injection during the 12-month follow-up. Wang and coworkers have found that intravenous injection of UC-MSCs (0.5 × 106 cells/kg) is feasible and well tolerated in patients with primary biliary cirrhosis (PBC) [93]. They exhibited that MSCs significantly decreased the level of ALP and GGT; however, there were no considerable changes in serum AST, ALT, total bilirubin, albumin, prothrombin time activity, or immunoglobulin M levels. Similarly, Zhang et al. [94] have demonstrated that intravenous administration of 1.0 × 106 cells/kg UC-MSCs is safe and efficient for patients with ischemic-type biliary lesions after liver transplantation. According to their results, MSCs therapy reduced the serum ALP, GGT, and total bilirubin. In a randomized placebo-controlled phase I–II single-center study, nine patients that suffer from acute-on-chronic liver failure (ACLF) grades 2 and 3 were enrolled [95]. The experiment group (n = 4) received standard medical therapy along with five injections of 1 × 106 cells/kg of BMSC for 3 weeks. There were no transplant-related adverse events; however, one patient in the experiment group showed hypernatremia and a gastric ulcer, after the third and fifth administrations, respectively. Furthermore, MSCT revealed a considerable improvement in CP, model for end-stage liver disease (MELD), and ACLF (grade 3 to 0). Thus, MSCT is safe and viable in individuals with ACLF. In an open-label non-blinded randomized controlled study conducted by Lin et al. [96], 110 patients with hepatitis B virus (HBV)-related ACLF were enrolled in this trial. These patients were divided into two groups: control group (N = 54) was treated with standard medical therapy only and the intervention group (N = 56) was injected four times with 1.0–10 × 105 cells/kg allogeneic BMSCs, and then followed up for 6 months. There were no serious adverse events associated with transplantation. The results of that study demonstrated that MSCT significantly improved clinical laboratory measurements, such as serum total bilirubin, and MELD scores in comparison with control group. In addition, mortality from multiple organ failure and prevalence rate of serious infection in the intervention group was lower than that in the control group. Their results clearly established the safety and feasibility of the clinical use of peripheral administration of allogeneic BMSCs for subjects with HBV-associated ACLF, and markedly enhanced the survival rate through enhancing liver function and reducing the prevalence of severe infections.
In summary, MSCT can meaningfully ameliorate the clinical manifestations of these patients, reduce the liver fibrosis, and inhibit the development of disease.
Kidney regeneration
Hurt to renal cells can occur because of a wide range of ischemic and toxic insults and results in inflammation and cell death, which can lead to kidney damage. Inflammation has a significant role in the damage of renal cells, as well as following cellular regeneration processes [3, 145]. Various investigations have consistently demonstrated a supportive effect of MSC on acute and chronic renal injury [146]. Makhlough et al. declared that intravenous administration of 1–2 × 106 cells/kg into seven patients with chronic kidney disease failed to induce remission [101]. They indicated that variations in estimated glomerular filtration rate (eGFR) and serum creatinine during the 18-month follow-up were not statistically significant. Nonetheless, no severe adverse events were reported, and they could not assess the efficacy because of their study design. Authors postulated that limited sample size and lack of a control group led to the lack of success. A study conducted by Swaminathan et al. in 2021, has displayed the effect of allogeneic BMSCs in acute kidney injury patients. They have shown that treatment of MSCs with SBI-101 stimulated an immunotherapeutic response that initiated an enhanced phenotypic alteration from tissue injury to tissue repair [102]. In a single-arm phase I clinical trial carried out by Makhlough et al. [100], six patients with autosomal dominant polycystic kidney disease (ADPKD) were intravenously injected 2 × 106 cells/kg autologous BMSCs. The results of the study showed that the mean eGFR value declined and the level of serum creatinine enhanced during the 1-year follow-up. Moreover, no remarkable modifications in renal function parameters and blood pressure were observed during the year after intervention. However, there were no severe adverse events after 1-year follow-up. In addition, the authors indicated that there are some reasons for the lack of success, including small number of patients, absence of a comparison group, limited follow-up period, single dose administration, and they did not utilize htTKV as a surrogate endpoint. Abumoawad and colleagues have established that adipose MSCs enhanced blood flow, GFR and reduced inflammatory injury in poststenotic kidneys of individuals that suffer from atherosclerotic renovascular disease (ARVD) [99]. Their results illustrated that mean renal blood flow was considerably enhanced, and hypoxia, renal vein inflammatory cytokines, and angiogenic factors were considerably attenuated.
Heart regeneration
Heart disease is the first and most frequently diagnosed disease and the leading cause of disease death [147]. When cardiomyocytes are damaged via ischemic and other factors, the remaining viable cardiomyocytes have a restricted ability to proliferate and dead cardiomyocytes are changed by non-contractile fibrous tissue, leading to functional impairment that elicits the progression of heart failure. According to the developing number of patients with heart disease, there is a vital need to expand an innovative remedy to rescue deteriorating hearts. Regenerative medicine and cell therapy are the upcoming therapeutic opportunities for heart diseases. According to the literature, the transplantation of BM-derived cells and cardiac stem cells into deteriorating hearts appeared to provide functional benefits [148, 149].
In a study by Yagyu et al. [110], 8 individuals with symptomatic heart failure were infused with BMSCs. During the follow-up period, no serious adverse events were observed. There were no major differences in B-type natriuretic peptide, left ventricular ejection fraction (LVEF), and peak oxygen uptake at 2 months. The results of this study recommend further research regarding the feasibility and efficacy of MSCs. In a study by Gao et al. [107], 116 patients with acute myocardial infarction randomly received an intracoronary injection of WJ-MSCs. They indicated that MSCs therapy elevated the myocardial viability and perfusion within the infarcted territory. In addition, the LVEF was elevated and LV end-systolic volumes and end-diastolic volumes were decreased in the WJ-MSCs group.
Chan et al. demonstrated that intramyocardial infusion of autologous BMSCs in conjunction with transmyocardial revascularization or coronary artery bypass graft surgery was technically feasible and could be performed safely. The results showed that regional contractility in the cell-treated regions improved during the 1-year follow-up; also, the quality of life was improved along with a substantial decrease in angina scores at 12 month post-treatment [104]. In a study by Kaushal et al. [113], 12 participants with hypoplastic left heart syndrome were transplanted with allogeneic human MSCs (2.5 × 105 cells/kg). This study determined the safety, feasibility, and usefulness of MSC administration into the left ventricular myocardium. No serious adverse effects were reported during the trial. Mathiasen et al. observed that after BM-MSCT, left ventricular end-systolic volume was significantly reduced, also LVEF, stroke volume, and myocardial mass remarkably improved [103]. In addition, a major decrease in the amount of scar tissue and quality of life score was observed. No side effects were identified. In a randomized, double-blind, placebo-controlled, multicenter, phase II study, 100 patients with anterior ST elevation myocardial infarction received autologous BMSCs and atorvastatin (ATV) treatment. The results of that study represented the absolute change of LEVF within 12 months, improvement in cardiac function, induction of remodeling and regeneration, and improvement in quality of life [108]. Recently, Celis-Ruiz and coworkers conducted a study in which intravenous administration of adipose MSCs within the first 2 weeks of ischemic stroke onset is safe at 24 months of follow-up [106]. In a study conducted by Hare et al. [112], 37 non-ischemic dilated cardiomyopathy patients were divided into two groups and received 10 × 107 allogeneic and autologous BMSCs. Minnesota Living with Heart Failure Questionnaire score decreased in both groups. The major adverse cardiac event rate was lower in allo vs. auto. Also, TNF-α decreased, to a greater extent in allo vs. auto at 6 months. These results suggested the clinically meaningful efficacy of allogeneic vs. autologous BMSCs in non-ischemic dilated cardiomyopathy patients. Qayyum et al. have found that intra‑myocardial injections of autologous adipose MSCs ameliorated cardiac functions and unchanged exercise capacity, in contrast to deterioration in the placebo group [115].
Levy et al. indicated that after allogeneic BMSCs in patients with chronic stroke, Barthel Index scores increased. Moreover, electrocardiograms, laboratory tests, and computed tomography scans of chest/abdomen/pelvis suggest that BMSCs could alleviate the clinical symptoms in patients with stroke [90].
In sum, BMSC therapy can be an effective, achievable, and safe process that remarkably improves cardiac function and promotes patients’ quality of life.
Bone regeneration
Bone regeneration is a hot topic of research in clinical studies. Bone regeneration is a crucial problem in numerous cases, including bone fracture, defect, osteoarthritis, and osteoporosis, which should be resolved [150,151,152]. Autogenous bone grafts are considered the standard approach for bone formation by means of the participants’ own cells that stimulate osteoinductive, bone conductivity, and histocompatibility in bone diseases [153]. Nevertheless, there are some shortcomings of this procedure such as unpredictable absorption, extended recovery time, and patients commonly experience pain and nerve injury at the harvest area [154,155,156]. With the development of understanding bone tissue biology as well as recent approaches in the improvement in tissue regeneration, the application of MSC has become an attractive subject in augmenting bone tissue forming [157, 158].
In a pilot study by Jayankura and coworkers, allogeneic BMSCs were applied to treat 22 participants with bone fractures [128]. All participants received percutaneous implantation of autologous BMSCs (5 to 10 × 107 cells) into the fracture area. After intervention, Tomographic Union Score (TUS) and Global Disease Evaluation (GDE) score were improved, and pain at palpation at the fracture site was reduced. In addition, the ratio of blood samples comprising donor-specific anti-HLA antibodies enhanced at 6 months post-intervention. Three serious cell-related adverse events were reported. In another study by Shim and coworkers [129], intramedullary (4 × 107 cells) and intravenous (2 × 108 cells) infusion of WJ-MSCs in combination with teriparatide showed beneficial results in individuals with osteoporotic vertebral compression fractures. Their observation displayed that the mean visual analog scale, Oswestry Disability Index, and Short Form-36 scores meaningfully improved. They stated that WJ-MSCs in combination with teriparatide are viable and have a clinical profit for fracture healing by stimulating bone architecture.
Several studies investigated the effect of BMSCs in osteoarthritis (OA) patients. Chahal et al. carried out a clinical phase I/IIa trial that involved 12 individuals with late-stage Kellgren–Lawrence knee OA. These 12 patients were injected with a single intra-articular of 1 × 106, 10 × 106, and 50 × 106 BMSCs. The results showed that patients had improved Knee Injury and Osteoarthritis Outcome Score (KOOS) pain, symptoms, quality of life, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness relative to baseline. Moreover, cartilage catabolic biomarkers and MRI synovitis were meaningfully lower at higher doses and the levels of pro-inflammatory monocytes/macrophages and IL-2 reduced in the synovial fluid after intervention. No serious events had occurred [116]. Dilogo et al. have reported that UC-MSCs (10 × 106 cells) significantly decreased the WOMAC and could be a potentially new regenerative treatment for patients with knee OA [127]. In a study conducted by Hernigou et al. [117], 140 patients with OA received a subchondral infusion of BMSCs on one side and received total knee arthroplasty (TKA) on the contralateral knee. They demonstrated that subchondral MSCs had a significant effect on pain to postpone or avoid the TKA in the contralateral joint of patients with OA. In a phase II multicenter randomized controlled clinical trial, 60 OA patients received 10 × 107 cells of autologous BMSCs along with platelet-rich plasma and followed up for 12 months [119]. No serious adverse effects were observed after MSCs injection or during follow‑up. According to the observations, treatment with BMSC related to platelet-rich plasma was demonstrated to be a feasible alternative treatment for individuals with OA, along with clinical development at the end of follow-up. Similarly, Bastos et al. have reported that MSCs alone or in combination with platelet-rich plasma are safe and have an advantageous effect on symptoms in OA individuals [121]. They found that MSCs group and MSCs + platelet-rich plasma group can improve the pain, function and daily living activities, and quality of life subscales. Ten adverse events were reported in three participants in the MSCs group and in two of the MSCs + platelet-rich plasma group. PERS and colleagues reported another clinical phase Ia study that involved 19 individuals suffering from knee OA [123]. These 18 individuals were classified into three groups and received a single intra-articular administration of 2 × 106, 10 × 106, and 50 × 106 adipose MSCs. According to their results, individuals had experienced significant improvement in pain levels and function. There were no severe adverse events; however, 4 individuals experienced transient knee joint pain and swelling after local administration. In a long-term follow-up of a multicenter randomized controlled clinical trial by Espinosa et al. [120], 30 OA patients were administered the intra-articular infusion of two diverse doses of autologous BMSCs cells (10 × 106 or 10 × 107) versus hyaluronic acid in the treatment of OA. No adverse effects occurred after MSCT or during the 4-year follow‑up. Their results showed that intra-articular infusion of BMSCs together with hyaluronic acid is a safe and viable process that leads to a clinical and functional improvement in knee OA.
Overall, these data display that BMSCs can be a promising, safe and effective alternative for bone regeneration, significantly improve the clinical manifestation of patients, and inhibit development of diseases.
Wound regeneration
The skin has several layers along with different compounds and roles that work together to support internal organs and serve various biological roles. It has three main layers, the epidermis, the dermis, and the subcutaneous layer [159]. Generally, skin wound healing, triggered by tissue injury, includes four stages: hemostasis, inflammation, proliferation, and maturation. MSCs can assist in all stages of the wound healing process. The use of MSCs for the treatment of skin can improve the regeneration of skin and reduce scarring. MSCs exert their functions through migration into the skin damage site, suppressing inflammation, and increasing the growth and differentiation ability of fibroblasts, epidermal cells, and endothelial cells [160, 161]. As MSCs have exhibited wound healing in many preclinical studies, the application of MSCs for chronic wounds contributes to progress toward clinical trials. Falanga et al. have demonstrated that autologous BMSCs are an impressive and safe treatment method for wound healing [131]. The results of the study indicated a trend toward a reduction in ulcer size or complete wound closure by 4–5 months. No adverse events were noted. In a study by Zhou et al., 346 patients with skin wounds were administered adipose MSCs [132]. There were no adverse events during the trial. They reported that the granulation tissue coverage rate and thickness of granulation tissue were considerably ameliorated. In an open-label phase I/II study, sixteen participants with vocal fold scarring were administered a single dose of 0.5–2 × 106 cells autologous MSCs [137]. Video ratings of vocal fold vibrations and digitized analysis of high-speed laryngoscopy and phonation pressure threshold were considerably enhanced for 62–75% of the participants. Voice Handicap Index was meaningfully enhanced in eight participants, with the remaining experiencing no remarkable alteration. No serious adverse events or minor side effects were reported. Lonardi et al. observed that micro-fragmented adipose tissue improved skin tropism in patients with diabetic foot ulcer [135]. Furthermore, the results of studies have shown that adipose-derived stem cells had a beneficial effect on the full-thickness foot dorsal skin wound in diabetic mice with a considerably decreased ulcer area [162]. Recently, Huang et al. carried out a clinical study in which six subjects with intrauterine adhesion and four with cesarean scar diverticulum enrolled in this trial [136]. They found that intrauterine injection of UC-MSCs improved the endometrial thickness, cesarean scar diverticulum, and the volume of the uterus.
Conclusion
In the last decades, optimizations of isolation, culture, and differentiation procedures have permitted MSCs to improve closer to clinical uses for improving disorders and various tissue regeneration. MSCs have some important characteristics that make them preferred candidates to use for regenerative medicine: immunomodulatory capability valuable to improve immune system abnormalities, paracrine or autocrine roles that produce growth factors, and the vital potential to differentiate into various cells. Several clinical trials have reported that both autologous and allogeneic MSCs are valuable sources for tissue forming. Particularly, autologous MSCs signify the chief sources examined safe for administration and minimization of immunological threat, regardless of the lack of reported grievances concerning allogeneic MSC-based therapy. According to the studies described in this literature, administration of MSCs appear to be more effective and the usefulness of MSC therapy in bone and heart disorders has been broadly established. In terms of safety, no significant relationship was found between the MSC therapy and incidence of cancer and infection. Intravenous injection of MSCs is the most widely used form of administration and the dosage commonly fluctuates between 1 × 106 cells/kg and 2 × 108 cells/kg. According to the literature works mentioned in this review, the repeated administration of MSCs suggests being more beneficial than a single injection. In addition, the effectiveness of MSCs therapy in osteoarthritis disorder has been widely established. Long-term follow-up studies exhibited that serum tumor markers did not enhance before and 3 years after MSCs therapy. Nevertheless, there is still a lack of reliable scientific data on the mechanisms whereby the MSC therapy improves the numerous disorders that can develop the MSC modification and increase their prospective clinical application.
Availability of data and materials
Not applicable.
Abbreviations
- ALS :
-
Amyotrophic lateral sclerosis
- AIS :
-
Association impairment scale
- ALSFRS :
-
ALS functional rating scale
- ALSFRS-R :
-
ALSFRS-revised
- ACLF :
-
Acute-on-chronic liver failure
- ADPKD :
-
Autosomal dominant polycystic kidney disease
- ATV :
-
Atorvastatin
- BM :
-
Bone marrow
- BMSCs :
-
Bone marrow mesenchymal stem cells
- COX2 :
-
Cyclooxygenase 2
- CP :
-
Child–Pugh
- DCs :
-
Dendritic cells
- ESCs :
-
Embryonic stem cells
- eGFR :
-
Estimated glomerular filtration rate
- FVC :
-
Forced vital capacity
- GDE :
-
Global Disease Evaluation
- HSCs :
-
Hematopoietic stem cells
- HBV :
-
Hepatitis B virus
- HGF :
-
Hepatocyte growth factor
- IPSCs :
-
Induced pluripotent stem cells
- IDO :
-
Indoleamine 2,3-dioxygenase
- ICOSL :
-
Inducible co-stimulator ligands
- ISCSCI-92 :
-
International Standards for Neurological and Functional Classification of Spinal Cord
- KOOS :
-
Knee injury and osteoarthritis outcome score
- LVEF :
-
Left ventricular ejection fraction
- MiRNAs :
-
MicroRNAs
- MSCs:
-
Mesenchymal stem cells
- MSCT:
-
Mesenchymal stem cells transplantation
- MELD:
-
Model for end-stage liver disease
- NO:
-
Nitric oxide
- OA:
-
Osteoarthritis
- PSCs:
-
Pluripotent stem cells
- PGE2:
-
Prostaglandin E2
- SCI:
-
Spinal cord injury
- SSEP:
-
Somatosensory evoked potentials
- SCIM-III:
-
Spinal cord independence measure
- Tregs:
-
Regulatory T cells
- TUS:
-
Tomographic Union Score
- TKA:
-
Total knee arthroplasty
- WSs:
-
Weakness scales
- WOMAC:
-
Western Ontario and McMaster Universities Osteoarthritis Index
References
Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63–80.
Swart JF, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol. 2017;13(4):244–56.
Abbaszadeh H, et al. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: a new horizon of stem cell therapy. J Cell Physiol. 2020;235(12):9230–40.
Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019;6:34.
Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013: 496218.
Rao M. Stem cells and regenerative medicine. Stem Cell Res Ther. 2012;3(4):27.
Ilic D, Ogilvie C. Concise Review: Human Embryonic Stem Cells-What Have We Done? What Are We Doing? Where Are We Going? Stem Cells. 2017;35(1):17–25.
Zakrzewski W, et al. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68.
Chen Y, et al. Dental-derived mesenchymal stem cell sheets: a prospective tissue engineering for regenerative medicine. Stem Cell Res Ther. 2022;13(1):38.
Kanemura H, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS ONE. 2014;9(1):e85336–e85336.
Souied E, Pulido J, Staurenghi G. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;377(8):792–3.
Rong X, et al. Antler stem cell-conditioned medium stimulates regenerative wound healing in rats. Stem Cell Res Ther. 2019;10(1):326.
Hong H, et al. Dental follicle stem cells rescue the regenerative capacity of inflamed rat dental pulp through a paracrine pathway. Stem Cell Res Ther. 2020;11(1):333.
Chimutengwende-Gordon M, Khan WS. Advances in the use of stem cells and tissue engineering applications in bone repair. Curr Stem Cell Res Ther. 2012;7(2):122–6.
Yu Y, et al. Human embryonic stem cell-derived cardiomyocyte therapy in mouse permanent ischemia and ischemia-reperfusion models. Stem Cell Res Ther. 2019;10(1):167.
Jin L, et al. Mesenchymal stem cells ameliorate myocardial fibrosis in diabetic cardiomyopathy via the secretion of prostaglandin E2. Stem Cell Res Ther. 2020;11(1):122.
Chugh RM, et al. Mesenchymal stem cell therapy ameliorates metabolic dysfunction and restores fertility in a PCOS mouse model through interleukin-10. Stem Cell Res Ther. 2021;12(1):388.
Saldaña L, et al. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther. 2019;10(1):58.
Friedenstein AJ, Piatetzky S II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–90.
Abbaszadeh H, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: a novel therapeutic paradigm. J Cell Physiol. 2020;235(2):706–17.
Chang D, et al. Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res Ther. 2021;12(1):384.
Horwitz EM, et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393–5.
Caplan AI. What’s in a name? Tissue Eng Part A. 2010;16(8):2415–7.
Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445–51.
Lee BC, et al. PGE2 maintains self-renewal of human adult stem cells via EP2-mediated autocrine signaling and its production is regulated by cell-to-cell contact. Sci Rep. 2016;6:26298.
Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9.
Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14.
Meirelles LDS, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–13.
Ghorbani F, et al. Renoprotective effects of extracellular vesicles: a systematic review. Gene Reports. 2022;26: 101491.
Tang Y, Zhou Y, Li H-J. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12(1):71.
Wu Y, et al. Adipose tissue-derived mesenchymal stem cells have a heterogenic cytokine secretion profile. Stem Cells Int. 2017;2017:4960831.
Mushahary D, et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93(1):19–31.
Barberini DJ, et al. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res Ther. 2014;5(1):25.
Abbaszadeh H, et al. Chronic obstructive pulmonary disease and asthma: mesenchymal stem cells and their extracellular vesicles as potential therapeutic tools. Stem Cell Res Ther. 2022;13(1):262.
Jiang XX, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6.
Marinescu C-I, Preda MB, Burlacu A. A procedure for in vitro evaluation of the immunosuppressive effect of mouse mesenchymal stem cells on activated T cell proliferation. Stem Cell Res Ther. 2021;12(1):319.
Malekpour K, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Reports. 2022;18(3):933–51.
Steens J, Klein D. Current strategies to generate human mesenchymal stem cells in vitro. Stem Cells Int. 2018;2018:6726185.
Beeravolu N, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;122:e55224.
Hmadcha A, et al. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol. 2020;8:43.
Aravindhan S, et al. Mesenchymal stem cells and cancer therapy: insights into targeting the tumour vasculature. Cancer Cell Int. 2021;21(1):158.
Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8.
Djouad F, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–44.
Swartzlander MD, et al. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials. 2015;41:79–88.
Rigotti G, et al. Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36(3):261–70.
Djouad F, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen-induced arthritis. Arthritis Rheum. 2005;52(5):1595–603.
Ge W, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant. 2009;9(8):1760–72.
Waterman RS, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4): e10088.
Miyagawa I, et al. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J Immunol. 2017;199(5):1616–25.
Lee H-J, et al. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci Rep. 2017;7(1):1–15.
Heo JS, Choi Y, Kim HO. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019;2019:7921760.
Morrison TJ, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86.
Melief SM, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31(9):1980–91.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
Beyth S, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–9.
Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72.
Glennie S, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7.
Naji A, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–48.
Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58-65.
Pourakbari R, et al. Identification of genes and miRNAs associated with angiogenesis, metastasis, and apoptosis in colorectal cancer. Gene Reports. 2020;18: 100552.
Tuli R, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278(42):41227–36.
Longobardi L, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21(4):626–36.
Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–39.
Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98(3):538–54.
Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015;30(5):580–9.
Quintanilha LF, et al. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res. 2014;44(10):E206–17.
Tang JM, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91(3):402–11.
Troncoso R, et al. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab. 2014;25(3):128–37.
Syková E, et al. Transplantation of Mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647–58.
Mazzini L, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A phase I clinical trial. Exp Neurol. 2010;223(1):229–37.
Siwek T, et al. Repeat Administration of bone marrow-derived mesenchymal stem cells for treatment of amyotrophic lateral sclerosis. Med Sci Monit. 2020;26: e927484.
Petrou P, et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73(3):337–44.
Oh KW, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6):590–7.
Staff NP, et al. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology. 2016;87(21):2230–4.
Barczewska M, et al. Umbilical cord mesenchymal stem cells in amyotrophic lateral sclerosis: an original study. Stem Cell Rev Rep. 2020;16(5):922–32.
Giordano R, et al. Autologous mesenchymal stem cell therapy for progressive supranuclear palsy: translation into a phase I controlled, randomized clinical study. J Transl Med. 2014;12:14.
Canesi M, et al. Finding a new therapeutic approach for no-option Parkinsonisms: mesenchymal stromal cells for progressive supranuclear palsy. J Transl Med. 2016;14(1):127.
Venkataramana NK, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155(2):62–70.
Zamani H, et al. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. 2022;60(1):63–70.
Honmou O, et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg. 2021;203: 106565.
Satti HS, et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. 2016;18(4):518–22.
Mendonça MV, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5(6):126.
Vaquero J, et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19(3):349–59.
Vaquero J, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20(6):806–19.
Hur JW, et al. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–64.
Albu S, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23(2):146–56.
Jaillard A, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. 2020;11(5):910–23.
Levy ML, et al. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50(10):2835–41.
Shichinohe H, et al. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): a study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017;17(1):179.
Law ZK, et al. The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: a phase 2 randomized controlled trial on safety, tolerability and efficacy. Cytotherapy. 2021;23(9):833–40.
Wang L, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28(Suppl 1):85–92.
Zhang YC, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19(2):194–9.
Schacher FC, et al. Bone marrow mesenchymal stem cells in acute-on-chronic liver failure grades 2 and 3: a phase I-II randomized clinical trial. Can J Gastroenterol Hepatol. 2021;2021:3662776.
Lin BL, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66(1):209–19.
Lanthier N, et al. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Res Ther. 2017;8(1):88.
Suk KT, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64(6):2185–97.
Abumoawad A, et al. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020;97(4):793–804.
Makhlough A, et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8(1):116.
Makhlough A, et al. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy. 2018;20(5):660–9.
Swaminathan M, et al. Pharmacological effects of ex vivo mesenchymal stem cell immunotherapy in patients with acute kidney injury and underlying systemic inflammation. Stem Cells Transl Med. 2021;10(12):1588–601.
Mathiasen AB, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22(5):884–92.
Chan JL, et al. Intramyocardial bone marrow stem cells in patients undergoing cardiac surgical revascularization. Ann Thorac Surg. 2020;109(4):1142–9.
Bolli R, et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail. 2021;23(4):661–74.
de Celis-Ruiz E, et al. Final results of allogeneic adipose tissue-derived mesenchymal stem cells in acute ischemic stroke (AMASCIS): a phase II, randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Cell Transplant. 2022;31:9636897221083864.
Gao LR, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162.
Xu JY, et al. Transplantation efficacy of autologous bone marrow mesenchymal stem cells combined with atorvastatin for acute myocardial infarction (TEAM-AMI): rationale and design of a randomized, double-blind, placebo-controlled, multi-center, Phase II TEAM-AMI trial. Regen Med. 2019;14(12):1077–87.
Bartolucci J, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121(10):1192–204.
Yagyu T, et al. Long-term results of intracardiac mesenchymal stem cell transplantation in patients with cardiomyopathy. Circ J. 2019;83(7):1590–9.
Florea V, et al. The impact of patient sex on the response to intramyocardial mesenchymal stem cell administration in patients with non-ischaemic dilated cardiomyopathy. Cardiovasc Res. 2020;116(13):2131–41.
Hare JM, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. 2017;69(5):526–37.
Kaushal S, et al. Study design and rationale for ELPIS: A phase I/IIb randomized pilot study of allogeneic human mesenchymal stem cell injection in patients with hypoplastic left heart syndrome. Am Heart J. 2017;192:48–56.
Xiao W, et al. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58(2):238–44.
Qayyum AA, et al. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J Transl Med. 2019;17(1):360.
Chahal J, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. 2019;8(8):746–57.
Hernigou P, et al. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int Orthop. 2021;45(2):365–73.
Hernigou P, et al. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop. 2021;45(2):391–9.
Lamo-Espinosa JM, et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med. 2020;18(1):356.
Lamo-Espinosa JM, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2018;16(1):213.
Bastos R, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3342–50.
Al-Najar M, et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. 2017;12(1):190.
Pers YM, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–56.
Freitag J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30.
Lee WS, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–11.
Matas J, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–24.
Dilogo IH, et al. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatol. 2020;30(5):799–807.
Jayankura M, et al. Percutaneous administration of allogeneic bone-forming cells for the treatment of delayed unions of fractures: a pilot study. Stem Cell Res Ther. 2021;12(1):363.
Shim J, et al. Safety and efficacy of Wharton’s jelly-derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: a phase I/IIa study. Stem Cells Transl Med. 2021;10(4):554–67.
Talaat WM, et al. Autologous bone marrow concentrates and concentrated growth factors accelerate bone regeneration after enucleation of mandibular pathologic lesions. J Craniofac Surg. 2018;29(4):992–7.
Falanga V, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–312.
Zhou L, et al. Efficacy of human adipose derived mesenchymal stem cells in promoting skin wound healing. J Healthc Eng. 2022;2022:6590025.
Moon KC, et al. Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes. 2019;68(4):837–46.
Qin HL, et al. Clinical evaluation of human umbilical cord mesenchymal stem cell transplantation after angioplasty for diabetic foot. Exp Clin Endocrinol Diabetes. 2016;124(8):497–503.
Lonardi R, et al. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: a randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res Ther. 2019;10(1):223.
Huang J, et al. Intrauterine infusion of clinically graded human umbilical cord-derived mesenchymal stem cells for the treatment of poor healing after uterine injury: a phase I clinical trial. Stem Cell Res Ther. 2022;13(1):85.
Hertegård S, et al. Treatment of vocal fold scarring with autologous bone marrow-derived human mesenchymal stromal cells-first phase I/II human clinical study. Stem Cell Res Ther. 2020;11(1):128.
Hardiman O, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17071.
van Es MA, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390(10107):2084–98.
Eli I, Lerner DP, Ghogawala Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39(2):471–88.
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359(9304):417–25.
Pajares M, et al. Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells. 2020;9(7):1687.
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912.
Hu C, et al. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020;11(1):377.
Baer PC, Koch B, Geiger H. Kidney Inflammation, Injury and Regeneration. Int J Mol Sci. 2020;21(3):1164.
Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014;127(1–4):75–80.
Virani SS, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143(8):e254–743.
Chien KR, et al. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019;37(3):232–7.
Murry CE, MacLellan WR. Stem cells and the heart-the road ahead. Science. 2020;367(6480):854–5.
Oryan A, Alidadi S. Reconstruction of radial bone defect in rat by calcium silicate biomaterials. Life Sci. 2018;201:45–53.
Qaseem A, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–39.
Čamernik K, et al. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res Ther. 2020;11(1):146.
Raghoebar GM, et al. Resorbable screws for fixation of autologous bone grafts. Clin Oral Implants Res. 2006;17(3):288–93.
Felice P, et al. Inlay versus onlay iliac bone grafting in atrophic posterior mandible: a prospective controlled clinical trial for the comparison of two techniques. Clin Implant Dent Relat Res. 2009;11(Suppl 1):e69-82.
Swan MC, Goodacre TE. Morbidity at the iliac crest donor site following bone grafting of the cleft alveolus. Br J Oral Maxillofac Surg. 2006;44(2):129–33.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408.
Fu J, et al. Systemic therapy of MSCs in bone regeneration: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12(1):377.
Gjerde C, et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res Ther. 2018;9(1):213.
Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20(8):847–56.
Hu MS, et al. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018;2018:6901983.
Marfia G, et al. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015;11(4):183–206.
Shi R, et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7(1):155.
Acknowledgements
The authors express their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through the Research Group Program under grant number RGP. 2/122/43.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RM, AM, AOZ, and MUH performed and wrote the manuscript; KHA, SHA, and NMH collected the references and designed the table and figures; IA, HHA, HHK, and RS modified the manuscript; and MEA, YFM, and HS designed the manuscript and approved the final manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors indicated no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Margiana, R., Markov, A., Zekiy, A.O. et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther 13, 366 (2022). https://doi.org/10.1186/s13287-022-03054-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03054-0