Abstract
Background
The slowly activated delayed rectifier potassium current (IKs) mediated by the KCNQ1 gene is one of the main currents involved in repolarization. KCNQ1 mutation can result in long-QT syndrome type 1 (LQT1). IKs does not participate in repolarization in mice; thus, no good model is currently available for research on the mechanism of and drug screening for LQT1. In this study, we established a KCNQ1-deficient human cardiomyocyte (CM) model and performed a series of microelectrode array (MEA) detection experiments on KCNQ1-mutant CMs constructed in other studies to explore the pathogenic mechanism of KCNQ1 deletion and mutation and perform drug screening.
Method
KCNQ1 was knocked out in human embryonic stem cell (hESC) H9 line using the CRISPR/cas9 system. KCNQ1-deficient and KCNQ1-mutant hESCs were differentiated into CMs through a chemically defined differentiation protocol. Subsequently, high-throughput MEA analysis and drug intervention were performed to determine the electrophysiological characteristics of KCNQ1-deficient and KCNQ1-mutant CMs.
Results
During high-throughput MEA analysis, the electric field potential and action potential durations in KCNQ1-deficient CMs were significantly longer than those in wild-type CMs. KCNQ1-deficient CMs also showed an irregular rhythm. Furthermore, KCNQ1-deficient and KCNQ1-mutant CMs showed different responses to different drug treatments, which reflected the differences in their pathogenic mechanisms.
Conclusion
We established a human CM model with KCNQ1 deficiency showing a prolonged QT interval and an irregular heart rhythm. Further, we used various drugs to treat KCNQ1-deficient and KCNQ1-mutant CMs, and the three models showed different responses to these drugs. These models can be used as important tools for studying the different pathogenic mechanisms of KCNQ1 mutation and the relationship between the genotype and phenotype of KCNQ1, thereby facilitating drug development.
Similar content being viewed by others
Introduction
Long-QT syndrome (LQTS) is a cardiogenetic disorder that can cause life-threatening arrhythmias and is associated with sudden cardiac death [1, 2]. Long-QT syndrome type 1 (LQT1) is the most prevalent subtype, accounting for approximately 40–50% of all patients with LQTS [3]. Studies have reported that LQT1 is caused by loss-of-function mutations in KCNQ1 that encodes the α subunit of the cardiac Kv7.1 potassium channel mediating the slowly activated delayed rectifier potassium current (IKs) [4,5,6,7,8,9]. To date, numerous mutations in KCNQ1 have been considered responsible for hereditary LQT1, and the type and location of KCNQ1 mutation are associated with varying clinical severities [10,11,12,13]. However, the clinical phenotype of the different mutations and the underlying mechanisms remain poorly understood.
Studies have demonstrated that mutations at the N-terminus of KCNQ1, such as Y111C, L114P, and P117L, could affect it transport [14]. Moreover, mutations on the C-loop inactivate the IKs mediated by protein kinase A (PKA), causing the inward calcium ion current to increase without confrontation during β-adrenergic stimulation [8]. These studies have provided direct evidence demonstrating that the types of mutation are differently associated with physiological activities and mechanisms; this provides the theoretical basis for drug target screening. However, most experiments have been performed almost exclusively on animal models [15, 16]. Although KCNQ1-knockout mice presented with the Jervell and Lange–Nielsen phenotype [17, 18], the mouse heart repolarization K+ current is a fast, slow-transient outward current, and delayed rectified voltage-gated K+ current, which cannot reflect the effect of KCNQ1 deficiency in humans [19]. Hence, the establishment of KCNQ1-mutant human embryonic stem cell-derived cardiomyocytes (CMs) is necessary for the mechanistic exploration and drug screening for LQT1 caused by KCNQ1 mutations.
In recent years, the establishment of disease models and drug screening using human induced pluripotent stem cell-derived CMs (hiPSC-CMs) has become a promising therapeutic approach for cardiovascular diseases [20]. In this study, we developed KCNQ1-deficient, KCNQ1L114P/+, and KCNQ1R190Q/+ human myocardial models using CRISPR/Cas9 system to investigate a well-defined genotype–phenotype correspondence. KCNQ1-deficient cells showed serious QT prolongation, irregular rhythm, early post-depolarization (EAD), and IKr current insensitivity. KCNQ1L114P/+ CMs showed a significantly longer QT delay than KCNQ1R190Q/+ CMs. Our results showed that MgCl2, propranolol, and amiodarone could reverse the abnormal phenotype caused by KCNQ1 deficiency or mutations separately. The results showed that those models can well reflect the disease phenotype and contribute to the drug screening and accurate treatment of KCNQ1 mutation-related diseases.
Methods
Cell culture and cardiac differentiation of hESC
The hESC line was purchased from Cellapy (Beijing, China) was routinely maintained in the presence of PSCeasy medium (Cellapy, China) on six-well plates (Corning, USA) coated with 5% Matrigel (Corning, USA). Medium was changed every day and passaged every 2–3 days with EDTA (Cellapy, China). The cells were grown in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. The hESCs were differentiated when they reached 70–80% confluence. Medium was changed to the basal differentiation medium. For day 0 to day 2, medium was changed to the basal medium C01 (Cellapy, China). For day 2 to day 4, medium was changed to the basal differentiational medium C02 (Cellapy, China). On day 4, medium was changed to the basal differentiational medium C03 (Cellapy, China) and changed medium every other day. Contracting cells were noted from day 9.
Genome editing
KCNQ1 single-stranded guide RNA (sgRNA) (ATGCTACACGTCGACCGCCA,CAGCCGCCCCCAGAGGCCCA,GCTCGAGGAAGTTGTAGACG) was designed using an online tool (https://www.synthego.com). We electroporated the epiCRISPR vector and sgRNA (100 µl electrotransformation solution (Cellapy, China) plus 2.5 µg KCNQ2 gRNA plasmid) into the cells using the 4D nuclear receptor system and the CA137 programme (Lonza, Germany). The transfected cells were seeded in 6-well plates and cultured overnight in PSCeasy medium 10 μM of Rho kinase inhibitor Y-27632. The medium was changed the next day. Drug (puromycin) selection was initiated after 72 h of transfection at a lower concentration of 0.1 µg/ml for the first hour and then at 0.3 µg/ml until the transfected lines were stable. The surviving cells were collected in 48-well plates and amplified for polymerase chain reaction (PCR) screening. The point mutation cells were prepared by epi-ABEmax/epi-AncBE4max/epi-ABEmax-NG/epi-AncBE4 max-NG plasmid. The plasmid was transfected using Lipofectamine 3000, and then, the transfected cells were selected by blasticidin. The specific methods can refer to the previous research.
Drug treatment
100 mM 293B, 100 µM isoproterenol (ISO), 5 µM propranolol, 100 µM amiodarone 100 µM MgCl2 (Selleck, USA) were diluted in C05 (Cellapy, China). hESC-CMs were treated with 293B, ISO, Propranolol for 12 h. hESC-CMs were treated with Amiodarone, MgCl2 for 30 min.
RNA extraction and RT-PCR
Total RNA from cells was extracted by using TRIZOL Reagent (Invitrogen, USA). An amount of 2 µg total RNA was reversed to cDNA by using the GoScript Reverse Transcription System (Promega, USA). Quantitative RT-PCR involved use of SYBR Green II (Takara, Japan) in the iQ5 system (Bio Rad, Hercules, CA). A comparative CT method was used to analyze the relative changes in gene expression. The results were expressed as relative to the data of GAPDH transcripts (internal control). Primer sequences are listed in Additional file 1: Table S1.
Immunofluorescent staining (IF) and imaging analyses
The cells were plated on 20 mm coverslips coated with 5% Matrigel and were fixed with 4% PFA for 15 min. Then, after washing with PBS three times for 5 min, the cells were permeabilized with 0.2% Triton X-100 (Sigma, USA) for 15 min and blocked with 3% BSA (Sigma, USA) for 1 h at room temperature. After that cells were incubated with primary antibodies, overnight at 4 °C. Then, cells were washed by PBS and incubated for 1 h at room temperature in the dark with secondary antibodies (Invitrogen, USA). Cells were washed again as above, mounted with Fluoroshield Mounting Medium with DAPI (4, 6 diamino-2-phenylindole). Images were taken under a Confocal Microscope (Leica DMI 4000B, German). The antibody and their appropriate dilution are provided in Additional file 1: Table S2.
Western blot (WB) analysis
Protein from hESC-CMs was extracted by using a Protein Extraction Kit (Promega, USA). The protein concentration of the supernatant was measured by BCA method. The 30 µg protein was separated on 10% SDS-PAGE and transferred to PVDF membrane at 300 mA for 90 min, which was blocked with 5% albumin bovine (BSA) at room temperature for 1 h, then incubated at 4 °C overnight with the primary antibodies, then with IR dye-conjugated secondary antibodies (LI-COR, USA) for 1 h at room temperature. GAPDH was used as an internal control. Blots were exposed and analyzed with use of an Odyssey infrared imaging system (LI-COR Biosciences, USA). The antibody and their appropriate dilution are provided in Additional file 1: Table S2.
Flow cytometry
The hESC-CMs under different treatments were singularized with CardioEasy Human Cardiomyocyte Digestive Fluid (Cellapy, China). Observe that most of the clones are detached from the bottom of the plate under the microscope, gently pipette the cells and suck them out, centrifuge, and wash three times with PBS. The cells were stained with different antibodies, filtered through the 300 mesh filter, and immediately analyzed by FACS (Beckman, USA). The cell count is generally 1–2 million. The results were analyzed with Flow Jo X program.
Microelectrode array (MEA) analysis
hESC-CMs were digested in CardioEasy Human Cardiomyocyte Digestive Fluid (Cellapy, China), after which 2 × 104 cells were plated on a microelectrode array (MEA) pre-coated with 5% Matrigel (Cellapy, China). The next day, 300 μl medium was added to each well. After the hESC-CMs resumed spontaneous beating, the experimental data were recorded on a Maestro EDGE (Axion Biosystems, Inc., Atlanta, USA) according to the MEA manual. Cardiac Analysis Tool, AxIS Navigator, AxIS data export tool, and Origin were used to analyze the data.
Statistical analysis
Results are expressed as mean ± SD. Statistical analysis was performed with GraphPad Prism 8.00 for Windows. Two-sided unpaired Student’s t test was used to compare 2 groups with normal distribution. One-way ANOVA was used to compare 3 or more groups. All tests for normality and homogeneity of variance were passed before t test and one-way analysis of variance. P values of less than 0.05 were used to denote statistical significance. *P < 0.05; **P < 0.01, ***P < 0.001; NS, not significant.
Results
Establishment of the KCNQ1-knockout and KCNQ1-mutant hESC-CM models
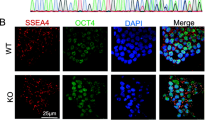
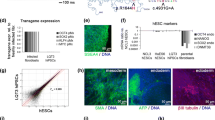
We used the CRISPR/Cas9 system to establish a KCNQ1-deficient H9 hESC cell model. We designed a highly specific sgRNA targeting KCNQ1 and electroporated hESC H9 cells with a plasmid containing sgRNA and Cas9 element. Subsequently, the transfected cells were screened using puromycin, and the genotype of the surviving clones was determined using the Sanger sequencing method. Sequencing results revealed that a homozygous clone with a 2-basepair(bp) mutation in KCNQ1 was obtained (KCNQ1R190Q/+) (Additional file 2: Fig. S1A). The pluripotency of KCNQ1R190Q/+ was identified using the appropriate markers, and the karyotype and tumorigenic characteristics of stem cells did not change (Fig. S1B–E) KCNQ1R190Q/+ and other hESCs (KCNQ1L114P/+ and KCNQ1−/−) that were established in our previous work were induced to differentiate into CMs using small molecules with clear chemical compositions (Additional file 3: Fig. S2A) [21]. Immunofluorescence staining of CMs for 30 days showed normal expression of troponin T (TNNT2) and α-actin (Additional file 3: Fig. S2B). Flow cytometry revealed that the TNNT2 positivity rate of H9 hESC wild type (WT) and KCNQ1−/− CMs was close to 86% (Additional file 3: Fig. S2C and S2D). We tested the cell viability of the four kinds of hESC-CMs by CCK8, and the results showed that there was no difference in the cell viability of the four kinds of hESC-CMs (Additional file 3: Fig. S2E). Western blot analysis confirmed the absence of the KCNQ1 protein in KCNQ1−/− CMs (Fig. 1A, B, Additional file 3: Fig. S2F). Furthermore, the expression of KCNQ1 in KCNQ1L114P/+ CMs significantly decreased compared with that in WT and KCNQ1R190Q/+ CMs, suggesting the abnormal KCNQ1 transport of KCNQ1L114P/+ CMs.
The baseline of four CMs. A, B Western blotting showed the expression of KCNQ1 in four hESC cells and quantitative analysis. C MEA recorded representative FPD waveforms on four CMs. D Representative FPD signal and quantitative analysis of four CMs. E MEA recorded representative rhythm traces on four CMs. F MEA quantitative analysis showed the proportion of EAD in four CMs. Results are presented as means ± standard error of the mean (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
KCNQ1−/−, KCNQ1L114P/+, and KCNQ1R190Q/+ CM models reflect the LQT phenotype
Differences between KCNQ1−/−, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs were observed at the multicellular level using the high-throughput Maestro Edge microelectrode array (MEA) system [22, 23]. The field potential duration (FPD) was calculated as the time between depolarization and repolarization marked by the beat time and the repolarization peak or T-wave, respectively. FPD can reflect the duration of myocardial QT interval. FPD statistics showed no difference between KCNQ1R190Q/+ and WT CMs. Moreover, the FPD of KCNQ1L114P/+ CMs was only slightly prolonged, whereas that of KCNQ1−/− CMs was significantly prolonged (Fig. 1C, D). This slight prolongation in KCNQ1L114P/+ CMs may be due to the lack of repolarization IKs caused by abnormal KCNQ1 transport, which is consistent with the decreased expression of KCNQ1 in KCNQ1L114P/+ CMs as observed through Western blot analysis. Irregular rhythm and EADs are precursors of ventricular arrhythmias in LQT; therefore, we also used MEA to analyze the rhythm of the three models. The results indicated that KCNQ1−/− CMs show obvious arrhythmia and that the proportion of EADs significantly increased compared with WT CMs. KCNQ1L114P/+ and KCNQ1R190Q/+ CMs also showed obvious arrhythmia (Fig. 1E, F).
Response to IKs-specific blocker
Moreover, we tested the effects of the IKs-specific blocker chromanol 293B on the FPD of the three models [24]. We used the ratio of FPD after dosing to that at baseline (FDP'/FDP) to indicate the extent of FPD change. A value of > 1 indicates that the FPD is prolonged, whereas a value of < 1 indicates a shortened FPD. Interestingly, the FPD of WT, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs showed prolongation after treatment with 100-mM 293B. In contrast, the FPD of KCNQ1−/− CMs did not show significant prolongation as that of KCNQ1L114P/+ and KCNQ1R190Q/+ CMs after treatment with 293B (Fig. 2A, B). Meanwhile, the FDP'/FDP of WT, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs was significantly higher than that of KCNQ1−/− CMs; however, the FPD prolongation of the knockout CMs is the least obvious (Fig. 2C). These results indicate that the KCNQ1 mutation and knockout models were successfully established.
Four CMs in response to 293B. A, B Representative FPD and quantitative analysis of four CMs after treatment with 100-mΜ 293B. C Ratio of the FDP to baseline of four CMs after 293B treatment. Results are presented as means ± standard error of the mean (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Responses to MgCl2
Mg2+ is the main coenzyme for potassium ion transfer inside and outside a cell. Mg2+ supplementation can increase potassium ion transport, increase the intracellular potassium concentration, and increase stability of cell membrane and electrocardiogram. Therefore, we observed changes in the FPD of the four CMs after MgCl2 treatment. MgCl2 treatment can shorten the FPD of all three models, with the FPD shortening of KCNQ1−/− CMs being the most significant (Fig. 3A–C). This suggests that magnesium supplementation is essential for LQT treatment with different mechanisms. However, EAD cannot be eliminated (Fig. 3D, E).
Four CMs in response to MgCl2. A, B Representative FPD and quantitative analysis of four CMs after treatment with 1-mM MgCl2. C Ratio of the FDP to baseline of four CMs after MgCl2 treatment. D Representative traces of EAD in the FPD of four CMs after treatment with MgCl2. E Quantitative analysis showed the proportion of EAD in four CMs after treatment with MgCl2. Results are presented as means ± standard error of the mean (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Responses to isoproterenol
The occurrence of LQT1 is often associated with sympathetic nerve excitement (such as due to exercise or emotional agitation); therefore, sympathetic nerve excitement was simulated using isoproterenol (ISO) treatment [25]. ISO is a β-agonist that binds to β-AR and activates cAMP–PKA-dependent downstream signals, which then promotes the phosphorylation of several target proteins, including the L-type calcium channel and lysine receptor. Subsequently, PKA phosphorylates these proteins and increases the calcium concentration in the sarcoplasm, resulting in the activation of the crossbridge and further enhancement of the contraction of CMs [26]. After treatment with 100-μM ISO, the FPD of WT, KCNQ1L114P/+, and KCNQ1−/− CMs was significantly shortened compared with the baseline (Fig. 4A–C). This phenotypic change was caused by the agonistic effect of ISO. However, the FPD of KCNQ1R190Q/+ CMs did not appear to be significantly shortened, suggesting that KCNQ1R190Q/+ CMs are not sensitive to ISO. This is consistent with the results of previous studies demonstrating that mutations in the C-loop, such as those in KCNQ1R190Q/+ CMs, may indeed inactivate Kv7.1’s response to PKA stimulation [12]. Furthermore, WT CMs did not show arrhythmia after ISO treatment, whereas KCNQ1L114P/+ CMs and KCNQ1R190Q/+ CMs both showed arrhythmia aggravation (Fig. 4D, E). Both point mutation cells showed the arrhythmia phenotype under β-adrenergic stimulation, which was consistent with the phenotype where LQT1 was more easily induced under sympathetic excitation; this indicates that the point mutation model can reflect the response of the myocardium to sympathetic excitation.
Four CMs in response to ISO. A, B Representative FPD and quantitative analysis of four CMs after treatment with 100-uM ISO. C Ratio of the FDP to baseline of four CMs after ISO treatment. D Representative traces of EAD in the FPD of four CMs after treatment with ISO. E Quantitative analysis showed the proportion of EAD in four CMs after treatment with ISO. Results are presented as means ± standard error of the mean (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Responses to propranolol
β-blockers can directly decrease β-adrenergic signaling and show antiarrhythmic effects [26]. Propranolol, a β-blocker, is one of the most common drugs used in the clinical treatment of LQT. Therefore, we subjected the CM models to this drug and examined the responses. The results showed that the three CM models showed prolongation of the FPD (Fig. 5A–C). Moreover, the slowing of the heart rhythm and arrhythmia in KCNQ1−/−, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs was significantly decreased compared with the baseline values (Fig. 5D, E). Propranolol can improve the arrhythmia phenotype of the three KCNQ1-mutant myocardial models, including KCNQ1−/− CMs, indicating that propranolol has therapeutic effects on LQT1 through multiple mechanisms.
Four CMs in response to propranolol. A, B Representative FPD and quantitative analysis of four CMs after treatment with 5-uM propranolol. C Ratio of the FDP to baseline of four CMs after propranolol treatment. D Representative traces of EAD in the FPD of four CMs after treatment with propranolol. E Quantitative analysis showed the proportion of EAD in four CMs after treatment with propranolol. Results are presented as means ± standard error of the mean (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Responses to amiodarone
We also tested the response of the three models to other common LQT medications used clinically. Amiodarone is a type III multi-ion channel blocker that can selectively prolong the repolarization time of the myocardium and is suitable for various ventricular arrhythmias [27]. After treatment with 100-μM amiodarone, WT, KCNQ1L114P/+, KCNQ1R190Q/+, and KCNQ1−/− CMs showed significant FPD prolongation; however, KCNQ1−/− CMs had the smallest FPD extension (Fig. 6A–C). Amiodarone alleviated the arrhythmia phenotype of KCNQ1L114P/+ CMs but weakened the pulsation of KCNQ1−/− CMs (Fig. 6D, E). These results suggest that amiodarone has a good therapeutic effect on LQT1 with different mutations; however, it is not suitable for treating patients with KCNQ1 large fragment deletion.
Four CMs in response to Amiodarone. A, B Representative FPD and quantitative analysis of four CMs after treatment with 100-uM amiodarone. C Ratio of the FDP to baseline of four CMs after amiodarone treatment. D Representative traces of EAD in the FPD of four CMs after treatment with amiodarone. E Quantitative analysis showed the proportion of EAD in four CMs after treatment with amiodarone. Results are presented as means ± standard error of the mean (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Discussion
KCNQ1 mutation is strongly correlated with LQT1. In this study, we used MEA to investigate the response of different genotypes of KCNQ1 mutations to different drugs to determine the underlying mechanisms of different KCNQ1 mutations. Our results indicated that KCNQ1-deficient and KCNQ1-mutant cells showed varying serious QT prolongation and irregular rhythm and that these can be corrected using IKs-specific, β, and multi-ion channel blockers. These results suggest that the novel hiPSC-CM models are helpful tools in the determination of pathogenic mechanisms and drug screening for LQT1 induced by KCNQ1 mutations.
Congenital LQTS is a life-threatening arrhythmic syndrome and is the leading cause of sudden death among young people [28]. The typical characteristics of LQTS are prolongation of the QT interval on electrocardiography and the presence of syncope or cardiac arrest mainly due to emotional or physical stress. The three main genotypes of LQTS—LQT1, LQT2, and LQT3—account for 80–90% of all 15 gene mutations found in patients with LQTS [14]. As the main genetic genotype of LQT, LQT1 is caused by mutations in the slow potassium (K+ outward current channel encoded by KCNQ1 [29]). KCNQ1 encodes the α-subunit of the K+ channel Kv7.1, producing a depolarized IKs current that increases through sympathetic activation and is critical for QT adaptation as the heart rate increases [30]. When IKs is defective, the QT interval cannot be appropriately shortened during tachycardia, resulting in high-grade arrhythmia. Homozygous mutations or compound heterozygous mutations in KCNQ1 can lead to Jervell and Lange–Nielsen syndrome, which is characterized by decreased inner ear IKs and deafness [31]. There are more than 100 pathogenic heterozygous mutations in KCNQ1, each with a different effect on the polymeric K+ channel. Mutant and WT protein subunits may assemble together and have a significant negative effect on the current. Alternatively, certain mutant subunits may fail to co-assemble with the WT peptide, resulting in the loss of function that reduces IKs by ≤ 50% (haploinsufficiency). The latter may also be due to mutations interfering with intracellular subunit transport, preventing the mutant peptide from reaching the cell membrane.
The complex mutation mechanism increases the difficulty of providing precise treatment for LQT1; therefore, establishing an effective model is essential to enable mechanism exploration and drug screening. Due to the huge difference in the cardiac functions of mice and humans, IKs does not participate in repolarization in mice, making it difficult to use mice models. HiPSC-CMs can simulate human cardiac action potential, which is a good application prospect in disease modeling. Models constructed using patient-derived hiPSC-CMs have been shown to be effective [32]. However, patient-derived hiPSC-CMs cannot accurately reflect the gene–phenotype relationship due to the influence of background genes. Moreover, obtaining patient-derived cardiac muscles is difficult. Therefore, the use of gene editing to artificially prepare myocardium with point mutation for disease simulation has good application prospects. In previous studies, our research group constructed KCNQ1L114P/+ and KCNQ1R190Q/+ models using BaseEditor’s method.
KCNQ1L114P/+ and KCNQ1R190Q/+ are serious pathogenic mutations in the LQT1 phenotypes. The pathogenic mechanism of LQT1 caused by KCNQ1 mutation mainly includes the following categories: defects in ion permeation, channel gating, trafficking defect, KCNQ1–KCNE1 interaction, PKA-mediated signaling pathway, PIP2 binding, and calmodulin binding [33]. KCNQ1L114P/+ is a mutation at the N-terminus of KCNQ1 that affects the upper membrane transport of KCNQ1 [21], whereas KCNQ1R190Q/+ is a mutation located on the C-ring of KCNQ1. Studies have proven that such mutations may reduce the sensitivity of KCNQ1 to PKA, leading to the inability of IKs to significantly increase when stimulated by adrenaline, thus inducing arrhythmias. The electrophysiological phenotypes of the two models were preliminarily detected and found to be able to reflect the disease phenotypes. Furthermore, due to the diverse pathogenic mechanisms of KCNQ1, there is no effective human KCNQ1 deletion model to clarify the direct phenotype of KCNQ1. Large fragment KCNQ1 deletion cases have been reported, and the development of a KCNQ1 deletion model helps improve the treatment of this disease. Even though KCNQ1-knockout mice were present and seen to exhibit the Jervell and Lange–Nielsen syndrome, the QT prolongation phenotype was thought to be mediated by extracardial factors because IKs did not participate in the mouse repolarization process [12]. Therefore, we developed an hESC-CM model without KCNQ1.
In this study, the MEA results showed that the FPD of KCNQ1−/− CMs was significantly prolonged compared with that of WT CMs, indicating that the absence of IKs prolonged the repolarization time course of CMs. The FPD of KCNQ1L114P/+ CMs was prolonged, whereas that of KCNQ1R190Q/+ CMs was not different from that of WT CMs. This suggests that the L114P mutation decreases the upper membrane of KCNQ1, whereas R190Q does not cause QT prolongation at baseline. Furthermore, KCNQ1−/− CMs showed arrhythmias even at baseline, suggesting that the complete loss of IKs has a severe effect on myocardial action potentials. When treated with 293B, the FPD of KCNQ1−/− CMs was not significantly prolonged, whereas that of KCNQ1L114P/+ and KCNQ1R190Q/+ CMs was, indicating that this insensitivity was due to the deletion of Kv7.1 channel encoded by KCNQ1. The baseline phenotype initially reflected the success of model construction and the phenotype of the disease. A schematic of the mechanism can be seen in Fig. 7.
LQT1 often occurs during exercise and emotional arousal; therefore, we used ISO to simulate sympathetic excitation. After ISO treatment, WT, KCNQ1−/−, and KCNQ1L114P/+ CMs showed significant shortening of the FPD, indicating that they responded to ISO’s excitement. However, KCNQ1R190Q/+ CMs showed insensitivity to ISO stimulation, confirming that this mutation indeed causes the passivation of Kv7.1 channel to PKA stimulation. Under ISO stimulation, all models showed aggravation of the arrhythmia phenotype, partially simulated LQT1 caused by KCNQ1 mutation under sympathetic excitation. Subsequently, we used common clinical LQT drug treatments to explore the response of different mutations to different drugs. The use of propranolol significantly prolonged the FPD of the three types of CMs and maintained cardiac rhythm stability in KCNQ1−/−, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs, indicating that β-blockers have good therapeutic effects on LQT with different mechanisms. Amiodarone has a greater prolongation effect on the FPD in the three models; however, amiodarone treatment may result in reduced pulsation of KCNQ1−/− CMs, suggesting that it is not be suitable for treating patients with large fragment KCNQ1 deletion. MgCl2 treatment can reduce the FPD of the three models and has a good effect on heart rhythm stability; this reflects the importance of magnesium supplementation for patients under treatment for LQT.
This study established a hESC-CM model of KCNQ1 deletion, clarified the relationship between the genotype and phenotype of KCNQ1, and bridged the gap in terms of a human model of KCNQ1 deletion. Moreover, KCNQ1−/−, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs showed different responses to different drug interventions, and their phenotypic changes were consistent with the mechanisms proposed in previous studies, indicating that the artificial absence and pitting myocardial model can reflect the LQT phenotype. As a good disease model, it shows great potential for application in precision treatment and drug screening. However, because the phenotype of hESC-CMs is closer to immature myocardium, there are some differences between hESC-CMs and adult myocardium in electrophysiological phenotype. This difference makes the hESC-CMs model unable to fully simulate the pathological phenotype and drug response of human myocardium. At the same time, we use the 2D myocardial differentiation method in this study, and the complete heart structure is not constructed, which may also lead to the difference between the drug response of this model and that under normal physiological conditions. These problems limit the application of the model in the clinical field. In the follow-up study, we will consider constructing engineered myocardial tissue (EHT) model to better approach the physiological phenotype.
Conclusion
In this study, we developed a KCNQ1 defect model using the CRISPR/Cas9 system. Simultaneous electrophysiological detection was performed on KCNQ1−/−, KCNQ1L114P/+, and KCNQ1R190Q/+ CMs. The KCNQ1−/− CM model showed significant QT interval prolongation, arrhythmia, and sensitivity to other ion channel blockers. This model can be used as an important tool to improve our understanding of the basic pathological mechanism of KCNQ1 dysfunction, define the genotype–phenotype correspondence, and promote drug development. Furthermore, under different intervention conditions, the phenotypes of KCNQ1L114P/+ and KCNQ1R190Q/+ CMs showed different responses, suggesting that 114 and 190 have different pathogenic mechanisms. It provides an effective model for studying the different pathogenic mechanisms of LQT and confirms the feasibility of preparing a single-gene genetic disease model through gene editing.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- IKs:
-
Delayed-rectifier potassium current
- hESC:
-
Human embryonic stem cell
- LQTS:
-
Long-QT syndrome
- MEA:
-
Microelectrode array
- CM:
-
Cardiomyocyte
- hiPSC-CMs:
-
Human induced pluripotent stem cell-derived CMs
- EAD:
-
Early post-depolarization
- sgRNA:
-
Single-stranded guide RNA
- ISO:
-
Isoproterenol
- FPD:
-
Field potential duration
- WT:
-
Wild type
References
Schwartz PJ, Ackerman MJ, Antzelevitch C, Bezzina CR, Borggrefe M, Cuneo BF, et al. Inherited cardiac arrhythmias. Nat Rev Dis Primers. 2020;6(1):58.
Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120(18):1761–7.
Schwartz PJ, Crotti L, Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5(4):868–77.
Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12(1):17–23.
Crotti L, Odening KE, Sanguinetti MC. Heritable arrhythmias associated with abnormal function of cardiac potassium channels. Cardiovasc Res. 2020;116(9):1542–56.
Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384(6604):78–80.
Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–3.
Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67(6):915–28.
Sun J, MacKinnon R. Cryo-EM structure of a KCNQ1/CaM complex reveals insights into congenital long QT syndrome. Cell. 2017;169(6):1042-50 e9.
Moss AJ, Shimizu W, Wilde AA, Towbin JA, Zareba W, Robinson JL, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115(19):2481–9.
Kapa S, Tester DJ, Salisbury BA, Harris-Kerr C, Pungliya MS, Alders M, et al. Genetic testing for long-QT syndrome: distinguishing pathogenic mutations from benign variants. Circulation. 2009;120(18):1752–60.
Barsheshet A, Goldenberg I, O-Uchi J, Moss AJ, Jons C, Shimizu W, et al. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to beta-blocker therapy in type 1 long-QT syndrome. Circulation. 2012;125(16):1988–96.
Schwartz PJ, Moreno C, Kotta MC, Pedrazzini M, Crotti L, Dagradi F, et al. Mutation location and IKs regulation in the arrhythmic risk of long QT syndrome type 1: the importance of the KCNQ1 S6 region. Eur Heart J. 2021;42(46):4743–55.
Dahimene S, Alcolea S, Naud P, Jourdon P, Escande D, Brasseur R, et al. The N-terminal juxtamembranous domain of KCNQ1 is critical for channel surface expression: implications in the Romano-Ward LQT1 syndrome. Circ Res. 2006;99(10):1076–83.
Casimiro MC, Knollmann BC, Ebert SN, Vary JC Jr., Greene AE, Franz MR, et al. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci USA. 2001;98(5):2526–31.
Casimiro MC, Knollmann BC, Yamoah EN, Nie L, Vary JC Jr., Sirenko SG, et al. Targeted point mutagenesis of mouse Kcnq1: phenotypic analysis of mice with point mutations that cause Romano-Ward syndrome in humans. Genomics. 2004;84(3):555–64.
Ergul Y, Kafali HC, Cilsal E, Yukcu B, Yaman I, Cetinkaya Isik F, et al. Prevalence of Jervell–Lange Nielsen syndrome in children with congenital bilateral sensorineural hearing loss. Turk Kardiyol Dern Ars. 2021;49(5):368–76.
Torrado M, Fernandez G, Ganoza CA, Maneiro E, Garcia D, Sonicheva-Paterson N, et al. A cryptic splice-altering KCNQ1 variant in trans with R259L leading to Jervell and Lange-Nielsen syndrome. NPJ Genom Med. 2021;6(1):21.
Tosaka T, Casimiro MC, Rong Q, Tella S, Oh M, Katchman AN, et al. Nicotine induces a long QT phenotype in Kcnq1-deficient mouse hearts. J Pharmacol Exp Ther. 2003;306(3):980–7.
Wang Y, Liang P, Lan F, Wu H, Lisowski L, Gu M, et al. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J Am Coll Cardiol. 2014;64(5):451–9.
Qi T, Wu F, Xie Y, Gao S, Li M, Pu J, et al. Base editing mediated generation of point mutations into human pluripotent stem cells for modeling disease. Front Cell Dev Biol. 2020;8:590581.
Clements M, Thomas N. High-throughput multi-parameter profiling of electrophysiological drug effects in human embryonic stem cell derived cardiomyocytes using multi-electrode arrays. Toxicol Sci. 2014;140(2):445–61.
Ban K, Wile B, Cho KW, Kim S, Song MK, Kim SY, et al. Non-genetic purification of ventricular cardiomyocytes from differentiating embryonic stem cells through molecular beacons targeting IRX-4. Stem Cell Rep. 2015;5(6):1239–49.
Lerche C, Bruhova I, Lerche H, Steinmeyer K, Wei AD, Strutz-Seebohm N, et al. Chromanol 293B binding in KCNQ1 (Kv7.1) channels involves electrostatic interactions with a potassium ion in the selectivity filter. Mol Pharmacol. 2007;71(6):1503–11.
Swan H, Viitasalo M, Piippo K, Laitinen P, Kontula K, Toivonen L. Sinus node function and ventricular repolarization during exercise stress test in long QT syndrome patients with KvLQT1 and HERG potassium channel defects. J Am Coll Cardiol. 1999;34(3):823–9.
Johnson DM, Antoons G. Arrhythmogenic mechanisms in heart failure: linking beta-adrenergic stimulation, stretch, and calcium. Front Physiol. 2018;9:1453.
Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22(2):240–54.
Steinberg C. Diagnosis and clinical management of long-QT syndrome. Curr Opin Cardiol. 2018;33(1):31–41.
Retraction of: Systematic evaluation of KCNQ1 variant using ACMG/AMP guidelines and risk stratification in long QT syndrome type 1. Circ Genom Precis Med. 2021;14(2):e000079.
Swartz KJ. Sensing voltage across lipid membranes. Nature. 2008;456(7224):891–7.
Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, et al. The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation. 2006;113(6):783–90.
Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471(7337):225–9.
Bohnen MS, Peng G, Robey SH, Terrenoire C, Iyer V, Sampson KJ, et al. Molecular pathophysiology of congenital long QT syndrome. Physiol Rev. 2017;97(1):89–134.
Acknowledgements
The authors thank the Cellapy Biological Technology Company (Beijing, CHN) for providing technical support for the hESC-CMs experiments.
Funding
This project has been supported by the National Natural Science Foundation of China (Grant Nos. 81970205, 82070272).
Author information
Authors and Affiliations
Contributions
MC, FL, YXS, TWG, YXJ, and WJL conceived the idea and designed the project. YXS, TWG, and YXJ performed most of the experiments and analyzed the data. HYW, MZ, MQ, and MQJ provided technical assistance. YXS, TWG, and YXJ wrote and revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Figure legends.
Additional file 2. Supplemental Figure 1.
KCNQ1 point mutation did not affect the pluripotency nature of hESC.
Additional file 3. Supplemental Figure 2.
KCNQ1 knockout and point mutations(L114P、R190Q)did not affect the ability of cardiac differentiation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, Y., Guo, T., Jiang, Y. et al. KCNQ1-deficient and KCNQ1-mutant human embryonic stem cell-derived cardiomyocytes for modeling QT prolongation. Stem Cell Res Ther 13, 287 (2022). https://doi.org/10.1186/s13287-022-02964-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02964-3