Abstract
Background
Human placenta-derived multipotent cells (hPDMCs) are isolated from a source uncomplicated by ethical issues and are ideal for therapeutic applications because of their capacity for multilineage differentiation and proven immunosuppressive properties. It is known that heat shock preconditioning induces the upregulation of heat shock proteins (HSPs), which enhance survival and engraftment of embryonic stem cells (ESCs) during transplantation in live animal models, although whether heat shock preconditioning has the same effects in hPDMCs is unclear.
Methods
The hPDMCs were isolated from placenta of healthy donors. The cells were treated with heat shock (43 °C, 15 min), followed by evaluation of cell viability. Furthermore, the HSPs expression was assessed by Western blot, qPCR. The reactive oxygen species (ROS) production and signal pathway activation were determined by flow cytometry and Western blot, respectively. The regulatory pathways involved in HSPs expression were examined by pretreatment with chemical inhibitors, and siRNAs of MAPK, Akt, and heat shock factor 1 (HSF1), followed by determination of HSPs expression.
Results
This study demonstrates that heat shock treatment induced ROS generation and HPSs expression in hPDMCs. Heat shock stimulation also increased p38 MAPK and Akt phosphorylation. These effects were reduced by inhibitors of ROS, p38 MAPK and Akt. Moreover, we found that heat shock treatment enhanced nuclear translocation of the HSF1 in hPDMCs, representing activation of HSF1. Pretreatment of hPDMCs with ROS scavengers, SB203580 and Akt inhibitors also reduced the translocation of HSF1 induced by heat shock.
Conclusions
Our data indicate that heat shock acts via ROS to activate p38 MAPK and Akt signaling, which subsequently activates HSF1, leading to HSP activation and contributing to the protective role of hPDMCs.
Similar content being viewed by others
Introduction
The ability of stem cells to self-renew and differentiate into multiple cell lineages and tissue types is crucial to regenerative medicine, where clinical investigations are seeking effective ways to repair, replace and regenerate cells, tissues and organs that are damaged, injured or diseased. Various biomedical approaches in regenerative medicine involve the use of stem cells, but current invasive procedures and ethical issues limit this therapeutic source, such as the isolation of stem cells from the human embryo [1, 2]. Alternative sources of human stem cells are needed that are safe, easily accessible and provide high cell yields.
It is suggested that umbilical cord blood, amniotic fluid and placental tissue are easily procurable sources of stem cells that do not involve the need for invasive procedures [3, 4]. Regenerative medicine investigations have used human placenta-derived multipotent cells (hPDMCs) as an useful replacement for embryonic stem cells (ESCs), in recognition of the fact that hPDMCs are capable of self-renewal, of differentiating into a variety of differentiated cells, and they possess immunomodulatory properties [5]. Moreover, not only are hPDMCs essential in embryonic and fetal development, but they can differentiate into multiple adult cell types and therefore hold enormous potential for stem cell therapy [6]. Indeed, hPDMSCs are considered to behave as young progenitor cells and they have a reputation of overcoming immunological barriers involving host immune responses in allogeneic transplantation [7, 8].
Heat shock proteins (HSPs) help to maintain cellular homeostasis and are upregulated under stressful physiological conditions, when they play a crucial role by refolding proteins into their correct structure and assist with protein assembly [9, 10]. Heat shock preconditioning induces the upregulation of HSPs, which enhance survival and engraftment of ESCs during transplantation in live animal models [11,12,13,14]. Human ESCs are more capable than differentiated cells at resisting oxidative stress and irradiation, which may be because of the upregulated levels of HSP expression in response to stressors [15]. This is supported by the finding that HSP overexpression enhances cell survival and engraftment during transplantation [14, 16]. A range of stressors, such as heat, anoxia, ischemia, heavy metal ions, ethanol, nicotine, surgical stress and viral agents, can activate the binding of the transcription factor heat shock factor-1 (HSF1) to the heat shock element (HSE) and upregulate levels of HSP expression, including HSP27, HSP70, and HSP90 [17, 18]. Evidence suggests that HSF1-induced mediation of HSP expression following hyperthermia and heat shock protects cells from the adverse consequences of stress [19]. Heat shock treatment induces the production of reactive oxygen species (ROS), triggering the activation of an intercellular signal transduction pathway [20,21,22]. ROS are crucial for cell growth, migration, differentiation and apoptosis, and are generated by various sources including stress, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and heat [20,21,22,23]. Importantly, ROS can act as signaling molecules for the activation of various signaling pathways [24] and heat shock induces ROS and HSP production by activating Akt and p38 MAPK signaling pathways in human esophageal microvascular endothelial cells (HEMECs) [20].
Little is known about the effects of heat shock treatment on hPDMCs. We therefore used hPDMCs to investigate the involvement of heat shock signaling pathways in HSP production. Our results show that heat activates the ROS/NADPH, p38 MAPK, Akt and HSF pathways, subsequently upregulating HSP production. These data help to elucidate the role of HSPs in modulating the survival of hPDMCs.
Materials and methods
Materials
Protein A/G PLUS-Agarose, anti-rabbit IgG-HRP secondary antibody, rabbit polyclonal antibodies against HSP90, HSP70, HSP27, p38 MAPK, JNK, ERK, Akt, HSF1 and β-actin, small interfering RNAs (siRNAs) against HSP90, HSP70 and HSP27 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The following rabbit polyclonal antibodies, including phospho-p38 MAPK (Thr180/Tyr182), phospho-SAPK/JNK (Thr183/Tyr185), phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), and phospho-Akt (Ser473), were obtained from Cell Signaling and Neuroscience (Danvers, MA, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell cultures
hPDMCs were isolated using enzyme treatment from term placentas (at 38–40 weeks’ gestation) obtained with informed consent from healthy donor mothers, following approval granted by the Institutional Review Board of Taipei Medical University (201005015). Briefly, the placental tissue was minced into small pieces, enzymatic digestion, then maintained in complete medium consisting of Dulbecco's Modified Eagle Medium (DMEM) with 20 mM HEPES and 10% Fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM glutamine. All cell culture materials were purchased from Invitrogen (Carlsbad, CA, USA).
The cells were maintained in incubator provide with humidified 5% CO2 atmosphere at 37 °C. The medium was renewed once or twice weekly. hPDMCs between the passages of 5 to 8 were used in all experiments [25]. The cell maintained in complete media was subjected to heat shock treatment in an oven at 43 °C for 15 min. For post-treatment recovery, the cells were maintained in an incubator at 37 °C until required for analysis [18].
Assessment of cell viability
3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was used for assessment of cell viability. The cells treated with heat shock were performed with MTT assay after 48 h post-treatment. The MTT (0.5 mg/ml) was added in wells and incubated for 2 h at 37 °C. Then, the dimethyl sulfoxide (DMSO) was added to dissolve formazan crystals. The absorbance at 550 nm (reference wavelength 650 nm) was measured using a microplate reader (Bio-Tek, Winooski, VT, USA).
Quantitative polymerase chain reaction (qPCR)
The hPDMCs grown in 6-well plates were treated with heat shock, followed by collecting total RNA using TRIzol kit (MDBio Inc., Taipei, Taiwan). The 2 µg RNA were subjected to reverse transcription to produce cDNA templates. qPCR was carried out using SYBR Green (KAPA Biosystems). The reactions were performed in triplicate using StepOnePlus (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instruction. The PCR probes used in qPCR reaction were obtained from Applied Biosystems. The expression level of target gene was normalized by the comparative threshold cycle (ΔΔCt) method. All the primers used in qPCR are list as follows:
Human HSP90, forward primer: 5’-AGAGCCTACGTTCCTGCACT-3’ and reverse primer: 5’-GACCATTCTTCTAGAGCATTCAGG-3’;
Human HSP70, forward primer: 5’-TAACCCCATCATCAGCGGAC -3’ and reverse primer: 5’-GAAGCTCCAAAACAAAAACAGCA-3’;
Human HSP27, forward primer: 5’-ACATTTGCTCGGTCACTCC-3’ and reverse primer: 5’-AAGCCGTGCTCATCTTGTCT-3’;
Human GADPH, forward primer: 5’-ACCCACTCCTCCACCTTTG -5’ and reverse primer:5’- CTGTAGCCAAATTCGTTGTCAT -3’.
Detection of reactive oxygen species (ROS)
Intracellular ROS were detected by using dihydrorhodamine 123 (DHR123). The cells (5 × 105) grown in 24-well plates were treated with heat shock. The cells were incubated with DHR123 (20 μM) for 10 min at 37 °C and cellular fluorescence intensity was detected using a flow cytometry analysis at 488 nm.
Western blot analysis
The cells grown in 6-well plates were treated with heat shock, followed by collecting cell lysates using RIPA lysis buffer. The 20 μg cell lysates were separated using 12% SDS-PAGE and then transferred to PVDF membranes. The membranes were incubated with 4% BSA solution for 1 h at room temperature. Subsequently, the membranes were incubated with primary antibodies (HSP90, HSP70, HSP27, p38 MAPK, JNK, ERK, Akt, HSF1 and β-actin) with 1:1000 dilution for 1 h at room temperature. After 3 washed with TBST, the membranes were incubated secondary antibody with 1:3000 dilution for 1 h at room temperature. Finally, the membranes were incubated ECL reagents (Amersham Pharmacia Biotech, Inc, USA) and the signals were detected with Kodak X-OMAT LS film (Eastman Kodak, Rochester, NY, USA).
siRNA transfection
siRNAs which specific for human HSP27, HSP70 and HSP90, and control siRNA, were obtained from Santa Cruz Biotechnology. Cells were transfected with 100 nM siRNAs using Lipofectamine 2000 (Invitrogen Life Technology, Waltham, MA, USA), according to the manufacturer's instructions [26].
Immunofluorescence staining
Cells grown in 12 mm coverslips were treated with heat shock and fixed with 4% paraformaldehyde at room temperature for 30 min. After washing with PBS for 3 times, the cells were permeabilized with 4% nonfat milk in PBS containing 0.5% Triton X-100. The cells were incubated with primary antibody (HSF-1) with 1:100 dilution and FITC-conjugated secondary antibody with 1:500 dilution for 1 h. Finally, the fluorescence was monitored under a Zeiss fluorescence microscope [27].
Statistical analysis
All values reported are means ± SEM. Comparisons between two samples were performed using the Student’s t test. Comparisons between more than two samples were performed using one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test for multiple comparisons. In all cases, differences were deemed significant when p < 0.05.
Results
Heat shock treatment induces HSP production in PDMCs
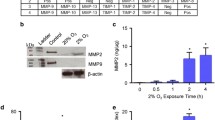
Heat shock pretreatment is beneficial for the survival and engraftment of transplanted cells [12,13,14]. We therefore used hPDMCs to investigate the involvement of heat signaling pathways in HSP production. Initial heat treatment of PDMCs did not affect cell viability (Fig. 1A). We examined levels of HSP expression in hPDMCs with Western blot and qPCR analysis. The PDMCs were treated with heat shock (43 °C) for 15 min, followed by determining the HSP production after 24 h post-treatment. The results indicated that heat shock treatment time-dependently induced HSP production (Fig. 1B, C). PDMCs were then transfected for 24 h with HSP90, HSP70 and HSP27, which inhibited HSP expression (Fig. 1D) and had no significant effect on cell viability (Fig. 1E). However, transfection with siRNAs of HSPs decreased the protective effects of HSPs upon cell survival after heat shock treatment (Fig. 1F). These data indicated that heat increases HSP production in human PDMCs and protects cell survival.
The effects of heat shock on cell viability and HSP production in PDMCs. A PDMCs were incubated with heat shock for 15 min, followed by determining cell viability by MTT assay after 48 h. B, C PDMCs were incubated with heat shock for 15 min; the total RNA and protein were collected after 0, 3, 6, 12, and 24 h. The expression levels of HSPs were determined by qPCR and Western blotting. D The HSPs siRNA or control siRNA were introduced into PDMCs for 24 h, follow by determining the protein expressions by Western blotting. E The PDMCs were treated as Fig. 1D described, follow by determining cell viability by MTT assay. F The HSPs siRNA or control siRNA were introduced into PDMCs for 24 h. Subsequently, the cells were treated with heat shock for 15 min. Forty-eight hours later, the cell viability was determined by MTT assay. Results are expressed as the mean ± SEM. *p < 0.05 compared with control
ROS and NADPH are involved in the heat-induced production of HSPs in hPDMCs
ROS production is triggered by different stressors such as oxidative stress, heavy metal stress and hyperthermia [22], as well as heat shock [20, 21]. Elevations in levels of superoxide anions (O2−), hydrogen peroxide (H2O2) and nitric oxide (NO) are observed in multiple cell lines and tumor tissues subjected to heat shock [28, 29]. When we sought to determine the involvement of ROS accumulation in heat shock-induced HSP production in PDMCs, DHR123-based FACS detection demonstrated increased levels of cellular ROS in PDMCs treated with heat shock (Fig. 2A). Pretreatment of cells with N-acetylcysteine (NAC), a direct scavenger of ROS, reduced the increase in HSP production induced by heat shock (Fig. 2B-C). NADPH oxidase is an important enzymatic source for the production of ROS under pathologic conditions [23], so we investigated the role of NADPH oxidase in heat shock-induced HSP production in hPDMCs. Pretreatment of cells with NADPH oxidase inhibitors (DPI and APO) inhibited HSP production induced by heat shock (Fig. 2B,C). In order to investigate whether heat shock-induced HSP production via ROS protects the survival of hPDMCs, we treated cells with ROS and NADPH oxidase inhibitors and found that these antagonized the protection conferred by heat shock upon hPDMC survival (Fig. 2D). These data implicate the involvement of ROS and NADPH oxidase activation in heat shock-induced HSP production in hPDMCs.
Heat shock-induced ROS production in PDMCs. A PDMCs were incubated with heat shock, and the flow cytometry was conducted to examine ROS production in different time points. B, C PDMCs were pretreated with DMSO vehicle, NAC (5 µM), DPI (5 µM) and APO (5 µM) for 30 min, and then stimulated with heat shock. The cells were collected at 48 h after the heat shock, and the mRNA and the protein expression of HSPs were examined by qPCR and Western blotting. D PDMCs were treated as described in Fig. 2B, and then, the cell viability was determined at different time points by MTT assay. Results are expressed as the mean ± SEM. *p < 0.05 compared with control; #, p < 0.05 compared with heat shock -treated group
p38 MAPK and Akt are involved in heat-induced production of HSPs in hPDMCs
Several reports have revealed that activation of Akt and p38 MAPK signal pathways was required for heat shock response in different cell types [20, 30, 31]. Therefore, we suggested that Akt and p38 signal proteins were the major components involved in heat shock response in stem cells as well, which was poorly described before. We then measured p38 MAPK phosphorylation in hPDMCs in response to heat shock and observed time-dependent phosphorylation of p38 MAPK, but not JNK or ERK (Fig. 3A). NAC, DPI and APO treatment all attenuated the increase in p38 MAPK phosphorylation induced by heat shock (Fig. 3B). Thirty minutes of pretreatment with a p38 MAPK inhibitor (SB203580, 10 µM) markedly inhibited heat shock-induced HSPs mRNA expression (Fig. 3C); no such effects were observed after pretreating the cells for 30 min with either a JNK inhibitor (SP600125, 10 µM) or ERK inhibitor (PD98059, 10 µM). Moreover, pretreating the PDMCs with the p38 MAPK inhibitor (SB203580) reduced levels of heat shock-induced protein expressions of HSPs, while the JNK inhibitor (SP600125) and ERK inhibitor (PD98059) had no such effects (Fig. 3D). In order to confirm whether heat shock-induced HSP production via p38 MAPK protects the survival of hPDMCs, we treated cells with the p38 MAPK inhibitor and found that this antagonized the protective effects on hPDMCs induced by heat shock, whereas JNK and ERK inhibitors had no such effects (Fig. 3E). Akt phosphorylation on Ser473 by a p38 MAPK-dependent signaling pathway causes enzymatic activation [32]; therefore, we evaluated Akt activation in hPDMCs after heat shock treatment. The result indicated that Akt phosphorylation was increased after heat shock (Fig. 4A). Treatment with p38 inhibitor but not JNK or ERK inhibitors obviously inhibited Akt phosphorylation (Fig. 4B), suggesting heat shock-induced Akt activation was regulated by p38. We further pretreated hPDMCs with the Akt inhibitor and found that antagonized heat shock-induced HSP production (Fig. 4C-D). Finally, pretreatment of Akt inhibitor also abolished protective effects induced by heat shock in hPDMCs. In summary, heat shock appears to act via the p38 MAPK and AKT-dependent signaling pathways and thereby enhances HSP production in PDMCs.
p38 MAPK is involved in heat shock-mediated HSPs production in PDMCs. A PDMCs were treated with heat shock, and p38 MAPK, JNK and ERK phosphorylation were assessed at different time points by Western blotting. B PDMCs were treated with DMSO vehicle, NAC (5 µM), DPI (5 µM) and APO (5 µM) for 30 min, followed by heat shock treatment. The treated cells were collected 15 min later, and p38 MAPK phosphorylation was determined by Western blotting. C, D PDMCs were pretreated with DMSO vehicle, SB203580 (10 µM), SP600125 (10 µM) and PD98059 (10 µM) for 30 min, followed by heat shock treatment. The treated cells were collected 24 h later, and gene expression of HSPs was assessed by qPCR and Western blotting. (E) PMDCs were pre-incubated with DMSO vehicle, SB203580 (10 µM), SP600125 (10 µM) and PD98059 (10 µM) for 30 min, followed by heat shock treatment. The cell viability was determined at different time points by MTT assay. Results are expressed as the mean ± SEM. *p < 0.05 compared with control; #, p < 0.05 compared with heat shock-treated group
Akt is responsible for HSP production after heat shock treatment. A PDMCs were treated with heat shock, and Akt phosphorylation was assessed at different time points by Western blotting. B PDMCs were pretreated with DMSO vehicle, SB203580 (10 µM), SP600125 (10 µM) and PD98059 (10 µM) for 30 min, followed by heat shock treatment, and Akt phosphorylation were examined by Western blotting. C, D PDMCs were pretreated with DMSO vehicle and Akti (10 µM) for 30 min, followed by heat shock treatment. The treated cells were collected 24 h later, and gene expression of HSPs was assessed by qPCR and Western blotting. E PDMCs were pretreated with DMSO vehicle and Akti (10 µM) for 30 min, followed by heat shock treatment, and the cell viability was evaluated at different time points by MTT assay. Results are expressed as the mean ± SEM. *, p < 0.05 compared with control; #, p < 0.05 compared with heat shock-treated group
The p38 MAPK and Akt signaling pathway involves HSF1 in heat shock-induced HSP production
Many types of stressors activate the binding of HSF1 to HSE elements and induce HSP expression (17, 18). The HSF1 pathway is therefore important in heat shock-induced HSP production in hPDMCs. Moreover, when subjected to stress, the HSF1 protein translocates from the cytosol to the cell nucleus [33]. We observed that heat shock enhanced HSF1 translocation from the cytosol to the cell nucleus (Fig. 5A). Similarly, pretreating hPDMCs with NAC, DPI, APO, SB203580 and Akt inhibitors reduced the heat shock-induced translocation of HSF1 into the nucleus (Fig. 5B–D). Our findings indicate that the heat shock-induced increase in HSP expression in PDMCs requires activation of the ROS, p38 MAPK, AKT and HSF1 pathways.
Heat shock-induced HSF1 activates through ROS, p38 MAPK/Akt pathway. A PDMCs were incubated with heat shock for indicated time intervals, and HSF1 expression in cytosol and nucleus was determined by Western blotting. B PDMCs were pretreated for 30 min with DMSO vehicle, NAC (5 µM), DPI (5 µM) and APO (5 µM) followed by stimulation with heat shock (43 °C, 15 min) for 60 min, and HSF1 expression in nucleus was determined by Western blotting. C PDMCs were pretreated for 30 min with DMSO vehicle, SB203580 (10 µM) and Akti (10 µM) followed by stimulation with heat shock (43 °C, 15 min) for 60 min, and HSF1 expression in nucleus was determined by Western blotting. D PDMCs were pretreated with DMSO vehicle, NAC, DPI, APO, SB203580, SP600125, PD98059 and Akti for 30 min followed by stimulation with heat shock for 60 min, and HSF1 immunofluorescence staining was examined (Scale bar = 50 μm). Results are expressed as the mean ± SEM. *p < 0.05 compared with control; #p < 0.05 compared with heat-treated group
Discussion
The isolation of hPDMCs from placental tissue is not complicated by ethical issues [8]. Such cells are capable of self-renewal, of multipotent differentiation and they possess immunomodulatory properties, making them desirable as an alternative source of ESCs in regenerative medicine [5]. Other advantages of hPDMCs include their ability to behave similarly to young progenitor cells and their capacity to overcome immunological barriers involving host immune responses in allogeneic transplantation [7, 8].
Damaged and diseased organs produce free radicals, inflammatory cytokines, toxins and active apoptotic signals, all of which reduce the efficacy of transplanted cells [34, 35]. Heat shock pretreatment can promote the survival of transplanted cells by inducing chaperone-mediated proteins [36, 37], including HSPs and nuclear/histone chaperones, which maintain protein folding homeostasis under both normal and stressful physiological conditions [9, 38]. Fever, nutritional deficiency, inflammation, oxidative stress and viral infection can activate the binding of HSF1 to HSE elements and induce HSP27, HSP70 and HSP90 expression (17, 18). HSPs are housekeeping proteins that are important for cell survival and show superiority over various differentiated cells in their capacity for self-renewal, differentiation and high stress tolerance [39, 40]. In this study, we found that heat shock (at 43 °C for 15 min) treatment increased HSP expression without apparently affecting the viability of hPDMCs. Although 45 °C for 15 min heat shock in PDMCs did not reduce cell viability as well, we have checked with previous studies, which indicated moderate/mild hyperthermia (i.e., heating at ≤ 43 °C) could induce thermotolerance by HSPs expression in cells [41]. However, at the temperature above 43 °C, the HSPs expression is diminished as well as its folding capacity [42, 43]. The unfold protein response is elevated and results in cell death. Our results here suggest that mesenchymal stem cells may have higher thermotolerance, but more investigations are needed to determine whether this is the case.
HSPs are proposed to modulate cell differentiation through various mechanisms. For instance, HSP27 was proposed as differentiation marker of skin keratinocytes and muscle cells [44, 45]. Moreover, several reports have indicated that HSP70 exhibited critical role in erythroblasts differentiation. HSP70 has been shown to bind to GATA-1 [46] or Apoptosis-inducing factor (AIF) [47], therefore protecting cell from apoptosis and allowing the differentiation of erythroblasts. HSP90 was also implicated in embryonic development and cell differentiation in different species, such as Drosophila melanogaster [48], Caenorhabditis elegans [49], zebrafish [50], and mice [51]. Here, we found these HSPs were increased after heat shock in PDMCs. These results suggest heat shock could regulate differentiation in PDMCs.
We found that heat shock elevated levels of HSP mRNA and protein expression via ROS induction of p38 and Akt signaling, which regulates the survival of hPDMCs. Our evidence is the first to show that heat shock treatment of hPDMCs induces increases in HSP expression and is protective of cell survival. These data indicate that the heat shock pathway is a mediator in the survival of hPDMCs.
Heat shock treatment has previously been shown to induce ROS production, which activates the intercellular signal transduction pathway [52]. In this study, we demonstrated that ROS production was required for heat shock-induced HSP production. Treating hPDMCs with a ROS inhibitor reduced heat shock-induced HSP production and inhibited the heat shock-mediated protection of hPDMC survival. Thus, it appears that ROS is involved in heat shock-induced HSP production and HSP release from hPDMCs.
Researchers have demonstrated that heat shock induces ROS and HSP production by activating Akt and p38 MAPK in HEMECs [20]. Our study found that heat shock enhanced p38 and Akt phosphorylation in hPDMCs and that heat shock-induced production of HSPs was effectively inhibited by treatment with a p38 or Akt inhibitor. Furthermore, treatment with a ROS inhibitor and a NADPH inhibitor blocked p38 activation induced by heat shock. These results were further confirmed when we observed that heat shock-induced protection of hPDMC survival was inhibited by a p38 or Akt inhibitor. Our data suggest that the p38/Akt pathway plays an important role in levels of HSP expression in PDMCs.
HSF1 has been reported as a major regulator in mediating expression of HSPs in response to heat shock. The signal pathways responsible for HSF1 activation are also summarized in previous article [53]. We found in this study that heat shock increased levels of HSF1 expression in the cell nucleus and that HSF1 activation contributed to heat shock-induced HSP production in hPDMCs. Thus, HSF1 is important in heat shock-induced HSP production. We also observed that treating hPDMCs with ROS, NADPH, p38 MAPK and Akt inhibitors reduced heat shock-induced HSF1 activity. It appears that the interactions between heat shock and these signaling molecules, as well as ROS, upregulate HSP expression via p38, Akt and HSF1 signaling in hPDMCs.
Conclusion
Our investigations into the signaling pathways involved in heat shock-induced HSP production in hPDMCs demonstrate that heat shock increases HSP production by inducing ROS expression and activating p38/Akt signaling, thereby enhancing HSF1 transcription activity which contributing to HSP production (Fig. 6). This evidence of a heat shock-mediated signaling pathway improves our understanding of the mechanisms underlying the survival of hPDMCs under hyperthermic conditions. This discovery could help to inform the development of more effective therapies in regenerative medicine.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- hPDMCs:
-
Human placenta-derived multipotent cells
- HSPs:
-
Heat shock proteins
- ESCs:
-
Embryonic stem cells
- ROS:
-
Reactive oxygen species
- HSF1:
-
Heat shock factor 1
- HEMECs:
-
Human esophageal microvascular endothelial cells
- siRNAs:
-
Small interfering RNAs
- qPCR:
-
Quantitative polymerase chain reaction
- NAC:
-
N-acetylcysteine
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide
- DHR123:
-
Dihydrorhodamine 123
- ANOVA:
-
One-way analysis of variance
References
Eichna DM, Brown KS, Breen A, Dean RB. Mucormycosis: a rare but serious infection. Clin J Oncol Nurs. 2008;12(1):108–12.
Atala A. Recent applications of regenerative medicine to urologic structures and related tissues. Curr Opin Urol. 2006;16(4):305–9.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301.
Zheng YB, Gao ZL, Xie C, Zhu HP, Peng L, Chen JH, et al. Characterization and hepatogenic differentiation of mesenchymal stem cells from human amniotic fluid and human bone marrow: a comparative study. Cell Biol Int. 2008;32(11):1439–48.
Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Mao N. Effect of human placenta derived mesenchymal stem cells on cord blood lymphocyte transformation. Zhonghua Yi Xue Za Zhi. 2005;85(24):1704–7.
Mathew SA, Naik C, Cahill PA, Bhonde RR. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol Life Sci. 2020;77(2):253–65.
Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, et al. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15(7):539–47.
Evangelista M, Soncini M, Parolini O. Placenta-derived stem cells: new hope for cell therapy? Cytotechnology. 2008;58(1):33–42.
Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79(2):425–49.
Wegele H, Wandinger SK, Schmid AB, Reinstein J, Buchner J. Substrate transfer from the chaperone Hsp70 to Hsp90. J Mol Biol. 2006;356(3):802–11.
Baugh J, Moussa O, Ryan CA, Nayak A, Laflamme R. Experimental implementation of heat-bath algorithmic cooling using solid-state nuclear magnetic resonance. Nature. 2005;438(7067):470–3.
Baumeister S, Ofer N, Kleist C, Terne P, Opelz G, Gebhard MM, et al. Reduction of skeletal muscle injury in composite tissue allotransplantation by heat stress preconditioning. Plast Reconstr Surg. 2004;114(7):1832–41.
Bouchentouf M, Benabdallah BF, Tremblay JP. Myoblast survival enhancement and transplantation success improvement by heat-shock treatment in mdx mice. Transplantation. 2004;77(9):1349–56.
Kim HP, Morse D, Choi AM. Heat-shock proteins: new keys to the development of cytoprotective therapies. Exp Opin Ther Targets. 2006;10(5):759–69.
Prinsloo E, Setati MM, Longshaw VM, Blatch GL. Chaperoning stem cells: a role for heat shock proteins in the modulation of stem cell self-renewal and differentiation? BioEssays. 2009;31(4):370–7.
Jayakumar J, Suzuki K, Khan M, Smolenski RT, Farrell A, Latif N, et al. Gene therapy for myocardial protection: transfection of donor hearts with heat shock protein 70 gene protects cardiac function against ischemia-reperfusion injury. Circulation. 2000;102(19 Suppl 3):III302–6.
Mivechi NF, Shi XY, Hahn GM. Stable overexpression of human HSF-1 in murine cells suggests activation rather than expression of HSF-1 to be the key regulatory step in the heat shock gene expression. J Cell Biochem. 1995;59(2):266–80.
Kuo HT, Chen HW, Hsiao HH, Chen HC. Heat shock response protects human peritoneal mesothelial cells from dialysate-induced oxidative stress and mitochondrial injury. Nephrol Dial Transplant. 2009;24(6):1799–809.
Tulapurkar ME, Asiegbu BE, Singh IS, Hasday JD. Hyperthermia in the febrile range induces HSP72 expression proportional to exposure temperature but not to HSF-1 DNA-binding activity in human lung epithelial A549 cells. Cell Stress Chaperones. 2009;14(5):499–508.
Banerjee Mustafi S, Chakraborty PK, Dey RS, Raha S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones. 2009;14(6):579–89.
Yu J, Liu F, Yin P, Zhao H, Luan W, Hou X, et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress. 2012.
Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95.
Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, et al. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280(21):20700–11.
Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194(1):7–15.
Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, et al. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24(11):2466–77.
Yang WH, Chang JT, Hsu SF, Li TM, Cho DY, Huang CY, et al. Bradykinin enhances cell migration in human chondrosarcoma cells through BK receptor signaling pathways. J Cell Biochem. 2010;109(1):82–92.
Wu MH, Lo JF, Kuo CH, Lin JA, Lin YM, Chen LM, et al. Endothelin-1 promotes MMP-13 production and migration in human chondrosarcoma cells through FAK/PI3K/Akt/mTOR pathways. J Cell Physiol. 2011.
Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1996;93(10):5116–21.
Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431(2):285–6.
Wang CC, Chen F, Kim E, Harrison LE. Thermal sensitization through ROS modulation: a strategy to improve the efficacy of hyperthermic intraperitoneal chemotherapy. Surgery. 2007;142(3):384–92.
Sirangelo I, Iannuzzi C, Vilasi S, Irace G, Giuberti G, Misso G, et al. W7FW14F apomyoglobin amyloid aggregates-mediated apoptosis is due to oxidative stress and AKT inactivation caused by Ras and Rac. J Cell Physiol. 2009;221(2):412–23.
Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, et al. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287(2):C494–9.
Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280(48):39962–9.
Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–97.
Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004;279(1):765–71.
Nakamura Y, Yasuda T, Weisel RD, Li RK. Enhanced cell transplantation: preventing apoptosis increases cell survival and ventricular function. Am J Physiol Heart Circ Physiol. 2006;291(2):H939–47.
Watanabe J, Kushihata F, Matsumoto K, Honda K, Matsuda S, Kobayashi N. Downregulation of cytokine release by heat preconditioning of livers used for transplantation in rats. Dig Dis Sci. 2005;50(10):1823–8.
Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16(9):833–8.
Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22(6):962–71.
Stromer T, Ehrnsperger M, Gaestel M, Buchner J. Analysis of the interaction of small heat shock proteins with unfolding proteins. J Biol Chem. 2003;278(20):18015–21.
Samali A, Holmberg CI, Sistonen L, Orrenius S. Thermotolerance and cell death are distinct cellular responses to stress: dependence on heat shock proteins. FEBS Lett. 1999;461(3):306–10.
Ito A, Fujioka M, Tanaka K, Kobayashi T, Honda H. Screening of cytokines to enhance vaccine effects of heat shock protein 70-rich tumor cell lysate. J Biosci Bioeng. 2005;100(1):36–42.
Lin FC, Hsu CH, Lin YY. Nano-therapeutic cancer immunotherapy using hyperthermia-induced heat shock proteins: insights from mathematical modeling. Int J Nanomed. 2018;13:3529–39.
Ishiwatari S, Suzuki T, Hitomi T, Yoshino T, Matsukuma S, Tsuji T. Effects of methyl paraben on skin keratinocytes. Journal of applied toxicology : JAT. 2007;27(1):1–9.
Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res. 2001;266(2):213–21.
Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445(7123):102–5.
Lui JC, Kong SK. Heat shock protein 70 inhibits the nuclear import of apoptosis-inducing factor to avoid DNA fragmentation in TF-1 cells during erythropoiesis. FEBS Lett. 2007;581(1):109–17.
Yue L, Karr TL, Nathan DF, Swift H, Srinivasan S, Lindquist S. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics. 1999;151(3):1065–79.
Inoue T, Hirata K, Kuwana Y, Fujita M, Miwa J, Roy R, et al. Cell cycle control by daf-21/Hsp90 at the first meiotic prophase/metaphase boundary during oogenesis in Caenorhabditis elegans. Dev Growth Differ. 2006;48(1):25–32.
Krone PH, Evans TG, Blechinger SR. Heat shock gene expression and function during zebrafish embryogenesis. Semin Cell Dev Biol. 2003;14(5):267–74.
Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127(1):1–11.
Pucciariello C, Banti V, Perata P. ROS signaling as common element in low oxygen and heat stresses. Plant Physiol Biochem. 2012.
Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cellular and molecular life sciences : CMLS. 2005;62(1):10–23.
Acknowledgements
We thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital, for technical support during the study. A dominant-negative p38 MAPK mutant was provided by Dr. J. Han (Southwestern Medical Center, Dallas, TX, USA) and a dominant-negative JNK mutant was provided by Dr. M. Karin (University of California, San Diego, CA, USA). A dominant-negative ERK2 mutant was gifted by Dr. M. Cobb (Southwestern Medical Center, Dallas, TX, USA). A dominant-negative kinase-deficient form of Akt (K179A) was gifted by Dr. W.M. Fu (National Taiwan University, Taipei, Taiwan).
Funding
This work was supported by grants from the National Science Council of Taiwan (NSC 99–2628-B-341–004-MY3), Ministry of Science and Technology (MOST108-2314-B-002–211-MY3), the Shin-Kong Wu Ho-Su Memorial Hospital (SKH-8302–100-0401) and National Taiwan University Hospital (NTUH.101-N1963).
Author information
Authors and Affiliations
Contributions
JF Liu, PC Chen, and CH Hou involved in conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. TY Ling involved in acquisition of patient specimens, data analysis and interpretation. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The cell material used in this study was approved by the Institutional Review Board of Taipei Medical University (201005015).
Consent for publication
Not applicable.
Competing interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, JF., Chen, PC., Ling, TY. et al. Hyperthermia increases HSP production in human PDMCs by stimulating ROS formation, p38 MAPK and Akt signaling, and increasing HSF1 activity. Stem Cell Res Ther 13, 236 (2022). https://doi.org/10.1186/s13287-022-02885-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02885-1