Abstract
Background
Long-term preservation of adipose tissue is crucial for clinical applications. Researchers should consider both efficiency and biosafety when choosing a cryoprotective agent (CPA) for adipose tissue preservation. Glycerol has been applied as a nontoxic CPA for multiple tissues but not adipose tissue. We aimed to evaluate the efficacy of glycerol as a CPA for adipose tissue cryopreservation.
Methods
Fresh human adipose tissues were obtained from patients who underwent liposuction and divided into 1 mL samples. Each sample was randomly mixed with 1 mL of CPA: 60–100% glycerol, 0.25 mol/L trehalose or DMSO + FBS and cryopreserved in − 196 °C liquid nitrogen for one month. After thawing and elution, the tissues were immediately evaluated for activity and structural integrity in vitro. Then, 0.2 mL of each sample was transplanted subdermally to the nude mouse dorsum and harvested after one month for histological examination to assess the effect of the cryopreserved fat in transplantation.
Results
After cryopreservation, the samples treated with DMSO + FBS, trehalose, 60% and 70% glycerol had a more integrated structure than the samples in other groups. Tissues preserved with 70% glycerol had the highest G3PDH activity of 24.41 ± 0.70, comparable to 24.76 ± 0.48 in fresh tissue (p > 0.05). Adipose-derived stem cells (ASC) viability, proliferation and differentiation capability were also better preserved in 70% glycerol group. In vivo analysis showed that tissue preserved with 70% glycerol had a retention rate of 52.37 ± 7.53%, significantly higher than other groups. Histological observation demonstrated better structural integrity and viability in 70% glycerol group. Compared to the DMSO + FBS and trehalose groups, the glycerol groups showed lower tissue inflammation.
Conclusion
Glycerol (70%) is efficient in adipose tissue cryopreservation. Glycerol-based CPAs, which are nontoxic and show biosafety, are a promising solution for clinical tissue cryopreservation.
Similar content being viewed by others
Background
Autologous fat transplantation is considered an ideal method for soft tissue surgery and repair in plastic and reconstructive surgery due to its bioavailability and biocompatibility [1]. Fat grafting for reconstruction or aesthetic surgery usually requires multiple transplantations to achieve the treatment goals due to its unpredictable absorption rates [2]. Preserving adipose tissue after a single liposuction enables multiple reinjections at the proper time, thus eliminating repeated liposuction, increasing patient acceptance, and reducing costs and suffering. Moreover, adipose tissue contains many adipose-derived stem cells (ASCs), which have multipotent mesenchymal differentiation potential [3]. Addressing the problem of long-term preservation of adipose tissue can also provide a new solution for stem cell storage.
Cryopreservation has been studied both clinically [4, 5] and experimentally [6, 7] as a solution for tissue and cell preservation. Cryoprotective agents (CPAs) are necessary to reduce the damage caused by ice crystals formed during freezing [8]. Adipose tissue, as a composite tissue, requires CPAs that can effectively preserve multiple cell types, especially adipocytes containing large oil drops. However, in previous studies, researchers often applied traditional cellular CPAs that were either cytotoxic [9] or lacked evidence of efficacy [10].
Glycerol is a molecule that protects cells from freezing injury mainly by reducing intracellular ice crystal formation and osmotic pressure differences [11]. This molecule has been studied as a CPA for composite tissues such as testicular tissue [11], ovarian tissue [12], bones and cartilage [13]. As the backbone of triglycerides contained in adipocytes [14], glycerol has a potential advantage in adipose tissue cryopreservation.
Therefore, in this study, we sought to evaluate the feasibility and efficacy of glycerol-based CPAs for adipose tissue cryopreservation, thus looking for a safe and reliable CPA for future clinical use.
Methods
Harvest and preparation of adipose tissue
Human adipose tissues were obtained from discarded tissue from 5 healthy female patients (aged 21–46 years) who underwent abdominal liposuction. Written informed consent was obtained from all patients. This study was approved by the Ethics Committee of Shanghai Ninth People's Hospital and complied with the principles of the Declaration of Helsinki.
The adipose tissue was washed to remove free oil and liquid as previously reported [15]. The middle layer of pure adipose tissue was obtained and divided into 1 mL samples (Fig. 1).
Schematic representation of the cryopreservation and experimental methods. Aspirated fat was centrifuged at 500 rpm for 3 min. The middle layer of adipose tissue was mixed with different cryoprotective agents (CPAs) at a 1:1 volume ratio. The mixtures were then cryopreserved with a programmed protocol and stored in liquid nitrogen at − 196 °C. After 1 month of cryopreservation, the tissues were thawed and eluted. In vitro and in vivo studies were then conducted
Cryopreservation of adipose tissue
Prepared adipose tissue samples were randomly divided into the following groups: (1)–(5) 60%, 70%, 80% and 90% glycerol-PBS solutions (volume/volume, V/V) and 100% pure glycerol (Bio-Rad Laboratories, Hercules, USA); (6) 0.25 mol/L trehalose(Solarbio, Beijing, China)-PBS solution as a nontoxic CPA [15]; (7) 90% fetal bovine serum (Gibco, Grand Island, USA) and 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, USA) (FBS + DMSO) (V/V), as a positive control; and (8) no CPA (blank). Each group contained 75 mL of adipose tissue (15 mL from each volunteer, 6 volunteers in all). One milliliter of adipose aspirate was mixed with 1 mL of CPA solution at room temperature in a 5 mL cryogenic vial.
Programmed cryopreservation using a controlled-rate freezing container (Thermo Scientific, Waltham, USA) with a cooling rate of − 1 °C/min was applied in this study. Mixed samples were kept in a freezing container at − 80 °C for at least 12 h before transfer to − 196 °C liquid nitrogen as the standard protocol [7].
Thawing and elution
After one month of cryopreservation, the samples were removed from liquid nitrogen and immediately placed in a 37 °C water bath until they were thoroughly thawed [7]. Five milliliters of PBS was added slowly to the cryopreserved tissues and mixed for 3 min to wash the tissue. The mixed samples were centrifuged at 500 rpm for 3 min to separate and remove the liquid in the lower layer. This procedure was repeated twice to thoroughly remove the CPAs.
Fat transplantation model
All animal experiments were performed in accordance with the Animal Use Committee of the Shanghai Jiao Tong University animal guidelines and was approved by the Animal Ethics Committee of Shanghai Ninth People's Hospital. Glycerol solution (90%) and glycerol (100%) demonstrated poor cryoprotective effects in vitro and were eliminated in the in vivo studies; thus, 6 groups were studied (60% glycerol, 70% glycerol, 80% glycerol, FBS + DMSO, trehalose and blank). Twenty-four BALB/c male mice (6 weeks old, weighing 25–30 g) were randomly divided into 6 groups (4 mice/8 sides each group).
After anesthesia, prepared fat tissue (0.2 mL) was injected into one side of the nude mouse dorsum using a blunt-tip cannula. Each mouse was injected on both sides of the dorsum. Mice were killed, and the grafted fat samples were harvested and weighed after 4 weeks. Samples were divided into half for histological studies and the other half for RNA extraction.
Biochemical activity assessment by G3PDH assay
The glyceraldehyde-3-phosphate dehydrogenase (G3PDH) activity assay kit (MAK208-1KT; Sigma-Aldrich, St. Louis, USA) was assessed the cellular activity of adipose tissues [16]. Briefly, tissue samples (10 mg) were homogenized in 200 mL of ice-cold GPDH assay buffer and then centrifuged. The supernatant was mixed with the reaction mixture, and the absorbance at 450 nm was measured with a microplate reader (BioTek, Winooski, USA) for 60 min at 37 °C. The G3PDH activity of cryopreserved tissue was calculated and compared with the fresh tissue as a control group.
Stromal vascular fraction (SVF) isolation and viability count
The SVF was isolated from 2 mL of cryopreserved adipose tissue. Briefly, the adipose tissue was digested with collagenase for 30 min at 37 °C. Cell suspension was filtered and centrifuged and resuspended in medium. Total SVF cell numbers were measured, followed by trypan-blue staining to identify dead cells. Calcein blue acetoxymethyl (AM) /propidium iodide (PI) stain was conducted by flow cytometry to confirm the live/dead cell results (For detailed methods, see Additional file 1). Viability rate were calculated as living cells divided by total SVF cell count.
ASCs culture and surface marker identification
Isolated SVF cells from different groups were subcultured to the 3rd passage for obtaining ASCs. The cells were cultured in low-glucose DMEM (Thermo Fisher, Waltham, USA) containing 10% FBS and 1% penicillin–streptomycin solution (GE Healthcare, Freiburg, Germany) until the confluence was 90%. ASCs were identified using cytofluorometry analysis. For detailed method of flow cytometry, see Additional file 1.
ASCs proliferation assessment
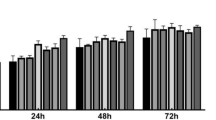
A Cell Counting Kit-8 (CCK-8) assay was conducted to examine the ASCs proliferative [17]. Briefly, a total of 10 µL of CCK-8 reagent was added to each well on days 1, 3, 5 and 7. After incubation for 30 min at 37 °C, absorbance at 450 nm was measured using a microplate Reader (BioTek, Winooski, USA). Cell proliferation was further calculated using a standard curve.
ASCs multi-lineage differentiation assessment
The multi-lineage differentiation capacity of the ASCs was detected as previously described [18]. Adipogenic differentiation was cultured at a concentration of 106 cells/mL in the adipogenic induction medium followed by culture for 14 days. Adipogenesis was assessed by staining with 0.5% Oil red O. For osteogenic differentiation, cells were cultured in osteogenic-inducing medium for 3 weeks and stained with alizarin red S. In chondrogenic differentiation, cells were maintained in chondrogenic medium for up to 5 weeks and stained with Alcian blue. All kits were purchased from Cyagen (Santa Clara, USA) and were conducted according to manufacturer’s protocol. Images were quantified using ImageJ 1.x (National Institutes of Health, USA). For detailed method of multi-lineage differentiation assessment, see Additional file 1.
Furthermore, multi-lineage differentiation was assessed by conducting quantitative real-time PCR for specific mRNAs.
Morphology assessment
Adipose tissues were fixed immediately in 4% paraformaldehyde, embedded in paraffin and sectioned at 6 μm for histological examination under a microscope (Nikon, Tokyo, Japan). The histological analysis was performed by H&E staining. Fibrosis was characterized by the presence of collagen fiber bundles and fibroblasts around adipocyte clusters, and vacuole was characterized as the cysts. The fibrotic and vacuole area was measured by ImageJ 1.x (National Institutes of Health, USA) [area (%) = area/total area of the same section * 100%].
For immunofluorescence study, the sections were washed and incubated at 4 °C overnight with the primary antibody against perilipin (1:200, Abcam, Cambridge, United Kingdom). The sections were then incubated with the secondary antibody incubation at 37 °C for 1 h. DAPI (Sigma-Aldrich, St. Louis, USA) was used for DNA counterstaining; 5 random views were imaged under a microscope at high magnification (Nikon, Tokyo, Japan).
RT-PCR
mRNA extraction and purification were executed by the TRIzol method following the standard protocol [19]. Relative gene expression was calculated by the ∆∆CT method and normalized to human TATA-box binding protein (TBP) for analysis. Primers are listed in Table 1. For detailed method of RT-PCR, see Additional file 1.
Statistical analysis
All data were collected from at least three independent replications. Experimental data were analyzed using GraphPad Prism 6 (GraphPad Software, San Diego, USA). Data comparison among multiple groups was performed using one-way analysis of variance (ANOVA) and Tukey’s post hoc test. Continuous variables are shown as the mean ± SD. p < 0.05 was considered statistically significant.
Results
The 70% glycerol group showed the highest tissue bioactivity in the G3PDH assay
After thawing and elution, the bioactivity of cryopreserved adipose tissue was evaluated by the G3PDH assay and compared with that of freshly harvested adipose tissue. G3PDH activity was 24.76 ± 0.48 in fresh adipose tissue and 24.41 ± 0.70 in the 70% glycerol group. No significant difference was found in the G3PDH activity between these two groups (p > 0.99), suggesting well-preserved tissue bioactivity in the 70% glycerol group.
The 60% glycerol (12.23 ± 0.61), 80% glycerol (15.93 ± 0.10), 90% glycerol (18.03 ± 2.47), FBS + DMSO (18.85 ± 1.49) and trehalose (18.38 ± 0.53) groups had moderately preserved G3PDH activity. These groups showed significant differences compared with the fresh group and the 70% glycerol group. The 100% glycerol group (3.49 ± 1.38) and blank group (0.92 ± 0.11) had the lowest G3PDH activity (Fig. 2A).
Bioactivity and morphological assay of the cryopreserved adipose tissue. A G3PDH assays in the CPA groups. All CPA groups were compared with fresh tissue. No significant difference was found between the 70% glycerol and fresh groups. B Images show HE-stained fat graft sections of 8 groups one month after fat grafting. All samples were cryopreserved at − 196 °C for 1 month. Necrosis was obvious in the blank group and the 80% glycerol group. A cyst is shown in the blank group. Scale bar: 250 µm. *p < 0.05, **p < 0.01, ***p < 0.001
The 60% and 70% glycerol, trehalose and F + D groups showed better histological features in vitro than the other groups
HE staining was performed in vitro after thawing and elution (Fig. 2B). The preserved adipose tissue showed varying degrees of tissue damage associated with freezing damage. In the CPA groups, the tissues from the DMSO + FBS, trehalose, 60% glycerol and 70% glycerol groups had a more integrated structure, while those from the 90% glycerol and 100% glycerol groups presented obvious tissue shrinkage and necrosis. Tissues from the blank group showed the most deteriorated structure.
ASCs were better preserved in the 70% glycerol group
The total of isolated SVF cells from 2 mL of adipose tissue were counted (Fig. 3A). Cell count was the highest in the 70% glycerol group [(10.28 ± 1.13) × 105 cells/mL)] and the FBS + DMSO group [11.3 ± 3.87 (× 105 cells/mL)], with no significant difference. The 60%, 90% and 100% glycerol groups, as well as the blank group, showed significantly lower counts of live SVF cells.
Stromal vascular fraction (SVF) cell viability and function. A The total number of SVF cells obtained from 2 mL of adipose tissue from different CPA groups. B Viability of the SVF cells. C CK-8 assay. D Left: Proliferation of ASCs. Growth density and morphology of the cells in the 70% glycerol group was better. Right: Oil red O staining showed that the 70% glycerol group had more red lipid droplets after adipogenic differentiation. E Quantification of lipid droplets areas after adipogenic differentiation. Scale bar: 250 µm. NS, no significance; *p < 0.05; **p < 0.01; ***p < 0.001
Viability of SVF cells was studied with trypan-blue stain identifying dead cells (Fig. 3B). 70% and 80% glycerol groups had the highest SVF cell viability (72.67 ± 5.80% and 61.63 ± 3.92%), with no significant difference found (p = 0.147). Compared to the 70% group, the FBS + DMSO (48.37 ± 5.53%), trehalose (58.83 ± 5.61%) and 60% glycerol groups (47.76 ± 4.55%) had significantly lower SVF cell viability (p < 0.001, FBS + DMSO vs 70% glycerol, 60% glycerol vs 70% glycerol; p = 0.04, trehalose vs 70% glycerol). The 90% glycerol (38.6 ± 2.95%) and 100% glycerol (33.13 ± 4.96%) groups had the worst SVF cell viability (Fig. 3B). Calcein blue AM/PI stain was also conducted to verify SVF cell viability, which had shown consistent results (Additional file 1: Figs. S4, S5).
Fat preserved in 70% glycerol showed better ASC morphology and better-preserved proliferation and multi-lineage differentiation ability than that in other groups
After sub-cultivation, the adherent ASCs presented a typical spindle-like shape. The results of the CCK-8 assay indicated that ASCs from the 70% glycerol group had a higher cell proliferation rate than those from the other cryopreserved groups (Fig. 3C, D, Additional file 1: Fig. S2A). ASCs from 70% glycerol group expressed negative CD45 and CD31, positive CD90, CD44, CD73 and CD105, indicating undifferentiated ASCs (Additional file 1: Fig. S1).
After adipogenic induction, lipid droplets areas were 19.91 ± 2.51%, 16.29 ± 2.27%, 14.66 ± 2.80%, 6.34 ± 2.15% in 70% glycerol, trehalose, FBS + DMSO and blank groups, respectively (Fig. 3D, Additional file 1: Fig. S2A). mRNA expression of PPARG and ADIPOQ was significantly higher in 70% glycerol group compared with trehalose and FBS + DMSO groups, further suggesting better adipogenic differentiation capacity (Additional file 1: Fig. S3). Blank group was eliminated from RT-PCR analysis because of too few cDNA acquired from digestion. Osteogenic and chondrogenic differentiation presented consistent results by morphology and RT-PCR analysis (Additional file 1: Figs. S2B, S3).
Tissues from the glycerol and trehalose groups had higher retention rates after transplantation
Four weeks after fat grafting, the transplanted tissues were harvested. Vessels were observed around the grafted adipose tissue, suggesting successful vascularization (Fig. 4A). The weight of the grafted fat was measured and calculated as volume retention (Fig. 4B). Tissues from the FBS + DMSO group had a retention rate of 19.52 ± 3.86%, while that in the blank group was 18.74 ± 4.05. These two groups had a similar retention rate (p > 0.99), which was significantly lower than that of the other groups. The 60% glycerol (51.87 ± 6.13), 70% glycerol (52.37% ± 7.53), 80% glycerol (47.69% ± 6.89) and trehalose groups (46.11% ± 12.26) all presented higher retention rates, with no significant difference among them (Fig. 4C).
Adipose tissue preserved in 70% glycerol showed better histological features after transplantation than other tissues
The 70% glycerol group demonstrated a well-preserved tissue structure, with few areas of cysts and fibrosis. Adipocytes were plump and well organized. The blank group showed a disorganized tissue structure, in which fibrosis and vacuoles occupied most areas of the grafted tissue instead of normal adipocytes. Other groups generally demonstrated preserved structural integrity. However, among them, the 60% glycerol group showed more cysts; the 80% glycerol group and FBS + DMSO group demonstrated more fibrotic areas. The trehalose groups had a preserved general structure, but interstitial fibrosis was severe (Fig. 5A).
Adipose tissue histology after fat graft. A Representative images of HE-stained fat grafts four weeks after grafting. Preserved tissue construction and integrity were observed in the 60%, 70% and 80% glycerol groups, while the 60% glycerol group had more obvious vacuole cyst (△) formation. The trehalose group demonstrated apparent fibrosis and inflammatory infiltration (*). Fibrosis, inflammation (*) and vacuole formation (△) were detected in the FBS + DMSO group. The blank group demonstrated a disorganized structure, fibrosis and inflammatory (*) infiltration. (Scale bar: 250 µm.) B The fibrotic areas of grafted fat samples. C The vacuole areas of grafted fat samples. *p < 0.05, **p < 0.01, ***p < 0.001
Histological quantification of the grafted cryopreserved tissue was performed. The fibrotic area was 12.70% ± 3.26 in the 70% group, with no significant difference compared with that of the 80% glycerol group (20.02% ± 1.77, p = 0.958) and the trehalose group (35.95% ± 4.17, p = 0.207). The fibrotic area was larger in the 60% glycerol group (41.70% ± 23.42, p = 0.048) and the FBS + DMSO group (47.53% ± 6.88, p = 0.024) than in the 70% glycerol group. The blank group had a fibrotic area up to 53.38% ± 16.39 (Fig. 5B).
The vacuole area was also calculated (Fig. 5C). The 70% glycerol group had the lowest vacuole rate (11.35% ± 3.97). The vacuole rates were moderately higher in the 80% glycerol group (16.49% ± 9.71, p = 0.920) and the trehalose group (15.40% ± 7.23, p = 0.978), with no significant difference from that in the 70% glycerol group. The other three groups, the 60% glycerol (35.35% ± 10.50), FBS + DMSO (31.49% ± 4.02) and blank groups (30.14% ± 6.41), all had significantly larger vacuole areas.
Perilipin is a reliable indicator of living adipocytes [20]. The results showed that samples from the 70% glycerol group had the most preserved structure of mature adipocytes, which were round in shape and had a continuous membrane. In contrast, perilipin-positive adipocytes were lower in number or lost the oil-containing structures of the other groups (Fig. 6).
Expression of proinflammatory cytokine genes was lower in the glycerol groups than the other groups
QPCR analyses evaluated the inflammatory and apoptotic activities of grafted adipose tissues. Overall, the expression of the proinflammatory cytokines IL-1, IL-6 and TNF-α was higher in the FBS + DMSO group than in the glycerol groups. Among all glycerol groups, the 70% glycerol group showed the lowest level of proinflammatory gene expression. The relative expression of IL-1 and TNF-α in the fat grafts from the 70% glycerol group was significantly lower than that in the fat grafts from the 60% and 80% glycerol groups (Fig. 7A, C). The expression of IL-1, IL-6 and TNF-α in the trehalose group was similar to that in the 70% glycerol group.
The expression of Caspase-9 was significantly higher in the blank group than in the other groups. No significant difference was found in Caspase-9 expression among the glycerol groups, trehalose group and FBS + DMSO group (Fig. 7D).
Discussion
Proper storage of harvested adipose tissue after thawing and grafting avoids repeated liposuction procedures, thus increasing patient acceptance, reducing patient suffering, and saving time and costs [20]. Current studies regarding the efficacy and reliability of adipose tissue cryopreservation are controversial [4, 5, 21]. Lack of proper CPA is the main obstacle.
Cryopreservation of isolated cells or uniform tissues has been widely studied and applied. However, cryopreservation of composite tissues is a challenge. Different cells in the tissue may have various cryopreservation requirements [13]. Because the structure of the tissue is integrated, it is more difficult for CPAs to infiltrate the tissue thoroughly, especially for adipose tissue.
Several studies have reported that using DMSO or DMSO + FBS as CPAs was effective in protecting cell survival and retention volume of the adipose tissue compared with the absence of CPA [9, 22,23,24,25]. However, the use of DMSO and FBS has the risk of toxicity and zoonotic infection [18]. Furthermore, due to the integrity and complexity of tissue compared with isolated cells, thorough elution of DMSO may be difficult [26, 27]. Our study also found that DMSO + FBS had increased fibrosis and inflammation in cryopreserved adipose tissue.
Our study revealed that glycerol at an optimal concentration is an effective agent for adipose tissue cryopreservation. Among all concentrations tested, 70% glycerol solution appeared to be most potent in preserving tissue bioactivity. The tissue from the 70% glycerol group showed comparable activity with fresh tissue when assessed by the G3PDH assay. In vivo studies also demonstrated that the cryopreserved adipose tissue from the 70% and 80% glycerol groups had a higher retention rate and less inflammation and fibrosis after transplantation than the adipose tissue from the DMSO + FBS group. This study also showed that groups with decreased tissue activity had more in vivo fibrosis and inflammation, which is consistent with previous studies showing that oil cyst formation, chronic inflammation and progressive calcification are the results of fat necrosis [28]. Moreover, the SVF isolated from adipose tissue contains a large number of ASCs [25]. The quantity and activity of SVF cells may explain the preserved volume and structure after grafting.
The mechanism underlying the advantages of glycerol in adipose tissue preservation may be as follows: compared with other tissues, adipose tissue has unique characteristics because it is mainly comprised of adipocytes, which contain large amounts of triglycerides. The chemical similarity of glycerol and triglyceride may help cryopreservation. Studies have shown that glycerol, as the backbone of triglycerides stored in adipocytes, is transported outward through glycerol transporters [14]. With an increased concentration of glycerol in the extracellular matrix, glycerol efflux might be inhibited, thus better preserving the structure and bioactivity of adipocytes [29]. Moreover, glycerol is nontoxic and generally nonallergenic to cells and tissues, characteristics that make it suitable for clinical use [13].
We evaluated the cryopreserved tissue morphology, bioactivity and immunohistochemical staining. Histological observation was critical to determine the structural features of the tissue. Immunofluorescence provided further information on cell viability and plasma membrane continuity. Bioactivity in our study was mainly studied by G3PDH assay. The G3PDH assay was conducted on the entire tissue without digestion, and assessed cellular function of fatty tissues due to adipocyte specificity that previously reported [30]. Our results showed that when tissue bioactivity deteriorated, increased levels of fibrosis and inflammation were observed after transplantation. Although the volume retention rate did not decrease sharply, the integrity of the adipose tissue structure was destroyed, with a large area of oil cysts and fibrosis [20].
In this study, we cryopreserved adipose tissue for 1 month. Previous studies on adipose tissue cryopreservation used various freezing durations, from days to 2 months [2, 6, 7, 9, 20, 31]. Whether cryopreservation duration would affect the viability of cells and tissues remains controversial. While Ntai et al. reported that there was no difference in the viability of pluripotent stem cells cryopreserved for 7 days and for 30 days [32], Guttridge et al. indicated that cryopreservation duration may affect the viability of cryopreserved blood cells [33]. Moreover, there are no present study concerning the effect of storage time on complex tissues, such as adipose tissue. Thus, the impact of storage duration on adipose tissue cryopreservation may be required in future research. Another limitation is that although glycerol is nontoxic and preclinical research has shown good efficacy, further clinical trials are required to evaluate its clinical efficiency.
Conclusions
Our study demonstrated that glycerol solutions can serve as CPAs for long-term cryopreservation of adipose tissue. Among all concentrations studied, 70% glycerol solution was superior at preserving adipose tissue bioactivity, reducing the retention rate, and preventing tissue fibrosis and inflammation in cryopreserved adipose tissue. Furthermore, glycerol is a nontoxic and nonimmunogenic agent and thus is safer and more compatible with clinical use than other CPAs. Future studies are required to further prove the safety and optimize the reliability of adipose tissue cryopreservation using this method.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASC:
-
Adipose-derived stem cell
- CCK8:
-
Cell Counting Kit-8
- CPA:
-
Cryoprotective agent
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- G3PDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
References
Vallejo A, Garcia-Ruano AA, Pinilla C, Castellano M, Deleyto E, Perez-Cano R. Comparing efficacy and costs of four facial fillers in human immunodeficiency virus-associated lipodystrophy: a clinical trial. Plast Reconstr Surg. 2018;141(3):613–23.
Ha KY, Park H, Park SH, Lee BI, Ji YH, Kim TY, et al. The relationship of a combination of human adipose tissue-derived stem cells and frozen fat with the survival rate of transplanted fat. Arch Plast Surg. 2015;42(6):677–85.
Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118(3 Suppl):121S-S128.
Erol OO, Agaoglu G. Facial rejuvenation with staged injections of cryopreserved fat and tissue cocktail: clinical outcomes in the past 10 years. Aesthet Surg J. 2013;33(5):639–53.
Butterwick KJ, Bevin AA, Iyer S. Fat transplantation using fresh versus frozen fat: a side-by-side two-hand comparison pilot study. Dermatol Surg. 2006;32(5):640–4.
Li BW, Liao WC, Wu SH, Ma H. Cryopreservation of fat tissue and application in autologous fat graft: in vitro and in vivo study. Aesthet Plast Surg. 2012;36(3):714–22.
Conti G, Jurga M, Benati D, Bernardi P, Mosconi E, Rigotti G, et al. Cryopreserved subcutaneous adipose tissue for fat graft. Aesthet Plast Surg. 2015;39(5):800–17.
Shu Z, Gao D, Pu LL. Update on cryopreservation of adipose tissue and adipose-derived stem cells. Clin Plast Surg. 2015;42(2):209–18.
Cui XD, Gao DY, Fink BF, Vasconez HC, Pu LL. Cryopreservation of human adipose tissues. Cryobiology. 2007;55(3):269–78.
Atik B, Ozturk G, Erdogan E, Tan O. Comparison of techniques for long-term storage of fat grafts: an experimental study. Plast Reconstr Surg. 2006;118(7):1533–7.
Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20(6):1676–87.
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–91.
Bakhach J. The cryopreservation of composite tissues: principles and recent advancement on cryopreservation of different type of tissues. Organogenesis. 2009;5(3):119–26.
Lebeck J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J Mol Endocrinol. 2014;52(2):R165–78.
Pu LL, Cui X, Fink BF, Cibull ML, Gao D. Cryopreservation of adipose tissues: the role of trehalose. Aesthet Surg J. 2005;25(2):126–31.
Basel-Vanagaite L, Zevit N, Har Zahav A, Guo L, Parathath S, Pasmanik-Chor M, et al. Transient infantile hypertriglyceridemia, fatty liver, and hepatic fibrosis caused by mutated GPD1, encoding glycerol-3-phosphate dehydrogenase 1. Am J Hum Genet. 2012;90(1):49–60.
Rao W, Huang H, Wang H, Zhao S, Dumbleton J, Zhao G, et al. Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Appl Mater Interfaces. 2015;7(8):5017–28.
Zhang TY, Tan PC, Xie Y, Zhang XJ, Zhang PQ, Gao YM, et al. The combination of trehalose and glycerol: an effective and non-toxic recipe for cryopreservation of human adipose-derived stem cells. Stem Cell Res Ther. 2020;11(1):460.
Bortolotto JW, Margis R, Ferreira AC, Padoin AV, Mottin CC, Guaragna RM. Adipose tissue distribution and quantification of PPARbeta/delta and PPARgamma1-3 mRNAs: discordant gene expression in subcutaneous, retroperitoneal and visceral adipose tissue of morbidly obese patients. Obes Surg. 2007;17(7):934–40.
Mashiko T, Wu SH, Kanayama K, Asahi R, Shirado T, Mori M, et al. Biological properties and therapeutic value of cryopreserved fat tissue. Plast Reconstr Surg. 2018;141(1):104–15.
Lidagoster MI, Cinelli PB, Levee EM, Sian CS. Comparison of autologous fat transfer in fresh, refrigerated, and frozen specimens: an animal model. Ann Plast Surg. 2000;44(5):512–5.
Qiu KMWM, Liang YC, Luo JS, Xie HJ. The viability and the transplanted survival rate analysis of fat granule in cryopreservation with different frozen conditions. Basic Clin Med. 2014;34(04):536–40.
Xiao BLY, Yu S, Chen SZ, Zhang XS, Tian WY. Selection of cryoprotector in preservation of fat granule at different frozen conditions. Chin J Aesthet Med. 2005;4(1):397–9.
Sari E, Bakar B, Sarkarati B, Bozdogan O, Cavusoglu T. Effectiveness of dimethylsulfoxide on the survival and volume preservation of autologous fat graft tissue: a preliminary study. Aesthet Surg J. 2016;36(2):NP58–67.
Bae YC, Song JS, Bae SH, Kim JH. Effects of human adipose-derived stem cells and stromal vascular fraction on cryopreserved fat transfer. Dermatol Surg. 2015;41(5):605–14.
Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49(4):469–76.
Nakamura Y, Obata R, Okuyama N, Aono N, Hashimoto T, Kyono K. Residual ethylene glycol and dimethyl sulphoxide concentration in human ovarian tissue during warming/thawing steps following cryopreservation. Reprod Biomed Online. 2017;35(3):311–3.
Mineda K, Kuno S, Kato H, Kinoshita K, Doi K, Hashimoto I, et al. Chronic inflammation and progressive calcification as a result of fat necrosis: the worst outcome in fat grafting. Plast Reconstr Surg. 2014;133(5):1064–72.
Martins SV, Madeira A, Lopes PA, Pires VM, Alfaia CM, Prates JA, et al. Adipocyte membrane glycerol permeability is involved in the anti-adipogenic effect of conjugated linoleic acid. Biochem Biophys Res Commun. 2015;458(2):356–61.
Pu LL, Cui X, Fink BF, Cibull ML, Gao D. Long-term preservation of adipose aspirates after conventional lipoplasty. Aesthet Surg J. 2004;24(6):536–41.
Shoshani O, Ullmann Y, Shupak A, Ramon Y, Gilhar A, Kehat I, et al. The role of frozen storage in preserving adipose tissue obtained by suction-assisted lipectomy for repeated fat injection procedures. Dermatol Surg. 2001;27(7):645–7.
Ntai A, La Spada A, De Blasio P, Biunno I. Trehalose to cryopreserve human pluripotent stem cells. Stem Cell Res. 2018;31:102–12.
Guttridge MG, Soh TG, Belfield H, Sidders C, Watt SM. Storage time affects umbilical cord blood viability. Transfusion. 2014;54(5):1278–85.
Acknowledgements
We thank American Journal Experts (AJE) for English language editing.
Funding
This study was supported by National Natural Science Foundation of China (81971848; 81620108019); Clinical Research Plan of SHDC (SHDC2020CR1019B; SHC2020CR402); Shanghai Municipal Key Clinical Specialty (shslczdzk00901); Innovative research team of high-level local universities in Shanghai (SSMU-ZDCX20180700).
Author information
Authors and Affiliations
Contributions
PQZ, SBZ and QFL initiated and designed the study and protocol. PQZ and PCT conducted experiments. PQZ and PCT contributed to data collection, data analysis and data interpretation. YMG, XJZ and DNZ. YX and SBZ performed liposuction surgeries. PQZ wrote the first draft of the manuscript. SBZ and QFL critiqued and modified the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shanghai Ninth People's Hospital and complied with the principles of the Declaration of Helsinki. All participants provided written informed consent for the scientific use of aspirated adipose tissue. All animal experiments were performed in accordance with the Animal Use Committee of the Shanghai Jiao Tong University animal guidelines.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary materials, including supplemental methods and the supplemental figures S1–S5. The supplemental methods contain the protocols of following experiments: Live/dead cell stain and flow cytometry, RT-PCR, flow cytometry and surface marker identification, ASCs multi-lineage differentiation assessment, quantify lipid droplets area, and immunofluorescence study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, PQ., Tan, PC., Gao, YM. et al. The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res Ther 13, 152 (2022). https://doi.org/10.1186/s13287-022-02817-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02817-z