Abstract
Background
Stem cell therapy is becoming an emerging therapeutic option for chronic liver disease (CLD). However, whether stem cell therapy is more effective than conventional treatment remains questionable. We performed a large-scale meta-analysis of randomized controlled trials (RCTs) to evaluate the therapeutic effects and safety of stem cell therapy for CLD.
Methods
We systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov databases for the period from inception through March 16, 2020. Primary outcomes were all-cause mortality and adverse events related to stem cell therapy. Secondary outcomes included the model for end-stage liver disease score, total bilirubin, albumin, alanine aminotransferase, prothrombin activity, and international normalized ratio. The standardized mean difference (SMD) and odds ratio (OR) with 95% confidence interval (CI) were calculated using a random-effects model.
Results
Twenty-four RCTs were included and the majority of these studies showed a high risk of bias. The meta-analysis indicated that compared with conventional treatment, stem cell therapy was associated with improved survival and liver function including the model of end-stage liver disease score, total bilirubin, and albumin levels. However, it had no obvious beneficial effects on alanine aminotransferase level, prothrombin activity, and international normalized ratio. Subgroup analyses showed stem cell therapy conferred a short-term survival benefit for patients with acute-on-chronic liver failure (ACLF), a single injection was more effective than multiple injections, hepatic arterial infusion was more effective than intravenous infusion, and bone marrow-derived stem cells were more effective than those derived from the umbilical cord. Thirteen trials reported adverse events related to stem cell therapy, but no serious adverse events were reported.
Conclusions
Stem cell therapy is a safe and effective therapeutic option for CLD, while patients with ACLF benefit the most in terms of improved short-term survival. A single injection administration of bone marrow-derived stem cells via the hepatic artery has superior therapeutic effects.
Similar content being viewed by others
Background
Chronic liver disease (CLD), mainly those arising from hepatitis viral infection, toxic injury, alcohol abuse, metabolic disorders, or genetic defect, is an important global health concern. In China alone, it was estimated that more than 400 million people suffered CLD, primarily viral hepatitis, metabolic associated fatty liver disease, and alcoholic liver disease [1]. Given the natural history of CLD, these patients are at high risk of progressing to advanced fibrosis, cirrhosis, and cirrhosis-related complications including acute-on-chronic liver failure (ACLF) and hepatocellular carcinoma. Cirrhosis and liver cancer ranked the 11st and 16th among the leading causes of death worldwide in 2016, respectively, representing a death toll of more than 2 million [2]. Currently, liver transplantation (LT) is the ultimate curative treatment for end-stage liver disease. However, limited organ availability, high costs, transplant-associated complications, and lifelong immunological side effects preclude many patients from benefiting from LT [3, 4]. Therefore, people have been seeking alternative therapeutic strategies to LT.
Stem cell therapy is becoming an emerging therapeutic option for CLD with great potential, because it is a less invasive curative with potentially equal effect compared to LT [5, 6]. Although a growing number of clinical researches, ranging from early proof-of-concept studies to randomized controlled trials (RCTs), have been carried out to explore the safety and efficacy of stem cell therapy in a range of different settings of liver diseases, whether stem cell therapy is associated with better therapeutic effects than conventional treatment remains unknown and its safety profile as well [7, 8]. More importantly, regarding cell source (autologous or allogeneic; bone marrow or umbilical cord blood), administration dose, infusion route (intrahepatic, intrasplenic, or intravenous), and delivery frequency (singular or multiple), no standardized protocols have been published to date, although these factors are undoubtedly the leading ones among those influencing the therapeutic effects of stem cell therapy, and they can even cause a series of side effects [7, 9, 10].
Previous systematic reviews pooled analysis of both RCTs and non-RCTs [11,12,13], but studies of different designs should not be analyzed in unification. Furthermore, none of them has included all relevant randomized trials, while RCTs are assessed as the best corroboration of the efficacy of new treatments in evidence-based medicine. Thus, previously published systematic reviews only have limited power to determine whether patients with CLD can benefit from stem cell therapy. Therefore, we conducted a systematic review and meta-analysis of all currently available RCTs to provide a more comprehensive and quantitative understanding of the therapeutic effects and safety of stem cell therapy for treating CLD.
Methods
This systematic review and meta-analysis has been registered in PROSPERO (CRD42020175317). We followed the recommendations from the Cochrane Collaboration for systematic review and meta-analysis of RCTs and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement [14].
Search strategy
We searched RCTs involving CLD patients treated with implantation of all kinds of stem cells from electronic medical databases including MEDLINE (PubMed), Ovid EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov from initial period to March 16, 2020. Key searching terms were “liver disease,” “stem cells,” “stem cell transplantation,” and “randomized controlled trial.” MeSH terms and free-text terms, as well as variation of root words, were combined within each database. No language restrictions were applied during the searches. The reference list of the eligible articles and relevant review articles were also checked to identify additional studies. The detailed search strategies are outlined in Additional file 1: Table S1.
Study selection
Two reviewers (G-PZ, Y-ZJ) independently screened the titles and abstracts of retrieved publications. We retrieved the full-text articles of the studies that were deemed potentially eligible for a review as a whole. Any disagreements were resolved through discussion with a third reviewer (L-YS). The inclusion criteria were (1) RCTs, (2) patients diagnosed with CLD, (3) patients in the experimental group received stem cell therapy and patients in the control group were treated with conventional treatment, and (4) availability of clinical outcomes. Primary outcomes were all-cause mortality and adverse events related to stem cell therapy. Secondary outcomes included the model for end-stage liver disease (MELD) score, liver function parameters (total bilirubin (TBIL), albumin (ALB), and alanine aminotransferase (ALT)), and coagulation function (prothrombin activity (PTA) and international normalized ratio (INR)). Studies were excluded if (1) they were animal-based, review articles, or case reports or (2) their full-text or adequate information was not available. When duplicate reports from the same study were identified, only the one with more information was included.
Data extraction
Two authors (G-PZ, Y-ZJ) extracted data from included studies independently, and disagreements were resolved through a discussion with a third reviewer (L-YS). The following information was extracted from the included studies using a predefined data form: study characteristics (first author, year of publication, country, study design, enrollment period, number of participants in the experimental and control groups, and follow-up duration), patient characteristics (age, sex, and liver disease type), stem cells (number, type, delivery route, and frequency of administration), and outcome measures.
Quality assessment
The risk of bias for each included study was independently assessed by two authors (G-PZ, Y-ZJ) using the Cochrane Collaboration’s Risk of Bias tool [15]. The evaluation domains included selection bias (allocation sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other bias. For each domain, studies were judged as low, high, or unclear risk of bias according to the Cochrane Handbook.
Statistical analysis
By using a random-effects model, continuous and dichotomous outcome variables were calculated as standardized mean difference (SMD) and odds ratio (OR) with 95% confidence interval (CI), respectively. Heterogeneity between studies was assessed using Cochran’s Q test and I2 statistic. In case of substantial heterogeneity (I2 > 50%), a sensitivity analysis with omission of one study at a time was conducted. To explore the potential influence factors of stem cell therapy for treating CLD, pre-planned subgroup analyses based on liver disease type (ACLF and CLD without ACLF), cell type [bone marrow-derived mesenchymal stem cells (BM-MSCs), bone marrow-derived mononuclear stem cells (BM-MNCs), or umbilical cord-derived mesenchymal stem cells (UC-MSCs)], delivery route (peripheral intravenous infusion and hepatic arterial infusion), and frequency of administration (single injection and multiple injections) were performed. Where sufficient studies were available (the number of included studies ≥ 10), publication bias was evaluated based on the funnel plot, Egger’s test for continuous endpoints and Harbord’s test for dichotomous endpoints [16, 17]. If the funnel plot was asymmetrical, contour-enhanced funnel plots combined with trim and fill analysis was conducted to explore the source of publication bias [18, 19]. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the Review Manager software (version 5.3) and STATA 14 software (Stata Corp).
Results
Study selection
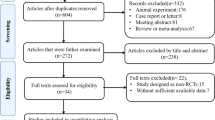
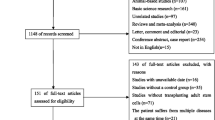
We identified a total of 2862 potentially eligible articles by searching the four databases and the reference lists of retrieved articles and relevant reviews, of which 431 were excluded due to duplication. After the title and abstract review, 2338 articles were further excluded, with 93 potentially relevant articles left. After a detailed assessment of the full texts, 69 papers were further excluded. Finally, 24 studies [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] were included in the present meta-analysis. The flow diagram of the selection of studies is listed in Fig. 1.
Study characteristics
The characteristics of the 24 included studies are presented in Table 1. These studies were published between 2010 and 2019 from Brazil (n = 1), China (n = 13), Egypt (n = 4), Iran (n = 3), South Korea (n = 1), Switzerland (n = 1), and the UK (n = 1). A total of 1359 patients were included, with 746 patients receiving stem cell therapy and 613 patients undergoing conventional treatment. The studies included patients with liver fibrosis (n = 1), liver cirrhosis (n = 17), and liver failure (n = 6). Stem cells were derived from the bone marrow (BM-MSCs; n = 8 and BM-MNCs; n = 6), umbilical cord (UC-MSCs; n = 8), and peripheral blood (PBSCs; n = 2), 15 of which involve autologous transplants while the remaining ones involve allogeneic transplants. Stem cells were delivered into the liver through the peripheral vein (n = 12), hepatic artery (n = 7), portal vein (n = 2), or multiple routes (n = 3). A single cell injection was adopted in 11 studies, multiple cell injections in 11 studies, and both (single and multiple injections) in 2 studies.
Risk of bias of the included studies
The majority of the included studies showed a high risk of bias, which mainly resulted from the lack of allocation concealment, absent blinding, and incomplete outcome data. Three studies [25, 39, 40] were considered to have an unclear risk of other bias because it is unclear whether there is free of for-profit bias. Further details are presented in Fig. 2.
Therapeutic safety and efficacy assessments
All-cause mortality
Seventeen studies (3452 participants) were included in the analysis of all-cause mortality (Fig. 3). Compared with conventional treatment, stem cell therapy was associated with significantly lower all-cause mortality, as indicated by decreased all-cause mortality at week 4 (OR = 0.24, 95% CI 0.11 to 0.51; P = 0.0002), week 12 (OR = 0.49, 95% CI 0.29 to 0.80; P = 0.005), and week 48 (OR = 0.56, 95% CI 0.37 to 0.87; P = 0.01).
We found substantial heterogeneity at week 24 only (I2 = 55%). By excluding one study [21], sensitivity analyses showed the heterogeneity among the remaining studies was eliminated (Additional file 2: Table S2). Publication bias was evaluated at weeks 4, 12, and 48, and the funnel plot and Harbord’s test indicated evident publication bias at week 48 (Additional file 3: Fig. S1). Symmetrical contour-enhanced funnel plot combined with trim and fill analysis suggested the asymmetry in the funnel plot was partly attributed to publication bias (Additional file 4: Fig. S2).
MELD score
Fifteen studies (3098 participants) were included in the analysis of MELD scores (Fig. 4). Before treatment, no significant difference was observed between the experimental and control groups (SMD = − 0.14, 95% CI − 0.28 to 0.00; P = 0.06). After treatment, stem cell therapy was associated with significantly lower MELD scores at week 2 (SMD = − 0.79, 95% CI − 1.44 to − 0.15; P = 0.02), week 8 (SMD = − 0.58, 95% CI − 0.84 to − 0.32; P < 0.0001), week 12 (SMD = − 0.37, 95% CI − 0.62 to − 0.12; P = 0.003), and week 24 (SMD = − 0.57, 95% CI − 0.92 to − 0.23; P = 0.001).
We found substantial heterogeneity at most of the time points (I2 = 57–86%). By excluding the results of Xu et al. [31] at week 4, Zekri et al. [34] at week 12, and Mohamadnejad et al. [27] at week 48, sensitivity analyses showed lowered heterogeneity among the remaining studies at each time point (Additional file 2: Table S2). Publication bias was evaluated at weeks 12 and 24, and the funnel plot and Egger’s test indicated no evident publication bias (Additional file 3 Fig. S1).
TBIL level
Nineteen studies (4708 participants) were included in the analysis of the TBIL level (Fig. 5). Before treatment, no significant difference was observed between the experimental and control groups (SMD = − 0.04, 95% CI − 0.17 to 0.09; P = 0.53). After treatment, stem cell therapy was associated with significantly lower TBIL levels at week 4 (SMD = − 0.31, 95% CI − 0.58 to − 0.05; P = 0.02), week 12 (SMD = − 0.43, 95% CI − 0.70 to − 0.17; P = 0.001), week 24 (SMD = − 0.40, 95% CI − 0.75 to − 0.05; P = 0.02), and week 48 (SMD = − 0.29, 95% CI − 0.51 to − 0.06; P = 0.01).
We found substantial heterogeneity at most of the time points (I2 = 62–88%). By excluding the results of Zhang et al. [25] at weeks 4, 8, and 12, Lin et al. [38] at weeks 12 and 24, and Shi et al. [24] at week 36, sensitivity analyses showed lowered heterogeneity among the remaining studies at each time point (Additional file 2: Table S2). Publication bias was evaluated at weeks 4, 12, and 24, and the funnel plot and Egger’s test indicated evident publication bias in the TBIL level at week 12 (Additional file 1: Fig. S1). Symmetrical contour-enhanced funnel plot combined with trim and fill analysis suggested the asymmetry in the funnel plot was not caused by publication bias (Additional file 1: Fig. S2).
ALB level
Seventeen studies (4173 participants) were included in the analysis of the ALB level (Fig. 6). Before treatment, no significant difference was observed between the experimental and control groups (SMD = − 0.02, 95% CI − 0.27 to 0.23; P = 0.88). After treatment, stem cell therapy was associated with significantly increased ALB levels at week 2 (SMD = 0.69, 95% CI 0.03 to 1.35; P = 0.04), week 4 (SMD = 0.40, 95% CI 0.13 to 0.66; P = 0.003), week 8 (SMD = 0.61, 95% CI 0.11 to 1.12; P = 0.02), week 24 (SMD = 0.62, 95% CI 0.03 to 1.21; P = 0.04), week 36 (SMD = 1.42, 95% CI 0.56 to 2.28; P = 0.001), and week 48 (SMD = 0.95, 95% CI 0.07 to 1.83; P = 0.03).
We found substantial heterogeneity at all time points (I2 = 59–92%). By excluding the results of Zekri et al. [34] at week 2, Salama et al. [30] at week 4, and Mohamadnejad et al. [27] at week 48, sensitivity analyses showed lowered heterogeneity among the remaining studies at each time point (Additional file 2: Table S2). Publication bias was evaluated at weeks 4, 12, and 24, and the funnel plot and Egger’s test indicated no evident publication bias (Additional file 3 Fig. S1).
ALT level
Sixteen studies (3670 participants) were included in the analysis of the ALT level (Fig. 7). Before treatment, no significant difference was observed between the experimental and control groups (SMD = − 0.08, 95% CI − 0.21 to 0.06; P = 0.26). After treatment, stem cell therapy was associated with significantly lower ALT levels only at week 12 (SMD = − 0.54, 95% CI − 0.91 to − 0.17; P = 0.004).
We found substantial heterogeneity at most of the time points (I2 = 54–90%). By excluding the results of Lin et al. [23] at week 2, Salama et al. [21] at week 4, Xu et al. [43] at week 8, and Shi et al. [24] at week 48, sensitivity analyses showed lowered heterogeneity among the remaining studies (Additional file 2: Table S2). Publication bias was evaluated at weeks 4, 12, and 24, and the funnel plot and Egger’s test indicated no evident publication bias (Additional file 3 Fig. S1).
Coagulation function (PTA and INR)
Ten studies with 2853 participants and 9 studies with 2151 participants were included in the analysis of the PTA level (Fig. 8) and INR level (Fig. 9), respectively. Before treatment, no significant difference in PTA level and INR level was observed between the experimental and control groups [(SMD = 0.04, 95% CI − 0.17 to 0.24; P = 0.71), (SMD = 0.13, 95% CI − 0.53 to 0.27; P = 0.53)]. After treatment, stem cell therapy was associated with significantly increased PTA level at week 24 (SMD = 0.51, 95% CI 0.09 to 0.94; P = 0.02) and lowered INR level at week 8 (SMD = − 0.53, 95% CI − 0.87 to − 0.19; P = 0.002).
We found substantial heterogeneity at most of the time points (I2 = 67–93%). By excluding the results of Salama et al. [21] at week 8 (PTA), Salama et al. [21] at week 4 (INR), and Zekri et al. [34] at week 48 (INR), sensitivity analyses showed lowered heterogeneity among the remaining studies (Additional file 2: Table S2). Due to the insufficient number of included studies, publication bias was not evaluated.
Subgroup analysis
We conducted subgroup analyses to explore whether the effects of stem cell therapy on mortality, MELD score, and TBIL, ALB, ALT, and PTA levels at weeks 4, 12, and 24 were influenced by different disease populations, cell type, delivery route, and administration frequency (Fig. 10).
Subgroup analyses by the liver disease type, cell type, delivery route, and frequency of administration. Red indicates a significant improvement in the stem cell therapy group compared with the conventional treatment group; blue indicates no significant improvement. CLD chronic liver disease, ACLF acute-on-chronic liver failure, BM-MSC bone marrow-derived mesenchymal stem cell, UC-MSC umbilical cord-derived mesenchymal stem cell, BM-MNC bone marrow-derived mononuclear stem cell
Liver disease type (ACLF versus CLD without ACLF)
Compared with the conventional treatment group, stem cell therapy was associated with lower all-cause mortality in the ACLF subgroup, as indicated by decreased all-cause mortality at week 4. Stem cell therapy was associated with more improved liver functions in the CLD without ACLF subgroup, as indicated by decreased MELD scores, decreased TBIL levels, and increased ALB levels at weeks 4, 12, and 24. Stem cell therapy was associated with more improved liver functions in the ACLF subgroup, as indicated by decreased MELD score at week 12, decreased ALT level at week 24, and increased PTA level at week 24.
Cell type (BM-MSCs, UC-MSCs versus BM-MNCs)
Compared with the conventional treatment group, stem cell therapy was associated with lower all-cause mortality in the BM-MSC and US-MCS subgroups, as indicated by decreased all-cause mortality at week 4. Stem cell therapy was associated with more improved liver functions in the BM-MSC subgroup, as indicated by decreased MELD score at week 24, decreased TBIL level at week 4, decreased ALT levels at week 12, and increased PTA levels at weeks 4 and 12. Stem cell therapy was associated with more improved liver functions in the BM-MNC subgroup, as indicated by decreased MELD score at week 24, decreased TBIL level at weeks 12 and 24, increased ALB levels at weeks 12 and 24, decreased ALT levels at week 4, and increased PTA levels at weeks 12 and 24. Stem cell therapy was associated with more improved liver functions in the UC-MSC subgroup, as indicated by decreased MELD score at week 12 and TBIL level at week 12.
Delivery route (peripheral vein versus hepatic artery)
Compared with the conventional treatment group, stem cell therapy was associated with lower all-cause mortality in the peripheral vein administration subgroup, as indicated by decreased all-cause mortality at weeks 4 and 12. Stem cell therapy was associated with more improved liver functions in the hepatic artery administration subgroup, as indicated by decreased MELD scores and TBIL levels at weeks 12 and 24; increased ALB levels at weeks 4, 12, and 24; and increased PTA levels at weeks 12 and 24. Stem cell therapy was associated with more improved liver functions in the peripheral vein administration subgroup, as indicated by decreased ALT level at week 24.
Frequency of administration (single injection versus multiple injections)
Compared with the conventional treatment group, stem cell therapy was associated with lower all-cause mortality in the multiple injection subgroup, as indicated by decreased all-cause mortality at weeks 4 and 12. Stem cell therapy was associated with more improved liver functions in the single injection subgroup, as indicated by decreased MELD scores and TBIL levels at weeks 12 and 24; increased ALB levels at weeks 4, 12, and 24; and increased PTA levels at weeks 4, 12, and 24. Stem cell therapy was associated with more improved liver functions in the multiple injection subgroup, as indicated by decreased MELD score at week 12 and decreased ALT levels at weeks 12 and 24.
Adverse events associated with stem cell therapy
Five studies [27, 32, 35, 37, 39] reported that there were no procedural complications after cell infusion, while thirteen studies [20,21,22, 24,25,26, 28, 31, 33, 36, 38, 40, 43] reported adverse events of stem cell therapy, including fever, transient shivering, local pain, ecchymosis/hematoma, rash, diarrhea, chest tightness, and constipation, most of which resolved spontaneously (Additional file 5: Table S3).
Discussion
In the present study, we produced a comprehensive meta-analysis of 24 randomized clinical trials to evaluate the therapeutic effects and safety of stem cell therapy in the treatment of patients with CLD. To our knowledge, it is the systematic review that includes the most RCTs up to now. Our study suggests compared with conventional treatment, stem cell therapy was associated with more favorable therapeutic effects, including lowered mortality and MELD scores, increased ALB levels, and decreased TBIL levels, while improvement in ALT, PTA, or INR was not evident. No serious adverse events related to the implantation of stem cells were reported. Overall, available evidence indicates that stem cell therapy is a safe and efficient treatment option for CLD.
Since safety is a major concern when initiating a new therapeutic strategy, our analysis evaluated the safety of stem cell therapy for treating CLD in terms of all-cause mortality and procedural adverse events. We find stem cell therapy significantly reduced all-cause mortality, with no serious adverse effect or death directly related to the implantation of stem cells themselves. Nevertheless, some potential risk of stem cell therapy must be cautiously considered, including immune reactivity, viral transmission, and tumorigenic potential [7, 44, 45]. Further high-quality clinical studies with larger sample size and longer follow-up period are still warranted to investigate the safety of stem cell therapy.
Liver disease population, cell type, delivery route, and injection frequency are highly variable among different studies, which will influence therapeutic effects of stem cell therapy [46]. Our subgroup analyses indicate that patients with ACLF had a short-term survival benefit from stem cell therapy, while other CLD patients had improved liver function. ACLF is a serious life-threatening disease and LT is the only effective treatment. Against this background, stem cell therapy can be a promising therapeutic option to temporarily support recipient through the limited survival time or waiting period until the spontaneous recovery of the native liver or availability of a suitable donor organ.
Of different cell types, MSCs especially show promise as an ideal cell resource for the treatment of liver disease [6, 44]. However, no clinical trial has directly compared different kinds of stem cells with regard to efficacy for CLD. Our study shows stem cells derived from the bone marrow (BM-MSCs and BM-MNCs) had superior therapeutic effects to UC-MSCs, which may be due to the different homing or migration ability of transplanted stem cells derived from different tissues [47].
Multiple infusions were considered to be associated with greater and sustained efficacy [34]. In contrast, one recent study demonstrated two-time injections of stem cells did not contribute to better therapeutic effects than a single injection [36]. This meta-analysis shows multiple injections only exerted greater beneficial effects on mortality and ALT levels, while a single administration could achieve more favorable effects, particularly on the MELD scores and TBIL, ALB, and TPA levels. Nonetheless, it is worth noting that the interval between the first and second infusions will have an important influence on the achievement of long-term therapeutic effects.
Across different trials, stem cells were delivered into the liver through peripheral intravenous, intrasplenic, hepatic arterial, or portal vein administrations. Our study shows hepatic arterial infusion was associated with better effects at more time points than intravenous infusion. It may be due to the different migration or homing ability of infused stem cells to the injured liver. Although peripheral intravenous infusion is an easy and convenient way with less-traumatic effects [30], systemic administration may cause the entrapment of a large number of cells within the capillaries, especially in the lungs [47]. In contrast, direct administration of cells into the liver through hepatic arterial infusion can significantly reduce the loss of cells in the circulation, thereby increasing the number of cells migrating to the injured sites [48, 49]. However, notably, hepatic arterial infusion is invasive and carries substantial risks including portal hypertensive bleeding and thrombosis following cell injection [50, 51].
Several limitations of the present meta-analysis are worth noting. Firstly, the majority of the included studies showed a high risk of bias. Secondly, the presence of substantial heterogeneity in some pooled estimate outcomes may hinder the establishment of robust conclusions and recommendations. Subgroup and sensitivity analyses did not provide a clear explanation, which suggested the heterogeneity may be due to true differences between studies. The degree of progression of liver disease (compensated or decompensated stage), liver disease types (viral hepatitis-related, autoimmune, alcoholic or other types of liver disease), and the purity, density, number, and quality of infused cells may be the key factors influencing the therapeutic efficacy of cell transplantation, which possibly contribute to some heterogeneity. However, available data did not allow us to assess whether these factors have an impact on outcomes. Thirdly, different trials evaluated therapeutic effects with different outcome parameters at different measurement time points, so it was difficult to summarize robust results using the limited statistical sample sizes at a specific time point.
Despite these limitations, our meta-analysis only included randomized clinical trials, while previous systematic reviews conducted pooled analyses of RCT and non-RCT. However, studies of different designs should not be analyzed in a combined manner; thus, our study theoretically could provide more reliable evidence than the previous ones, supporting stem cell therapy as a safe and effective treatment for CLD. Nonetheless, many factors still challenge the establishment of stem cell therapy as a definite treatment in patients with CLD [52]. The source, purity, density, and quality of stem cells and the dosage, route, and frequency of cell infusion are critical for therapeutic effects of stem cell therapy in the treatment of CLD. Hence, future preclinical and clinical researches should focus on the optimization of cell isolation, culture condition, and differentiation protocol; the determination of ideal cell source, cell dosage, injection frequency, and administration route; and the choice of therapeutic timing in various liver diseases. The prospects of stem cell therapy in the treatment of CLD will be determined by the outcomes of upcoming clinical studies.
Conclusion
This meta-analysis suggests stem cell therapy is a safe and effective therapeutic approach for patients with CLD, while patients with ACLF benefit most in terms of improved short-term survival. A single injection administration with bone marrow-derived stem cell has superior therapeutic effects, and hepatic artery injection is the optimum cell delivery approach. There are significant heterogeneity and high risk of bias in existing studies; therefore, further high-quality randomized clinical studies are still in demand to acquire more solid evidence for the safety and efficacy of stem cell therapy in the treatment of CLD.
Availability of data and materials
Availability of data and materials can be assessed both in the “Methods” section, the “Results” section, and the “Additional files” section.
Abbreviations
- CLD:
-
Chronic liver disease
- ACLF:
-
Acute-on-chronic liver failure
- LT:
-
Liver transplantation
- RCT:
-
Randomized controlled trial
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- MELD:
-
Model for end-stage liver disease
- TBIL:
-
Total bilirubin
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- PTA:
-
Prothrombin activity
- INR:
-
International normalized ratio
- SMD:
-
Standardized mean difference
- OR:
-
Odds risk
- CI:
-
Confidence interval
- BM-MSC:
-
Bone marrow-derived mesenchymal stem cell
- UC-MSC:
-
Umbilical cord-derived mesenchymal stem cell
- BM-MNC:
-
Bone marrow-derived mononuclear stem cell
- PBSC:
-
Peripheral blood stem cell
References
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–108.
Global Health Estimates 2016: Deaths by cause, age, sex, by country and by rsegion, 2000–2016. Geneva, World Health Organization. 2018;Available from: https://www.who.int/healthinfo/global_burden_disease/estimates/en/. Accessed 29 Apr 2020.
Jadlowiec CC, Taner T. Liver transplantation: current status and challenges. World J Gastroenterol. 2016;22(18):4438–45.
Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long-term quality of life. Liver Int. 2014;34(9):1298–313.
Iansante V, Chandrashekran A, Dhawan A. Philos Trans R Soc Lond B Biol Sci. 2018;373(1750):20170229. https://doi.org/10.1098/rstb.2017.0229.
Lee CW, Chen YF, Wu HH, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018;154(1):46–56.
Kang SH, Kim MY, Eom YW, Baik SK. Mesenchymal stem cells for the treatment of liver disease: present and perspectives. Gut Liver. 2019.
Hu C, Zhao L, Li L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther. 2019;10(1):199.
Meier RP, Muller YD, Morel P, Gonelle-Gispert C, Buhler LH. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013;11(3):1348–64.
Liao L, Shi B, Chang H, et al. Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7(1):106–16.
Sun A, Gao W, Xiao T. Autologous bone marrow stem cell transplantation via the hepatic artery for the treatment of hepatitis B virus-related cirrhosis: a PRISMA-compliant meta-analysis based on the Chinese population. Stem Cell Res Ther. 2020;11(1):104.
Zhao L, Chen S, Shi X, Cao H, Li L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res Ther. 2018;9(1):72.
Xue R, Meng Q, Li J, et al. The assessment of multipotent cell transplantation in acute-on-chronic liver failure: a systematic review and meta-analysis. Transl Res. 2018;200:65–80.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–57.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Lyra AC, Soares MBP, Da Silva LFM, et al. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22(1):33–42.
Salama H, Zekri AR, Bahnassy AA, et al. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol. 2010;16(42):5297–305.
Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23(10):936–41.
Lin H, Zhang Z, Shi M, et al. Evaluation on the efficacy of human umbilical cord derived-mesenchymal stem cell transplantation in liver cirrhosis patients with ascites in a prospective and control trial. Chinese Am J Infect Dis. 2012;30(4):204–8.
Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10):725–31.
Zhang YF, Li N, Zhai JS, Jiang LJ, Cao JN. Short-term effects of human umbilical cord-derived mesenchymal stem cells in treatment of patients with decompensated cirrhosis. Chin J Tissue Eng Res. 2012;16(14):2585–8.
Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112–20.
Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33(10):1490–6.
Spahr L, Chalandon Y, Terraz S, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8(1):e53719.
Wang QC, Zhang LY, Wang DL. Short-term effects of human umbilical cord-derived mesenchymal stem cells in treatment of patients with chronic liver failure. Chin J Gastroenterol Hepatol. 2013;22(1):22–4.
Salama H, Zekri AR, Medhat E, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5(3):70.
Xu L, Gong Y, Wang B, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29(8):1620–8.
Deng QZ, Cai T, Zhang S, Hu AR, Zhang XF, Huang JR. Effects of autologous peripheral stem cell transplantation on acoustic radiation force impulse in patients with HBV-related decompensated cirrhosis. Zhonghua gan zang bing za zhi [Chinese journal of hepatology]. 2015;23(11):832–6.
Li YY, Xu RN, Shi M, et al. A pilot study of human umbilical cord derived- mesenchymal stem cells transfusion in patients with acute-on-chronic liver failure. Chin J Cell Stem Cell. 2015;5(1):32–6.
Zekri AR, Salama H, Medhat E, et al. The impact of repeated autologous infusion of haematopoietic stem cells in patients with liver insufficiency. Stem Cell Res Ther. 2015;6(1):118.
Mohamadnejad M, Vosough M, Moossavi S, et al. Intraportal infusion of bone marrow mononuclear or CD133+ cells in patients with decompensated cirrhosis: a double-blind randomized controlled trial. Stem Cells Transl Med. 2016;5(1):87–94.
Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016;64(6):2185–97.
Fang XQ, Zhang JF, Song HY, et al. Effects of human umbilical cord mesenchymal stem cell therapy on the immune function and prognosis in patients with decompensated liver cirrhosis due to hepatitis B. Chin J Tissue Eng Res. 2017;21(17):2696–701.
Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66(1):209–19.
Wu YZ, Yang L, Zhai YF, Zhang HH, Huo LY. Therapeutic effect of autologous bone marrow mesenchymal stem cells on hepatic fibrosis, liver function, MELD score and 1-year survival rate in patients with decompensated hepatitis B. Chin J Tissue Eng Res. 2017;21(13):2049–55.
Zhang D. A clinical study of bone mesenchymal stem cells for the treatment of hepatic fibrosis induced by hepatolenticular degeneration. Genet MolRes. 2017;16(1): gmr16019352. https://doi.org/10.4238/gmr16019352.
Newsome PN, Fox R, King AL, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(1):25–36.
Esmaeilzadeh A, Ommati H, Kooshyar MM, et al. Autologous bone marrow stem cell transplantation in liver cirrhosis after correcting nutritional anomalies, a controlled clinical study. Cell J. 2019;21(3):268–73.
Xu WX, He HL, Pan SW, et al. Combination treatments of plasma exchange and umbilical cord-derived mesenchymal stem cell transplantation for patients with hepatitis B virus-related acute-on-chronic liver failure: a clinical trial in China. Stem Cells Int. 2019;2019:4130757.
Liu WH, Song FQ, Ren LN, et al. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 2015;19(3):511–20.
Owen A, Newsome PN. Mesenchymal stromal cell therapy in liver disease: opportunities and lessons to be learnt? Am J Physiol Gastrointest Liver Physiol. 2015;309(10):G791–800.
Kadyk LC, Collins LR, Littman NJ, Millan MT. Proceedings: moving toward cell-based therapies for liver disease. Stem Cells Transl Med. 2015;4(3):207–10.
Zhang Y, Li Y, Zhang L, Li J, Zhu C. Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis. Stem Cell Res Ther. 2018;9(1):59.
Zhang L, Li K, Liu X, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22(23):3074–86.
Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46(6):1935–45.
Mohamadnejad M, Namiri M, Bagheri M, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13(24):3359–63.
Salama H, Zekri AR, Zern M, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 2010;19(11):1475–86.
Pinheiro D, Dias I, Ribeiro Silva K, et al. Mechanisms Underlying Cell Therapy in Liver Fibrosis: An Overview. Cells. 2019;8(11):1339. https://doi.org/10.3390/cells8111339.
Acknowledgements
The authors would like to thank Shan-Shan Wu, PhD, for her help with the statistics and methodology.
Funding
This study was supported by grants from the Beijing Municipal Science & Technology Commission (Z181100001718220) and Capital’s Funds for Health Improvement and Research (No.2020-1-2024). The sponsors had no role in the design, execution, interpretation, or writing of the study.
Author information
Authors and Affiliations
Contributions
G-PZ conceptualized the topic, collected the data, performed the statistical analysis, and wrote the manuscript. Y-ZJ collected the data and performed the statistical analysis. L-YS participated in all stages of the review and supervised the conduct of the study. Z-JZ conceptualized the topic and supervised and facilitated the conduct of the study. All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval and consent to participate were not necessary because this study was a meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Search strategy
Additional file 2: Table S2.
Results of sensitivity analyses with omission of one study at a time
Additional file 3: Figure S1.
Funnel plots of mortality at weeks 4, 12, and 48; MELD at weeks 12 and 24; TBIL at weeks 4, 12 and 24; ALB at weeks 4, 12 and 24; and ALT at weeks 4, 12 and 24. Asymmetry was observed in the funnel plots of mortality at week 48 (P = 0.016) and TBIL at week 12 (P = 0.035).
Additional file 4: Figure S2.
Symmetrical contour-enhanced funnel plots for mortality at week 48 and TBIL at week 12. For mortality at week 48, three hypothetical studies were filled: two plotted in the area of statistical significance and one in the area of statistical non-significance, indicating that the asymmetry in the funnel plot was partly caused by publication bias. For TBIL at week 12, no hypothetical studies were filled, indicating that the asymmetry in the funnel plot was not caused by publication bias.
Additional file 5: Table S3.
Adverse events associated with stem cell therapy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, GP., Jiang, YZ., Sun, LY. et al. Therapeutic effect and safety of stem cell therapy for chronic liver disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther 11, 419 (2020). https://doi.org/10.1186/s13287-020-01935-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-020-01935-w