Abstract
Background
Bacterial infection of embryo culture medium is rare but may be detrimental. The main source of embryo culture contamination is semen. Assisted reproduction centers currently lack consensus regarding the methods for preventing and managing embryo culture infection. In our recent case, a successful pregnancy was achieved with intracytoplasmic sperm injection after failed conventional in vitro fertilization owing to bacterial contamination.
Case presentation
We present a case report of two consecutive in vitro fertilization–intracytoplasmic sperm injection cycles with photo and video documentation of the bacterial growth. A 36-year-old Hungarian woman and her 37-year-old Hungarian partner came to our department. They had two normal births followed by 2 years of infertility. The major causes of infertility were a closed fallopian tube and asthenozoospermia. Bacterial infection of the embryo culture medium was observed during in vitro fertilization and all oocytes degenerated. The source was found to be the semen. To prevent contamination, intracytoplasmic sperm injection was used for fertilization in the subsequent cycle. Intracytoplasmic bacterial proliferation was observed in one of the three fertilized eggs, but two good-quality embryos were successfully obtained. The transfer of one embryo resulted in a successful pregnancy and a healthy newborn was delivered.
Conclusion
Intracytoplasmic sperm injection may be offered to couples who fail conventional in vitro fertilization treatment owing to bacteriospermia, as it seems to prevent infection of the embryo culture. Even if bacterial contamination appears, our case encourages us to continue treatment. Nevertheless, the development of new management guidelines for the prevention and management of bacterial contamination is essential.
Similar content being viewed by others
Background
The occurrence of bacterial contamination in embryo culture medium is rare (0.23–0.68%) [1, 2], but it may have detrimental consequences such as reducing live birth rates [1], causing maternal pelvic infection [3], or epigenetic mutations in embryos [4]. Currently, there is no consensus regarding the management of bacterial contamination of embryo culture medium [5].
We present a case where conventional in vitro fertilization (IVF) treatment failed owing to the bacterial infection in the embryo culture medium. In the subsequent cycle, a successful pregnancy was achieved by using intracytoplasmic sperm injection (ICSI).
Case presentation
Informed consent was obtained from the couple for this case report.
A 36-year-old Hungarian woman and her 37-year-old Hungarian partner came to our department with secondary infertility. The investigation revealed tubal and male factors (Table 1).
As a first step intrauterine insemination was performed. Pregnancy was not achieved and, on the basis of the reduced sperm parameters (total progressively motile sperm count 1.1 million), we planned IVF treatment for the next cycle.
Following ovarian stimulation based on the gonadotropin-releasing hormone (GnRH) antagonist protocol, 16 oocytes were retrieved using transvaginal ultrasound-guided follicular puncture.
The male partner received written information regarding abstinence and hygiene rules, and then semen was collected by masturbation in a sterile container. Laboratory procedures were carried out using the standard protocol [6]. Semen was incubated at 37 °C. After liquefaction, the sample was homogenized and examined. The sample had a volume of 4.4 ml and a sperm concentration of 14.5 million/ml with progressive motility of 29%, without a significant number of round cells. Progressively motile spermatozoa were isolated by a combined method of density gradient centrifugation and swim-up technique. A two-layer SpermGrad (Vitrolife, Göteborg, Sweden) density gradient centrifugation was used according to the manufacturer’s instructions. Swim-up technique was applied following density gradient centrifugation to obtain a sample with high progressive motility.
After processing, the semen sample contained 1 million progressively motile spermatozoa, so we performed in vitro fertilization. Oocyte and embryo culture was performed in a culture media product line called “G-series” (Vitrolife) containing gentamicin.
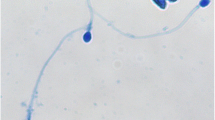
Following 16 h of coincubation of oocytes and sperm, a proliferation of rods was observed in the culture media and all oocytes degenerated (Fig. 1).
Samples were sent for microbiological culture. The control culture medium and cervical swab proved to be negative. The sperm suspension and the contaminated culture medium contained Escherichia coli (E. coli) and Staphylococcus hominis.
The patient was disappointed by the failed treatment, but had confidence in the department’s work.
On the basis of a thorough review of the literature, we planned ICSI treatment 3 months later. After ovarian stimulation using the antagonist protocol, 14 oocytes were retrieved by means of follicular aspiration. Semen was collected as discussed above. The volume of the semen sample was 1.8 ml and had a sperm concentration of 40.5 million/ml with progressive motility of 46%, without a significant amount of round cells. Progressively motile spermatozoa were isolated by density gradient centrifugation. After processing, the sample contained 0.3 million progressively motile spermatozoa. ICSI was performed for fertilization and embryo culture continued in an Embryoslide + group culture dish in an Embryoscope + time-lapse incubator (Vitrolife), which enables a precise evaluation of embryo morphology [7].
Out of 14 oocytes, 3 fertilized normally, 1 abnormally, and 10 were not fertilized. During pronucleus evaluation, bacterial proliferation was detected in one fertilized oocyte, which was recorded on a time-lapse video (Additional file 1. Intracellular proliferation of bacteria in a fertilized oocyte after ICSI). We did not observe any contamination in the other oocytes or culture medium. The fertilized but infected oocyte was removed from the culture plate, and its zona pellucida was opened under a microscope by using a microsurgical laser. A mass of bacteria emerged from the cell, which was captured on video (Additional file 2. Bacteria emerging from the infected oocyte after opening the zona pellucida). Microbiological analysis of the contaminated culture medium revealed E. coli in dominant germ counts and Cutibacterium acnes. The sperm culture also showed E. coli.
Because of the repeated positive semen cultures, the husband was referred to an andrologist, and 2 × 500 mg cefuroxime treatment was initiated.
Two of the fertilized oocytes were not contaminated and their further culture resulted in normal embryo development and good-quality blastocysts. Cervical culture proved to be negative for bacteria, fungi, and sexually transmitted diseases (Chamydia trachomatis, Neisseria gonorrheae, Trichomonas vaginalis, Mycoplasma genitalium, and Mycoplasma hominis by molecular testing). Given these points, one blastocyst-stage embryo was transferred on the fifth day after fertilization (Fig. 2).
The other embryo was cryopreserved.
Progesterone (3 × 200 mg vaginal suppositories) was given to support the corpus luteum function.
After positive human chorionic gonadotropin (hCG) tests, a viable singleton pregnancy was confirmed by vaginal ultrasound at 6 weeks of gestation. The woman’s gratitude was heightened by the challenging path to pregnancy. No complications occurred during routine prenatal care, and genetic testing of the fetus confirmed normal chromosomes. In February 2023, a healthy female newborn was delivered by cesarean section at 40 weeks of pregnancy. We intend to monitor the development of the child.
Discussion
Bacterial contamination of the embryo culture medium
The main source of bacterial contamination of the embryo culture medium is the semen sample [8]. Bacteriospermia is defined as a bacterial count greater than 103/ml [9]. It can occur in case of infection, contamination or urethral colonization, and the differentiation between these entities is challenging [10]. In addition, a further infection or chronic disease may worsen the prognosis of this condition [11]. Since our patient was symptomless and did not have abnormal physical findings or leukocytospermia, a clear diagnosis could not be established. Most researchers agree that bacteria can have a negative impact on sperm parameters [12]; however, the majority of studies demonstrated no significant difference in the effectiveness of IVF treatment in the presence or absence of bacteriospermia [13].
The poor fertilization rate, despite the use of ICSI, may be a reflection of the gametes’ quality [14].
Bacterial contamination of the culture dishes can result in the poor quality of developing embryos [2, 15]. Although direct infection and complete destruction of embryos by bacteria are unlikely, we observed total degeneration of all oocytes in our IVF cycle. After ICSI, we detected intracytoplasmic proliferation of bacteria in one of the fertilized oocytes, which may be explained by the direct injection of bacteria adhered to the spermatozoa. Tight adhesion to the sperm midpiece after centrifugation has been described with Staphylococcus saprophyticus, but not with E. coli [16].
As in our case, E. coli seems to be the most common cause of contamination of embryo cultures and it is usually resistant to the antibiotics used in culture media [2, 8]. E. coli can cause membrane defects and cytoplasmic vacuoles in spermatozoa [17]. These alterations affect the neck, the mid-piece, and the acrosomal membrane, which may result in immobilization and impaired acrosomal function. This hypothesis can explain the extremely poor fertilization rate we observed after ICSI.
Prevention of contamination
To prevent microbial contamination, we strictly follow the rules provided by the World Health Organization for the retrieval and processing of semen [18].
Routine microbial culture of semen before IVF allows direct detection, but it is costly [8] and the clinical relevance of positive cases is controversial [13]. Microbiological screening of semen is not routinely performed in our department. If the number of round cells in the sample is elevated, we assess the number of white blood cells and if the number exceeds 1 million/ml, patients are referred to an expert andrologist. We observed neither bacteria nor a significant amount of round cells using microscopy and there was no sign of inflammation. Owing to repeated bacterial contamination, the patient was referred to an andrologist, who initiated antibiotic treatment. Treatment of clinically symptomatic infections is obligatory, but in cases of symptomless alteration of the normal flora, it is questionable [5]. Despite antibiotic treatment and a negative control sperm culture, recurrent bacteriospermia can still occur [19].
Comparing the technique of fertilization, contamination of the embryo culture medium seems to be more common in IVF than in ICSI treatments [1, 2, 8, 15]. A possible explanation for this is that only one single sperm is used from the semen solution during ICSI. Although the use of ICSI is expensive and it is not always beneficial in cases of non-male factor infertility [20], some working groups have suggested that it may help to avoid contamination of the culture solution [8, 15]. On the basis of this, we planned ICSI treatment after the failed IVF cycle and were able to avoid bacterial infection in the culture medium. However, we observed intracytoplasmic infection in one fertilized oocyte. To the best of our knowledge, this phenomenon has not been described in literature so far.
Management of contamination
Regarding the management of contamination, there are only a few experimental cases available.
In a recent case report, a massive E. coli infection was detected in the embryo culture medium after ICSI; however, a healthy baby was born as a result of the treatment and the development was normal during the 2.5 year long follow-up [19]. The authors concluded that washing with gentamicin helped to remove the contamination and that freezing–devitrification might have contributed to the disappearance of the bacteria.
Shu et al. found that removal of the zona pellucida might help if there is still contamination after washing the embryos, as bacteria might adhere to the zona pellucida [15].
Interestingly, the three fertilized oocytes were cultured in the same well in our case, but the infection of the contaminated one remained intracellular and therefore did not affect the other two embryos. Some working groups advise against embryo transfer from contaminated culture medium [2, 21]. Du et al. discarded embryos if all of them were contaminated but did not contraindicate the transfer of normal embryos if there was only partial contamination [1]. Since the development of the two blastocysts was unaffected in our case, we considered the embryo transfer to be safe. This way we did not have to cancel the cycle and a healthy baby was born from the treatment.
Conclusion
For almost 30 years of our department’s operation, we have only experienced one case of microbial infection in the IVF system. This suggests that we are following our protocols correctly when it comes to prevention. In our recent case, we observed that after conventional IVF, there was massive bacterial proliferation in the embryo culture medium, while after ICSI, bacterial infection was only detected in one fertilized oocyte. The source of the infection was found to be the semen. Although we cannot further conclude from one case, the use of ICSI may be an effective and safe procedure to prevent contamination of embryo culture medium in the case of bacteriospermia. ICSI may be offered to couples who fail conventional IVF treatment because of chronic bacterial colonization. Although this appears to be a rare event, new guidelines regarding the management of bacterial contamination of embryo culture medium are needed. A proper management of embryo culture infection would help to reduce the need for canceling cycles, while also being safe for the patient. Although cost–benefit studies would be required, our case encourages us to continue assisted reproduction treatments even if bacterial contamination appears.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ART:
-
Assisted reproductive technology
- E. coli :
-
Escherichia coli
- DHEAS:
-
Dehydroepiandrosterone sulfate
- FSH:
-
Follicle-stimulating hormone
- hCG:
-
Human chorionic gonadotropin
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In vitro fertilization
- LH:
-
Luteinizing hormone
- SHBG:
-
Sexual hormone binding hormone
- TSH:
-
Thyroid-stimulating hormone
References
Du F, Luo H, Li R, et al. Microbe contamination during embryo cultures in an in vitro fertilization-embryo transfer system. Ann Obstet Gynecol. 2021;4:1015.
Kastrop PM, de Graaf-Miltenburg LA, Gutknecht DR, et al. Microbial contamination of embryo cultures in an ART laboratory: sources and management. Hum Reprod. 2007;22:2243–8.
Czernobilsky B. Endometritis and infertility. Fertil Steril. 1978;30:119–30.
Bierne H, Hamon M, Cossart P. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med. 2012;2: a010272.
Borges ED, Berteli TS, Reis TF, et al. Microbial contamination in assisted reproductive technology: source, prevalence, and cost. J Assist Reprod Genet. 2020;37:53–61.
Lehner A, Kaszas Z, Murber A, et al. Giant oocytes in human in vitro fertilization treatments. Arch Gynecol Obstet. 2015;292:697–703.
Joo K, Nemes A, Dudas B, et al. The importance of cytoplasmic strings during early human embryonic development. Front Cell Dev Biol. 2023;11.
Lin L-L, Guu H-F, Yi Y-C, et al. Contamination of ART culture Media-the role of semen and strategies for prevention. Taiwan J Obstet Gynecol. 2021;60:523–5.
Domes T, Lo KC, Grober ED, et al. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. 2012;97:1050–5.
Farahani L, Tharakan T, Yap T, et al. The semen microbiome and its impact on sperm function and male fertility: a systematic review and meta-analysis. Andrology. 2021;9:115–44.
Lee KW, Devaraj NK, Ching SM, et al. Effect of SGLT-2 inhibitors on non-alcoholic fatty liver disease among patients with type 2 diabetes mellitus: systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. Oman Med J. 2021;36: e273.
Pergialiotis V, Karampetsou N, Perrea DN, et al. The impact of bacteriospermia on semen parameters: a meta-analysis. J Family Reprod Health. 2018;12:73.
Berkes-Bara É, Nemes A, Joó K, et al. Indokolt-e a spermaminták bakteriológiai szűrése in vitro fertilizáció előtt? Orv Hetil. 2023;164:660–6.
The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod. BioMed. 2017;35:494–510.
Shu Y, Prokai D, Berga S, et al. Transfer of IVF-contaminated blastocysts with removal of the zona pellucida resulted in live births. J Assist Reprod Genet. 2016;33:1385–8.
Ghasemian F, Esmaeilnezhad S, Moghaddam MJM. Staphylococcus saprophyticus and Escherichia coli: tracking from sperm fertility potential to assisted reproductive outcomes. Clin Expe Reprod Med. 2021;48:142.
Diemer T, Huwe P, Michelmann H, et al. Escherichia coli-induced alterations of human spermatozoa. An electron microscopy analysis. Int J Androl. 2000;23:178–86.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva, 2021. https://www.who.int/publications/i/item/9789240030787. Accessed 08 Jun 2023.
Vaduva C, Cilica R, Nastase A, et al. A successful case of assisted reproduction after contamination of the culture medium with Escherichia coli. Eur Rev Med Pharmacol Sci. 2022;26:5932–8.
Fancsovits P, Lehner A, Kaszas Z, et al. Intracytoplasmic sperm injection does not improve the outcome of IVF treatments in patients with advanced maternal age or low oocyte number: a randomized controlled trial. J Gynecol Obstet Hum Reprod. 2023;52: 102625.
Campbell A, Best L. Infection in embryo culture medium. Assisted reproduction techniques: challenges and management options. 2021;509–514.
Acknowledgements
Not applicable.
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by PF, AN, and KJ; Analysis was performed by EBB and JU; The first draft of the manuscript was written by EBB; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Intracellular proliferation of bacteria in a fertilized oocyte after ICSI.
Additional file 2. Bacteria emerging from the infected oocyte after opening the zona pellucida.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Berkes-Bara, E., Nemes, A., Dudas, B. et al. Successful pregnancy with intracytoplasmic sperm injection after bacterial contamination of embryo culture in in vitro fertilization: a case report. J Med Case Reports 18, 247 (2024). https://doi.org/10.1186/s13256-024-04521-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04521-3