Abstract
Background
Tirofiban is a nonpeptide glycoprotein IIb/IIIa receptor antagonist used widely in patients subjected to percutaneous coronary intervention. While the usage of tirofiban sets an important clinical benefit, severe thrombocytopenia can occur with use of this agent.
Case presentation
A 76-year-old Chinese man was admitted with 1-month history of sudden onset of chest tightness. He was diagnosed as having subacute inferior myocardial infarction, and percutaneous coronary intervention was performed. After the procedure, patient received tirofiban at 0.15 µg/kg/minute for 4 h. A blood sample was obtained for a complete blood count; severe thrombocytopenia was reported according to routine orders at our hospital. All antiplatelet drugs including tirofiban, aspirin, and clopidogrel were immediately discontinued. The patient received platelet transfusions and was treated with immunoglobulin G. Two days later, the patient’s platelet count had increased to 75 × 109/L. There was a significant improvement after day 5, and the platelet count was 112 × 109/L. Seven days after the acute thrombocytopenia, he was discharged with normal platelet count.
Conclusions
Clinicians should be particularly aware of tirofiban-induced thrombocytopenia in routine practice.
Similar content being viewed by others
Background
Glycoprotein IIb/IIIa receptor antagonists (GPRAs), including abciximab, eptifibatide, and tirofiban, are widely used in the treatment of patients with acute coronary syndromes (ACS) [1]. These agents have been extensively studied in several randomized trials, which have demonstrated that the use of GPRAs may reduce the incidence of myocardial infarction (MI) and composite cardiac outcomes in patients subjected to percutaneous coronary intervention (PCI) [2]. However, severe thrombocytopenia can occur with use of these agents [3]. Drug-induced thrombocytopenia may result from a number of diverse etiologies; it is important for clinicians to make an accurate diagnosis to guide treatment decisions and to inform prognosis. Here, we report a case of severe thrombocytopenia within 4 hours of tirofiban administration after PCI for subacute inferior MI.
Case presentation
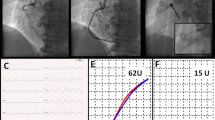
A 76-year-old Chinese man was admitted with 1-month history of sudden onset of chest tightness. A month previously, the symptom started while he was doing exercise. It was associated with diaphoresis and shortness of breath, without chest pain. The resting 12-lead electrocardiogram (ECG) showed abnormal Q waves in leads II, III, and aVF, and the cardiac troponin T was elevated. He was diagnosed as having ACS and was treated at a local hospital. Owing to the fact that the hospital was unable to perform coronary angiography (CAG), the patient was discharged home several days later on aspirin (100 mg qd), clopidogrel (75 mg qd), metoprolol (12.5 mg qd), and rosuvastatin (10 mg qn). On admission to our hospital, the patient still had a mildly elevated level of high-sensitivity cardiac troponin T (21.58 ng/L). His initial ECG showed abnormal Q waves in the inferior (II, III, and avF) leads (Fig. 1), which may indicate subacute inferior MI. His routine blood test was normal, including a platelet count of 201 × 109/L and a white blood cell count of 5.86 × 109/L. He had no history of blood dyscrasia and denied any history of smoking or drinking. The patient underwent CAG in our hospital after admission. CAG revealed evidence of left main (LM) and three-vessel coronary artery disease (Fig. 2). Low-dose heparin (2000 units) was given during the procedure. We consider the optimal revascularization technique for this patient to be coronary artery bypass graft (CABG) surgery and did not perform PCI. However, the patient decide to be treated with PCI rather than CABG. Four days later, CAG was performed again; multivessel coronary intervention was performed with drug-eluting stents and drug-coated balloon. The procedure lasted for about 2 hours, and the dose of heparin given was 6500 units. After the procedure, the patient was transferred in stable condition to the ward and treated by intravenous tirofiban at 0.15 µg/kg/minute. Post-PCI medications included aspirin 100 mg qd, clopidogrel 75 mg qd, metoprolol succinate 12.5 mg qd, rosuvastatin 10 mg qn, and benazepril 5 mg qd. A blood sample was obtained for a complete blood count 4 hours after the procedure, according to routine orders at our hospital. His platelet count was 21 × 109/L, which was confirmed by manual examination of the blood film. The patient’s hemoglobin level was 119 g/L. Tirofiban infusion was stopped by 4 hours, and other antiplatelet drugs including aspirin and clopidogrel were immediately discontinued. A heparin-induced thrombocytopenia (HIT) platelet factor 4 antibody test was performed, and the result was negative [4]. Over the next 12 hours, the patient received 10 unit platelet transfusions to prevent hemorrhage, and his platelet count had increased to 49 × 109/L. Another analysis completed later indicated that his platelet count was 37 × 109/L. Additionally, immunoglobulin G (10g) was given. Aspirin and clopidogrel were resumed the next day, and the patient received another 10 unit platelet transfusions. Two days later, the patient’s platelet count had increased to 75 × 109/L. The course of the patient’s platelet count is shown in Fig. 3. There was a significant improvement after day 5, and the platelet count was 112 × 109/L. Seven days after the acute profound thrombocytopenia, his platelet count was 138 × 109/L, and he was discharged with no hemorrhagic sequelae.
Discussion and conclusions
Tirofiban is a nonpeptide glycoprotein (GP) IIb/IIIa receptor antagonist used in patients subjected to PCI for the prevention of acute stent thrombosis and reduction of major adverse coronary events [5]. It inhibits platelet aggregation by preventing the attachment of fibrinogen and von Willebrand factor to the GP IIb/IIIa receptor on the thrombocyte surface [6]. While the usage of GP IIb/IIIa inhibitors sets an important clinical benefit, the reported incidence of thrombocytopenia induced by tirofiban ranges from 0.4% to 5.6% [7]. Thus, clinicians should be particularly aware of tirofiban-induced thrombocytopenia in routine practice.
Besides tirofiban, aspirin and clopidogrel are widely used as antiplatelet agents; they have also been reported to be associated with thrombocytopenia [8]. However, the described patient had used these two drugs for 1 month before admission, and his routine blood test showed a normal platelet count after admission. Therefore, the thrombocytopenia was not caused by the dual antiplatelet therapy.
The most well-known medication that can induce thrombocytopenia is heparin [9]. HIT is the most important complication of heparin therapy during PCI in cardiac patients. There are two types of HIT. Type I HIT is a transient, mild drop in platelet counts 48–72 hours after initiation of heparin therapy. It occurs because of direct heparin-induced platelet aggregation and is usually clinically harmless [7]. Type II HIT is an adverse immune-mediated reaction due to antibodies formed against heparin–platelet factor 4 complexes, which is usually associated with thrombosis risk. It is more severe than type I HIT and should be suspected when patients show a reduction in the platelet count to less than 100,000 per cubic millimeter or more than 50% of the baseline value 5–15 days after initiation of heparin therapy [10, 11]. The 4T's score (the severity of Thrombocytopenia, its Timing of heparin exposure, appearance of new Thrombosis, and differential diagnosis by exclusion of oTher causes) has been utilized as a clinical assessment tool to evaluate the likelihood of HIT [11, 12]. Our patient’s 4T's score was 3 points. Thus, the suspicion for HIT was low. In addition, we carried out an immunoassay to examine the presence of HIT antibodies; the negative result indicated that our patient did not have an HIT type II reaction. It is important to exclude HIT and other causes of thrombocytopenia to diagnose tirofiban-induced thrombocytopenia accurately and treat patients appropriately.
A recent study based on pre-procedural characteristics for early prediction of thrombocytopenia before patients were exposed to tirofiban has developed a simple risk model to predict thrombocytopenia associated with periprocedural tirofiban exposure [7]. Five independent risk factors, including age ≥ 65 years (2 points), white blood cell ≥ 12 × 109 /L (1 point), diabetes mellitus (2 points), congestive heart failure (2 points), and chronic kidney disease (1 point), were identified as risk factors in the scoring system. According to the scoring system, ≥ 7 points, 3–6 points, and ≤ 2 points indicate high risk, moderate risk and low risk. For our patient, this score only indicates a moderate risk (4 points), calculated based on age and congestive heart failure. Therefore, a further predictive model is still needed to help doctors identify high-risk patients in clinical practice [7].
The efficacy of tirofiban for patients with MI who undergo PCI was positively correlated with its dose; high dose can enhance the clinical effects, but also increase the hemorrhagic risk [13]. The appropriate dose could be adopted by reference to the specific conditions of patients under assessment of bleeding risk, and a common recommended clinical dose of 10 µg/kg may be appropriate for patients without high hemorrhagic risk, followed by continuous intravenous injection at 0.15 µg/kg/minute [13]. It is important to monitor platelet counts closely after initiation of tirofiban infusion [14]. For these patients, testing platelet counts before treatment, 2–4 h following the start of infusion, and at 24 h would detect most cases of acute thrombocytopenia [1, 15]. Discontinuation of tirofiban is usually sufficient for treatment of thrombocytopenia because it is cleared from the circulation within the first hours of cessation of the drug [14, 16].
In conclusion, this report demonstrates an example of acute severe thrombocytopenia induced by tirofiban and endorses the importance of platelet count monitoring after initiating therapy with this agent in clinical practice.
Availability of data and materials
Not applicable.
Abbreviations
- ACS:
-
Acute coronary syndromes
- CABG:
-
Coronary artery bypass graft
- CAG:
-
Coronary angiography
- ECG:
-
Electrocardiogram
- GP:
-
Glycoprotein
- GPRAs:
-
Glycoprotein IIb/IIIa receptor antagonists
- HIT:
-
Heparin-induced thrombocytopenia
- hs-cTnT:
-
High-sensitivity cardiac troponin T
- LAD:
-
Left anterior descending
- LCX:
-
Left circumflex artery
- LM:
-
Left main
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- RCA:
-
Right coronary artery
References
Huxtable LM, Tafreshi MJ, Rakkar AN. Frequency and management of thrombocytopenia with the glycoprotein IIb/IIIa receptor antagonists. Am J Cardiol. 2006;97:426–9.
Karvouni E, Katritsis DG, Ioannidis JP. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. J Am Coll Cardiol. 2003;41:26–32.
Sharma A, Ferguson C, Bainey KR. Thrombocytopenia in acute coronary syndromes: etiologies and proposed management. Can J Cardiol. 2015;31:809–11.
Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346–53.
Giordano A, Musumeci G, D’Angelillo A, Rossini R, Zoccai GB, Messina S, et al. Effects Of glycoprotein IIb/IIIa antagonists: anti platelet aggregation and beyond. Curr Drug Metab. 2016;17:194–203.
Ede H, Erkoç MF, Alüzüm H, Özdemir ZT, Erbay AR. Tirofiban induced anemia without thrombocytopenia. Int J Cardiol. 2015;179:500–1.
Yi YH, Yin WJ, Gu ZC, Fang WJ, Li DY, Hu C, et al. A simple clinical pre-procedure risk model for predicting thrombocytopenia associated with periprocedural use of tirofiban in patients undergoing percutaneous coronary intervention. Front Pharmacol. 2018;9:1456.
Hu Y, Yuan M, Lu X. Thrombocytopenia induced by both aspirin and clopidogrel in the same patient. Int J Clin Pharmacol Ther. 2013;51:228–31.
Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–92.
Cicco N, Gerken G, Frey M, et al. Heparin-induced thrombocytopenia. Intensivmed. 2000;37(Suppl 1):S099. https://doi.org/10.1007/s003900070012.
Lassila R, Antovic JP, Armstrong E, Baghaei F, Dalsgaard-Nielsen J, Hillarp A, et al. Practical viewpoints on the diagnosis and management of heparin-induced thrombocytopenia. Semin Thromb Hemost. 2011;37:328–36.
Liu W, Zhang C, Bai Q, Zhang Z. Rare heparin induced thrombocytopenia type I reaction in a hemodialysis patient: case report. Medicine (Baltimore). 2018;97: e13609.
Wang H, Feng M. Influences of different dose of tirofiban for acute ST elevation myocardial infarction patients underwent percutaneous coronary intervention. Medicine (Baltimore). 2020;99: e20402.
Elcioglu OC, Ozkok A, Akpınar TS, Tufan F, Sezer M, Umman S, et al. Severe thrombocytopenia and alveolar hemorrhage represent two types of bleeding tendency during tirofiban treatment: case report and literature review. Int J Hematol. 2012;96:370–5.
Shenoy C, Harjai KJ. Thrombocytopenia following percutaneous coronary intervention. J Interv Cardiol. 2011;24:15–26.
Kondo K, Umemura K. Clinical pharmacokinetics of tirofiban, a nonpeptide glycoprotein IIb/IIIa receptor antagonist: comparison with the monoclonal antibody abciximab. Clin Pharmacokinet. 2002;41:187–95.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (no. 81703213) and the Natural Science Youth Foundation of Jiangsu Province of China (no. BK20151034) to Z-MW.
Author information
Authors and Affiliations
Contributions
Z-MW wrote the manuscript. Z-MW, BW, and Y-FL analyzed and interpreted the patient data. BC and QS collected the relevant materials including the figures and medical records. D-FL performed the PCI procedure. L-SW was a major contributor in proof reading and manuscript correction. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, ZM., Wang, B., Li, YF. et al. Severe thrombocytopenia induced by tirofiban after percutaneous coronary intervention: a case report. J Med Case Reports 17, 430 (2023). https://doi.org/10.1186/s13256-023-04169-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04169-5