Abstract
Introduction
Ventricular septal defect (VSD) is one of the most common congenital cardiac anomalies. Patients with perimembranous VSD may have aortic regurgitation (AR) secondary to prolapse of the aortic cusp.
Case presentation
We present a case of 23-year-old White man with VSD, AR and ascending aortic aneurysm. The patient presented to outpatient clinic with weakness and gradual worsening shortness of breath for the past 5 years. Clinical examination revealed regular heart rhythm and loud continuous systolic-diastolic murmur (Lewin’s grade 6/6), heard all over the precordium, associated with a palpable thrill. The ECG showed right axis deviation, fractionated QRS in V1 and signs of biventricular hypertrophy. The chest X-ray showed cardiomegaly. Transthoracic and transesophageal echocardiograms showed a perimembranous VSD with moderate restrictive shunt (Qp/Qs = 1.6), aortic regurgitation (AR), and ascending aortic aneurysm. Other clinical and laboratory findings were within normal limits.
Conclusions
Perimembranous VSD, may be associated with aortic regurgitation and ascending aortic aneurysm as secondary phenomenon if it is not early diagnosed and successfully treated.
Similar content being viewed by others
Introduction
Ventricular septal defect (VSD) is one of the most common congenital cardiac anomalies both in children and adults [1]. VSD in childhood occurs in 50% of all congenital heart disease, and in adults, despite being rare. It is the second most prevalent congenital heart disease, after bicuspid aortic valve [2]. The cause of VSD and other congenital heart diseases is not fully understood, but it is now widely accepted that they are genetically mediated and potentially influenced by exogenic factors during fetal development, leading to disruption of the delicate and complex process of normal heart and great vessel morphogenesis.
VSDs can be classified according to their location, size, hemodynamic impact and accompaniment with other anomalies [3]. Because of the close anatomical relation of membranous interventricular septum (IVS) and the semilunar aortic valve, aortic regurgitation (AR) is seen in patients with perimembranous subtypes of VSD, caused by the secondary prolapse as a result of Venturi effect [4]. Delay in diagnosis and treatment of perimembranous VSD can cause physiological stress on the aorta and valve that can lead to AR and aneurysm.
Case presentation
We present a 23-years-old White man with VSD and AR, associated with ascending aortic aneurysm. The patient was admitted because of generalized. weakness and worsening exertional breathlessness, over the previous five years, with significant deterioration in last year. His personal and family history denied significant cardiovascular risk factors.
Clinical examination revealed regular heart rhythm, loud continuous systolic-diastolic murmur (Lewin’s grade 6/6) heard over the whole precordium with punctum maximum on the left sternal border, associated with a palpatory thrill.
A 12 lead ECG showed slight right axis deviation, fractionated QRS in V1 and electric criteria for biventricular hypertrophy (Fig. 1).
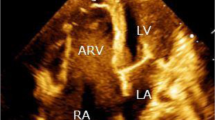
Chest X- ray showed enlarged cardiac silhouette suggestive of left ventricular hypertrophy. Other clinical and laboratory findings were within normal range. A transthoracic echocardiogram demonstrated a perimembranous VSD, with moderate restrictive shunt (Qp/Qs = 1.6), AR and ascending aortic aneurysm (Fig. 2, Additional file 1: Video S1). Similar findings were confirmed by transesophageal echocardiogram (Figs. 3, 4).
Discussion
Congenital VSD may be found as an isolated lesion or in combination with other cardiac anomalies [5]. As for all congenital heart disease, there are some genetical disorders responsible for the formation of VSDs during the intrauterine fetal development, which can lead to a disruption of a very sensitive and complex process of normal morphogenesis of heart and great vessels. These genetic disorders may also be related to teratogenic exogenous factors [6].
The interventricular septum (IVS) is quite a voluminous muscular structure that separates the two ventricles, despite the distinguishable basal membranous septum which is in close proximity to the atrio-ventricular and semilunar aortic valves. The muscular part of the IVS can be divided into inlet, trabecular and infundibular components [2, 5, 7].
Because of the close anatomical relation of the membranous IVS and the aortic valve, aortic regurgitation (AR) is seen in patients with perimembranous VSDs, being caused by secondary prolapse of one of the aortic valve cusps as a result of the Venturi effect [3, 5]. The natural history of these defects is a progressive deterioration of the semilunar cusp involved, resulting in worsening AR [8]. The absence of a muscular septum below the adjacent aortic valve cusp results in unopposed downward force on the cusp during diastole, hence cusp prolapse and AR. Yet larger VSDs, which are bordered by a correspondingly larger portion of the aortic annulus, rarely develop aortic valve prolapse or AR. This suggests that the lack of ventricular septal support alone is an inadequate explanation. If a lack of support were a principal cause of AR then it would follow that larger VSDs would, more commonly, result in AR. But according to the available literature, the opposite is true, indicating the predominance of the Venturi effect in the pathogenesis. Abnormal commissural structures have been observed in some cases of AR associated with a VSD [7].
Literature has disclosed the importance of an early diagnosis, follow-up and appropriate treatment of this condition as indicated in a certain stage of disease. Although some asymptomatic VSD are set for a very careful follow-up throughout childhood because, they were considered too small to warrant surgical intervention, there are some early evidence that the heart may be hyperkinetic, slightly overloaded, but `yet no distinct abnormality could be displayed by conventional diagnostic investigations [8]. Many adult patients with congenital VSD also develop ascending aortic dilation, but only few report the clinical features and surgical management of these patients [8].
We report a case of a 23 years man, with very lately diagnosed VSD, associated with AR and ascending aortic aneurysm. The patient had experienced generalized weakness and exertional breathlessness during the previous five years, with a marked decline in his symptoms in the last year. VSDs that result in congestive heart failure (HF) are routinely closed in infancy, whereas those associated with AR are nearly always in adults, as in our patient. The lack of early diagnosis and appropriate treatment worsens prognosis. VSD closure is indicated in cases of heart failure, large shunt, and elevated pulmonary artery pressure caused by hemodynamic changes, regardless of AR presence and severity. However, the development of AR may occur in the absence of congestive heart failure [9]. In the absence of a hemodynamically significant VSD, the indications for surgical repair become more complicated. Closure of the VSD, with or without aortic valve repair, is indicated for both perimembranous and subarterial VSDs when more than trivial AR is identified because of the progressive nature of AR. For patients with a subarterial VSD and AVP, VSD closure is indicated because of the high likelihood of progression of AVP and development of AR. Subarterial VSDs, larger than 5 mm should be closed regardless of the presence of aortic cusp prolapse, in order to prevent the development of AR. Furthermore, there is only a minimal chance for spontaneous closure of a subarterial VSD [10].
Types of surgical technique for VSD closure are few. In patients with doubly committed supracristal defect, in which the lack of support of the semilunar cusp seems to be the basic mechanism underlying the prolapse, simple closure of the defect with a patch should solve the problem. This technique would be ideal for cases with a small muscular ridge and conserved aortic ring. Highly evolved cases with a large prolapse require the placement of a double patch, one on the defect and the other on the aneurysm [11]. Owing to the anatomic relationship between the VSD and aortic structure, there might be some potential reoperations of aortic disease after VSD repair. So, it is extremely vital to address aortic disorder in patients with congenital VSD, especially when aortic cusps are involved [12].
Conclusion
Perimembraneous VSD, may be associated with aortic regurgitation and ascending aortic aneurysm as a secondary phenomenon, in case that it is not early diagnosed and successfully treated. Early diagnosis and management of these patients should prevent disease worsening and hemodynamic deterioration.
Availability of data and materials
Availability of supporting data.
References
Minette MS, Sahn DJ. Ventricular septal defects. Circulation. 2006;114:2190–7.
Jacobs JP, Burke RP, Quintessenza JA, et al. Congenital heart surgery nomenclature and database project: Ventricular septal defect. Ann Thorac Surg. 2000;69(suppl 4):S25–35.
Eroglu AG, Oztunc F, Saltik L, et al. Aortic valve prolapse and aortic regurgitation in patients with ventricular septal defect. Pediatr Cardiol. 2003;24:36–9.
Hoffman JI. Congenital heart disease: incidence and inheritance. Pediatr Clin N Am. 1990;37:25–43.
Hoffman JI, Somerville J, Williams RG, Webb GD. Task Force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–5.
Taqatqa AS, Vettukattil JJ. Atrioventricular septal defects: pathology, imaging, and treatment options. Curr Cardiol Rep. 2021;23(8):93.
Tomita H, Arakaki Y, Yagihara T, et al. Incidence of spontaneous closure of outlet ventricular septal defect. Jpn Circ J. 2001;65:364–6.
Rhodes LA, Keane JF, Keane JP, Fellows KE, Jonas RA, Castañeda AR, et al. Long follow-up (to 43 years) of ventricular septal defect with audible aortic regurgitation. Am J Cardiol. 1990;66:340–5.
Eroğlu AG, Oztunç F, Saltik L, Dedeoğlu S, Bakari S, Ahunbay G. Aortic valve prolapse and aortic regurgitation in patients with ventricular septal defect. Pediatr Cardiol. 2003;24(1):36–9. https://doi.org/10.1007/s00246-002-1423-6.
Dearani JA, Connolly HM, Martinez R, Fontanet H, Webb GD. Caring for adults with congenital cardiac disease: successes and challenges for 2007 and beyond. Cardiol Young. 2007;17(Suppl 2):87–96.
Umebayashi Y, Yuda T, Fukuda S, Moriyama Y, Iguro Y, Saigenji H, et al. Surgery for ventricular septal defect with aortic regurgitation. Kyobu Geka. 1993;46:1013–6.
Li H-Y, Yuan-Fei Zhao Lu, Dai S-J, Zhang H-J, Jiang W-J. Ascending aortic dilation in adult patients with congenital ventricular septal defect. An observational study. Medicine (Baltimore). 2018;97(15): e0383.
Acknowledgements
Thank you to Teuta Lumi, for the echocardiographic assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
IB performed the cardiac imaging and diagnosis. EH and BB drafted the manuscript. MYH and IB and GB editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical approval was not necessary as per the institutional law.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Coexistence of ventricular septal defect, aortic regurgitation, and ascending aortic aneurysm; or Concurrent ventricular septal defect, aortic regurgitation, and ascending aortic aneurysm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Haliti, E., Bytyçi, B., Henein, M.Y. et al. Ventricular septal defect associated with aortic regurgitation and ascending aortic aneurysm: a case report. J Med Case Reports 17, 446 (2023). https://doi.org/10.1186/s13256-023-04167-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04167-7