Abstract
Background
The BRCA2 gene is a well-known tumor suppressor gene implicated in breast and ovarian cancers. BRCA1/2 mutations can be sensitive to poly ADP-ribose polymerase (PARP) inhibitors such as olaparib. However, some of these patients develop resistance to this treatment and an essential factor contributing to acquired insensitivity is the occurrence of reversion mutations in the BRCA1/2 genes.
Case presentation
We report the case of a 65-year-old Brazilian female patient who had previously been diagnosed with metastatic lung carcinoma carrying a BRCA2 mutation that had extended to the central nervous system. Following disease progression, olaparib was administered, resulting in a stabilizing effect on her condition for ~ 30 months. During a routine follow-up, a new triple-negative breast tumor was found. Genetic testing revealed the presence of two distinct BRCA2 gene mutations in the breast tumor. The original mutation (p.Val220Ilefs4) led to a frameshift, culminating in the production of a truncated and non-functional BRCA2 protein; the second mutation, K437fs22, rectified the reading frame of exon 11. Consequently, Rad51 could properly bind to BRCA2—an essential protein crucial for DNA repair. This restoration resulted in a functional BRCA2 protein, effectively elucidating the clinical resistance observed in the new breast tumor in this case.
Conclusions
This case report highlights the clinical significance of comprehensive next-generation sequencing analyses for lung adenocarcinomas, both at diagnosis and upon progression. Such analyses enable informed decisions regarding targeted therapies and facilitate a deeper comprehension of resistance mechanisms.
Similar content being viewed by others
Background
Lung adenocarcinoma accounts for the highest number of cancer-related deaths worldwide, constituting 18% of them [1]. Among all lung cancer cases, adenocarcinoma makes up ~ 40% [2], and around 60% of adenocarcinomas carry at least one mutation [3]. The most prevalent mutations in patients with lung adenocarcinoma occur in the EGFR, KRAS, and ALK genes [3]. The significance of investigating these mutations lies in the availability of targeted therapies for some of them. These drugs have the potential to alter the natural course of the disease for responsive patients, leading to substantial enhancements in their survival and quality of life [4].
BRCA1/2 germinative mutations are the most common cause of hereditary breast and ovarian carcinomas, but they can also lead to other types of cancer, such as pancreatic and prostatic carcinomas [5]. Approximately 1% of lung adenocarcinomas can harbor BRCA1/2 mutations [6], which can be sensitive to poly ADP-ribose polymerase (PARP) inhibitors such as olaparib [7]. However, some of these patients develop resistance to this treatment, and an essential factor contributing to acquired insensitivity is the occurrence of reversion mutations in the BRCA1 or BRCA2 genes [8]. A reversion mutation is a genetic alteration that reinstates the functionality of a gene previously mutated [9].
We are reporting a case of a patient with a germline mutation in BRCA2 and a diagnosis of metastatic lung adenocarcinoma with long-standing disease control on olaparib. The patient developed a new triple-negative breast carcinoma while receiving this PARP inhibitor. Interestingly, next-generation sequencing (NGS) of the breast tumor revealed an additional BRCA2 mutation.
Case presentation
We present a case involving a 65-year-old Brazilian woman with a history of smoking and a family background of breast cancer. Her mother passed away at the age of 61 years due to breast cancer, and her maternal aunt also succumbed to breast cancer at the age of 51 years. This patient was diagnosed in August 2019 with lung PDL1 negative adenocarcinoma metastatic to the central nervous system (CNS) with three lesions (right frontal and left occipital and parietal), the largest of them 1 cm in diameter. She subsequently underwent stereotactic body radiation therapy (SBRT) of the three CNS lesions followed by a four-cycle regimen of chemotherapy involving pembrolizumab, carboplatin, and pemetrexed. This was followed by maintenance therapy consisting of pembrolizumab and pemetrexed.
She additionally underwent radiation therapy with a dose of 3000 cGy delivered in five fractions targeting the residual mass in the right upper lung. This approach aimed to enhance the effectiveness of immunotherapy. Maintenance therapy was continued until August 2020. Unfortunately, at this point, disease progression was observed in the central nervous system (CNS), characterized by the emergence of two new cerebellar lesions. In response, she underwent surgery to address the larger of the two lesions and subsequently received stereotactic body radiation therapy (SBRT) for the other lesion. The pathological examination of the cerebellar lesion revealed positivity for thyroid transcription factor-1 (TTF-1), cytokeratin 5, and Napsin, which was consistent with brain metastasis originating from her pulmonary adenocarcinoma.
We had not yet obtained the NGS study of the cerebellar lesion. Since the FoundationOne test of the lung lesion revealed mutations in both the BRCA2 and ATM genes (but not in EGFR and ALK, as presented in Table 1), we initiated her treatment with olaparib in November 2020. The initial dosage was three tablets of 150 mg per day, which was later reduced to two tablets daily due to severe fatigue. By February 2023, positron emission tomography/computed tomography (PET/CT) scan revealed a new lesion in her right breast. Despite her possession of a germline BRCA mutation (as detailed in Table 1), we did not pursue breast screening due to the metastatic nature of the lung carcinoma.
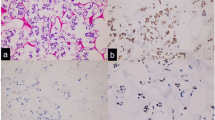
A biopsy of the breast lesion revealed a triple-negative invasive breast adenocarcinoma. Subsequently, she underwent a quadrantectomy, and the pathological report unveiled a 14 mm triple-negative breast carcinoma. Additionally, one sentinel lymph node displayed a 3 mm focus of carcinoma (Fig. 1). Given the overall stability observed in the PET scan results, the decision was made to continue olaparib treatment without interruption. The FoundationOne test results for the breast tumor are also presented in Table 1. In addition to her ongoing treatment, she also received adjuvant radiation therapy targeted at the right breast.
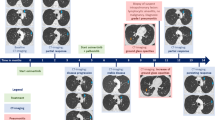
A Upper three images: Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET/CT) scan results before starting olaparib in October 2020. Lower three images of December 2020 after 1 month of olaparib showing a partial response in the mediastinal and lung nodules. B FDG PET/CT scan from February 2023 at the level of the right breast showing the appearance of a new hypermetabolic nodule in the right breast and no activity in previous lung and mediastinal nodules
We analyzed the impact of both mutations using the predictProtein software (freely available at https://predictprotein.org); our analysis revealed that the original BRCA2 mutation, c.658_659del (p.Val220Ilefs4), results in a frameshift, which in turn leads to the truncation and inactivation of the BRCA2 protein. Conversely, the second mutation, c.1310_1313del (K437fs22), restores the reading frame, initiating from the beginning of exon 10. Notably, exon 10 encompasses a DNA binding domain [10]. Exon 11, in turn, encompasses eight crucial functional domains referred to as BRC repeats, which directly interact with the recombinase RAD51 [10] (Fig. 2). In effect, this second mutation reinstates the functionality of BRCA2, which had been disrupted by the initial mutation.
Discussion and conclusions
This case highlights several intriguing aspects. The initial observation centers around the remarkable extended disease control achieved over a span of 30 months in this patient with olaparib, targeting her metastatic lung adenocarcinoma harboring a BRCA2 mutation. In existing literature, we encounter instances of transient responses in lung adenocarcinomas with BRCA2 mutations, ranging from 13 months [11] to 6 months [7]. This case exemplifies why individuals with lung adenocarcinoma, regardless of their smoking history, should ideally undergo comprehensive next-generation sequencing (NGS) to detect potentially rare yet actionable mutations, as underscored by the findings in this patient’s case.
Another interesting observation is that resistance to olaparib appeared in the breast tumor of this patient through a reversion mutation. Reversion mutations are characterized as secondary mutations, often manifesting as small deletions, within a mutant gene. These mutations work to restore the reading frame of the gene, thereby giving rise to a partially functional protein [8]. In this specific case, the original BRCA2 mutation led to a frameshift, culminating in the production of a truncated and nonfunctional BRCA2 protein. Subsequently, the occurrence of a second mutation reinstated the BRCA2 reading frame, thus enabling the functionality of exon 11 within this gene. Indeed, exon 11 of BRCA2 serves as the site where Rad51 binding takes place [8]. Thanks to the presence of this second mutation, the functionality of BRCA2, which had been compromised by the original mutation, was reinstated. Consequently, this mutation falls into the category of reversion mutations.
The reason behind the occurrence of this mutation in breast tissue, leading to the emergence of a new primary triple-negative breast tumor, rather than manifesting in one of the previously identified lung primary or metastatic sites, resulting in disease progression at any of those locations, remains elusive. Notably, Darabi et al. and Yonina et al. [12, 13] also presented findings indicating a higher occurrence of reversion mutations in breast and ovarian carcinomas compared with other tumor histologies. Whether this observation can be attributed to the elevated frequency of BRCA mutations in breast and ovarian cancers, or if there exists a tissue-specific context that facilitates a higher likelihood of reversion mutations, remains to be clarified. Of particular interest is the fact that the use of platinum salts, as seen in the treatment of this patient, has been linked to the occurrence of reversion mutations [13].
We interpreted the emergence of the new triple-negative breast tumor as the sole locus of disease that had become unmanageable. Consequently, we maintained the administration of olaparib, concurrently implementing surgery and radiation therapy to address the breast tumor. We chose not to introduce adjuvant chemotherapy, as we believed it had the potential to jeopardize the intricate task of managing the metastatic lung cancer, which had been effectively controlled using olaparib. Combining chemotherapy with a PARP inhibitor could escalate toxicity, potentially hindering patient compliance and undermining the control of the metastatic lung carcinoma.
Another promising way to detect a BRCA reversion mutation is through circulating cell-free DNA (cfDNA). In a study conducted at a single institution using a clinically validated 73-gene cfDNA assay, which assesses single-nucleotide variants and insertion-deletion mutations (indels) in BRCA1/2 and differentiates somatic/reversion from germline mutations with remarkable accuracy, the analysis extends beyond identifying germline and somatic BRCA1/2 mutations. This cfDNA analysis methodology also enables the detection of reversion BRCA1/2 mutations [14]. Among 828 individuals with advanced malignancies, including breast cancer, who underwent testing with the 73-gene cfDNA assay, 7.2% of the patients were found to carry one or more pathogenic BRCA1/2 mutations. Notably, both somatic and germline variants were identified. Furthermore, in 21.4% of patients harboring germline BRCA1/2 mutations, polyclonal reversion mutations were detected. These reversion mutations were most observed in individuals who had previously received a PARP inhibitor.
It is our assertion that individuals afflicted with metastatic lung adenocarcinoma should undergo thorough NGS procedures. These NGS assessments are indispensable for detecting infrequent yet exploitable mutations that can be effectively managed using tailored molecular-based therapies, thus augmenting the efficacy of disease control. Additionally, as the disease progresses, patients should pursue NGS analyses to unravel the intricate molecular mechanisms that underlie treatment resistance.
Availability of data and materials
Data will be made available under reasonable request.
Abbreviations
- cGy:
-
Centigray
- CNS:
-
Central nervous system
- NGS:
-
Next-generation sequencing
- PARP:
-
Poly ADP-ribose polymerase
- PET/CT:
-
Positron emission tomography/computed tomography
- SBRT:
-
Stereotactic body radiation therapy
References
Global Cancer Statistics 2020. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries-Sung-2021. CA: Cancer J Clin. https://doi.org/10.3322/caac.21660. Accessed 12 Jun 2023.
Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–44.
Bernicker EH, Miller RA, Cagle PT. Biomarkers for selection of therapy for adenocarcinoma of the lung. J Oncol Pract. 2017;13(4):221–7.
Majeed U, Manochakian R, Zhao Y, Lou Y. Targeted therapy in advanced non-small cell lung cancer: current advances and future trends. J Hematol OncolJ Hematol Oncol. 2021;14(1):108.
Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer. 2018;119(2):141–52.
Prevalence and clinical significance of pathogenic germline BRCA1/2 mutations in Chinese non-small cell lung cancer patients|Semantic Scholar. https://www.semanticscholar.org/paper/Prevalence-and-clinical-significance-of-pathogenic-Hu-Yang/38da866b63a16ffc2b53de6cbed1b9f75b69517f. Accessed 12 Jun 2023.
Wu C, Fan M, Hu Y. Response to olaparib in metastatic lung adenocarcinoma with germline BRCA2 mutation: a case report. Anticancer Drugs. 2022;33(1): e734.
Ganesan S. Tumor suppressor tolerance: reversion mutations in BRCA1 and BRCA2 and resistance to PARP inhibitors and platinum. JCO Precis Oncol. 2018;2:1–4.
Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–20.
Andreassen PR, Seo J, Wiek C, Hanenberg H. Understanding BRCA2 function as a tumor suppressor based on domain-specific activities in DNA damage responses. Genes. 2021;12(7):1034.
Zhang L, Wang J, Cui LZ, Wang K, Yuan MM, Chen RR, et al. Successful treatment of refractory lung adenocarcinoma harboring a germline BRCA2 mutation with olaparib: a case report. World J Clin Cases. 2021;9(25):7498–503.
Darabi S, Braxton DR, Xiu J, Carneiro BA, Swensen J, Antonarakis ES, et al. BRCA1/2 reversion mutations in patients treated with poly ADP-ribose polymerase (PARP) inhibitors or platinum agents. Medicina. 2022;58(12):1818.
Murciano-Goroff YR, Schram AM, Rosen EY, Won H, Gong Y, Noronha AM, et al. Reversion mutations in germline BRCA1/2-mutant tumors reveal a BRCA-mediated phenotype in non-canonical histologies. Nat Commun. 2022;13(1):7182.
Vidula N, Rich TA, Sartor O, Yen J, Hardin A, Nance T, et al. Routine plasma-based genotyping to comprehensively detect germline, somatic, and reversion BRCA mutations among patients with advanced solid tumors. Clin Cancer Res. 2020;26:2546–55.
Acknowledgements
None.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
ADG and AMM: conceptualization, resources, writing—original draft, analysis and interpretation of patient data regarding the BRCA2 reversion mutation. FLAF: resources and writing—original draft. BCAA: protein predication analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
del Giglio, A., da Costa Aguiar Alves, B., Murad, A.M. et al. Metastatic lung adenocarcinoma with BRCA2 mutation and longstanding disease control on olaparib, developing triple negative breast adenocarcinoma with additional BRCA2 reversion mutation: a case report. J Med Case Reports 17, 407 (2023). https://doi.org/10.1186/s13256-023-04139-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04139-x