Abstract
Background
Adenomatoid mesothelioma is a rare subtype of malignant mesothelioma that can be confused with adenomatoid tumors, which are classified as benign. The clinical features and optimal management of adenomatoid mesothelioma have not been elucidated in the literature. In this report, we present an extremely rare case of adenomatoid mesothelioma that developed on the peritoneal surface of the diaphragm as well as a literature review of adenomatoid mesothelioma in the abdominal cavity.
Case presentation
The patient was a 61-year-old Japanese woman who had undergone resection of a malignant peripheral nerve sheath tumor of the hand 18 years prior. She was diagnosed with clinical stage I lung adenocarcinoma on follow-up chest radiography. Simultaneously, a 20-mm enhancing nodule with slow growth on the right diaphragm was detected on contrast-enhanced computed tomography. She presented no specific clinical symptoms. At this point, the lesion was suspected to be a hypervascular tumor of borderline malignancy, such as a solitary fibrous tumor. After a left upper lobectomy for lung adenocarcinoma, she was referred to our department, and laparoscopic tumor resection was performed. Adenomatoid tumors were also considered based on the histopathological and immunohistochemical analyses, but we made the final diagnosis of adenomatoid mesothelioma using the results of the genetic profile. The patient remains alive, with no recurrence noted 6 months after surgery.
Conclusion
We encountered a valuable case of adenomatoid mesothelioma of peritoneal origin. There are some previously reported cases of adenomatoid mesothelioma and adenomatoid tumors that may need to be recategorized according to the current classification. It is important to accumulate and share new findings to clarify the clinicopathological characteristics and genetic status of adenomatoid mesothelioma.
Similar content being viewed by others
Background
Malignant mesothelioma is classified into three main histological subtypes as follows: epithelial, sarcomatoid, and biphasic. According to the recent World Health Organization classification of pleural tumors, adenomatoid mesothelioma is categorized as a rare subtype of epithelioid mesothelioma that should be distinguished from adenomatoid tumors, which are benign mesothelial tumors [1, 2]. It is reported that adenomatoid mesothelioma accounts for approximately 5% of pleural mesothelioma and can present various types of morphologic features, leading to difficulty in diagnosis [3]. Due to the rarity of reported cases, the clinical features and optimal management of adenomatoid mesothelioma have not been described yet. Herein, we report an extremely rare case of adenomatoid mesothelioma that developed on the peritoneal surface of the diaphragm as well as a literature review of adenomatoid mesothelioma in the abdominal cavity.
Case presentation
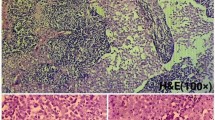
A 61-year-old Japanese woman underwent resection of a malignant peripheral nerve sheath tumor of the hand when she was aged 43 years and was followed up by radiological examination. Chest radiography revealed a mass lesion in the left upper lung 18 years later. She was a current smoker but had no history of asbestos exposure, and presented no specific clinical symptoms. As a result of a detailed examination, she was diagnosed with clinical stage I lung adenocarcinoma. Simultaneously, contrast-enhanced computed tomography (CT) detected a 20-mm enhancing nodule with slow growth on the right diaphragm (Fig. 1a). 18-Fluoro-2-deoxyglucose positron emission tomography revealed that the maximum standard uptake value of the nodule was 3.5 (Fig. 1b). Ultrasonography (US) revealed a low-echoic lesion, and early enhancement was observed on Sonazoid-enhanced US (Fig. 1c, d). These results indicated that the lesion was a hypervascular tumor of borderline malignancy, such as solitary fibrous tumor (SFT). After a left upper lobectomy for lung adenocarcinoma, the patient was referred to our department for surgical resection of the peritoneal tumor. Laboratory data at the time of presentation were as follows: white blood cell count, 7240 cells/μL; hemoglobin level, 14.2 g/dL; platelet count, 10.4 × 104 cells/μL; aspartate transaminase, 22 IU/L; alanine aminotransferase, 24 IU/L; total bilirubin, 0.57 mg/dL; albumin, 4.1 g/dL; creatinine, 0.58 mg/dL. Serum tumor markers, such as proteins induced by vitamin K absence or antagonist II and alpha-fetoprotein, were within the normal range (25 μg/mL and 2.1 ng/mL, respectively). Laparoscopic tumor resection was performed. Intraoperative findings are shown in Fig. 2. A thin pedunculated tumor was found to originate from the peritoneal surface of the right diaphragm. The tumor was compressing liver segment 8 but without apparent invasion. Well-developed capillary vessels were observed around the tumor. The pedicle of the tumor was clipped at its origin and divided, and a tumorectomy was completed. Gross examination showed a 28 × 20 x 11 mm3 brown–red tumor with a smooth cut surface (Fig. 3a). Histopathological examination revealed papillary architecture with focal small aggregates of mesothelial cells (Fig. 3b). Glandular lumen formation, indicative of an adenomatoid pattern, was partially observed. Immunohistochemical analysis showed that the tumor cells were positive for cytokeratin 5/6 (CK 5/6) and calretinin, and negative for carcinoembryonic antigen (CEA), thyroid transcription factor-1 (TTF-1), cluster of differentiation 34 (CD34), and signal transducer and activator of transcription 6 (STAT6) (Fig. 3c–h). In addition, hot-spot mutations in TNF receptor associated factor 7 (TRAF7) were not detected by Sanger sequencing, and the tumor cells displayed negative immunostaining for L1 cell adhesion molecule. Fluorescence in situ hybridization (FISH) showed no homozygous deletion of 9p21 or hemizygous deletion of NF2 (data not shown). Based on these results, the patient was diagnosed with localized adenomatoid mesothelioma. The postoperative course was uneventful, and the patient was discharged on the fourth postoperative day. She remains alive and is being monitored in an outpatient setting. No recurrence was noted 6 months after surgery.
Preoperative examination. a Contrast-enhanced computed tomography revealed a tumor (arrow) with marked enhancement in the arterial phase of the right diaphragm. b 18-Fluoro-2-deoxyglucose positron emission tomography image revealed that the maximal standard uptake value of nodule (arrow) was 3.5. c Ultrasonography showed a low-echoic lesion. d Early enhancement was observed in Sonazoid-enhanced ultrasonography
Macroscopic and microscopic findings of the resected tumor. a Macroscopic findings revealed a 28 × 20 × 11 mm3 brown–red tumor with a smooth cut surface. b Microscopic findings revealed papillary architecture with focal small aggregates of mesothelial cells. c Tumor cells were positive for cytokeratin 5/6. d Tumor cells were partially positive for calretinin. e Tumor cells were negative for carcinoembryonic antigen. f Tumor cells were negative for thyroid transcription factor-1. g Tumor cells were negative for cluster of differentiation 34. h Tumor cells were negative for signal transducer and activator of transcription 6
Discussion and conclusions
Malignant mesothelioma is a highly aggressive tumor arising from mesothelial cells lining the pleura, peritoneum, and pericardium. The prognosis differs among histological subtypes, and epithelioid mesothelioma contains several morphological subtypes that also have some impact on prognosis [4, 5]. Adenomatoid mesothelioma is usually classified as an architectural subtype of epithelioid mesothelioma. It can histologically mimic adenomatoid tumors and is sometimes difficult to distinguish from others [3]. We report herein a case of peritoneal adenomatoid mesothelioma originating in the diaphragm. There are several reports of pleural adenomatoid mesothelioma, but adenomatoid mesothelioma of peritoneal origin is extremely rare. To the best of our knowledge, this is the first report of peritoneal adenomatoid mesothelioma localized to the diaphragm.
In the present patient, we made the final pathological diagnosis of localized adenomatoid mesothelioma based on histochemical and immunohistochemical analyses and genetic profiling. Based on the tumor growth rate and enhancement pattern on contrast-enhanced CT, we first suspected an SFT. However, immunohistochemical staining was negative for CD34 and STAT6, ruling out the possibility of SFTs. Metastasis of lung adenocarcinoma was also ruled out based on the immunohistochemical staining patterns for positive mesothelial and negative epithelial markers (positive for CK5/6 and calretinin and negative for CEA and TTF-1). The tumor was thought to be an adenomatoid tumor or adenomatoid mesothelioma. Several reports indicate that adenomatoid tumors are defined by TRAF7 mutation; therefore, we examined TRAF7 hot-spot mutations, including codons 519, 521, 538, 561, and 577, using Sanger sequencing [6, 7]. TRAF7 mutations were not detected. Homozygous deletion of 9p21 and hemizygous deletion of NF2, which are characteristics of pleural mesothelioma, was also not detected by FISH. However, the frequency of these genetic alterations in peritoneal mesothelioma is reported to be less than that of pleural mesothelioma, which led to the final diagnosis of adenomatoid mesothelioma [8]. In addition to a pathological examination, a genetic approach may be necessary and useful for distinguishing adenomatoid mesothelioma from adenomatoid tumors.
We conducted a literature search of the PubMed database to understand the current status of adenomatoid mesothelioma in the abdominal cavity. We came across six cases, including our case, that reported an “adenomatoid mesothelioma” (Table 1) [9,10,11,12,13]. There was one man and five women, and none had a history of asbestos exposure. The sites of tumor origin included the uterus (n = 1), testis (n = 1), liver (n = 1), and peritoneum (n = 4), and multiple tumors were observed in patient 5. Recurrence was not observed in five out of six patients, but Mori et al. reported a recurrent case after curative resection (patient 5) [13]. We should also conduct a careful follow-up with our patient, although the optimal follow-up strategy has not been well established. Through a literature search, we found that there were patients in whom it was difficult to distinguish between adenomatoid tumors and adenomatoid mesothelioma. For instance, several reports mentioned adenomatoid mesothelioma as a “benign tumor.” However, considering that adenomatoid mesothelioma is categorized as a subtype of epithelial mesothelioma in the latest World Health Organization classification, it should be treated as a malignant tumor. In addition, there are several reports of adenomatoid tumors invading the surrounding organs. Adenomatoid tumors are classified as benign; therefore, these patients may need to be recategorized according to the current classification. It is necessary to clearly define adenomatoid mesothelioma not only from the viewpoint of morphology but also from the viewpoint of mutational status, such as TRAF7 mutation and immunostaining findings, which would enable us to examine the clinical characteristics of adenomatoid mesothelioma.
In conclusion, we encountered a case of adenomatoid mesothelioma of peritoneal origin. It is important to accumulate and share such experiences, which may lead to better characterization of this disease.
Availability of data and materials
Not applicable.
Abbreviations
- CT:
-
Computed tomography
- US:
-
Ultrasonography
- SFT:
-
Solitary fibrous tumor
- FISH:
-
Fluorescence in situ hybridization
- CEA:
-
Carcinoembryonic antigen
- TTF-1:
-
Thyroid transcription factor-1
- CD34:
-
Cluster of differentiation 34
- STAT6:
-
Signal transducer and activator of transcription 6
- TRAF7 :
-
TNF receptor associated factor 7
- CK 5/6:
-
Cytokeratin 5/6
References
Dacic S. Pleural mesothelioma classification-update and challenges. Mod Pathol. 2022;35:51–6.
Sauter JL, Dacic S, Galateau-Salle F, Attanoos RL, Butnor KJ, The CA, et al. World Health Organization classification of tumors of the pleura: advances since the 2015 classification. J Thorac Oncol. 2021. https://doi.org/10.1016/j.jtho.2021.12.014.
Weissferdt A, Kalhor N, Suster S. Malignant mesothelioma with prominent adenomatoid features: a clinicopathologic and immunohistochemical study of 10 cases. Ann Diagn Pathol. 2011;15:25–9.
Meyerhoff RR, Yang CF, Speicher PJ, Gulack BC, Hartwig MG, D’Amico TA, et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res. 2015;196:23–32.
Brcic L, Vlacic G, Quehenberger F, Kern I. Reproducibility of malignant pleural mesothelioma histopathologic subtyping. Arch Pathol Lab Med. 2018;142:747–52.
Goode B, Joseph NM, Stevers M, Van Ziffle J, Onodera C, Talevich E, et al. Adenomatoid tumors of the male and female genital tract are defined by TRAF7 mutations that drive aberrant NF-kB pathway activation. Mod Pathol. 2018;31:660–73.
Itami H, Fujii T, Nakai T, Takeda M, Kishi Y, Taniguchi F, et al. TRAF7 mutations and immunohistochemical study of uterine adenomatoid tumor compared with malignant mesothelioma. Hum Pathol. 2021;111:59–66.
Offin M, Yang SR, Egger J, Jayakumaran G, Spencer RS, Lopardo J et al. Molecular characterization of peritoneal mesotheliomas. J Thorac Oncol. 2021.
Bisset DL, Morris JA, Fox H. Giant cystic adenomatoid tumour (mesothelioma) of the uterus. Histopathology. 1988;12:555–8.
Lins CM, Elias J Jr, Cunha AF, Muglia VF, Monteiro CR, Valeri FV, et al. MR imaging features of peritoneal adenomatoid mesothelioma: a case report. Clinics (Sao Paulo). 2009;64:264–9.
Okuda T, Ogino Y, Yamashita S, Ishii H, Kin S, Nagata A, et al. Diagnostic laparoscopy identifies a peritoneal adenomatoid-like mesothelioma masquerading as ovarian cancer: a case report. Eur J Gynaecol Oncol. 2014;35:91–4.
Yang LH, Yu JH, Xu HT, Lin XY, Liu Y, Miao Y, et al. Mesothelioma of the tunica vaginalis testis with prominent adenomatoid features: a case report. Int J Clin Exp Pathol. 2014;7:7082–7.
Mori D, Kido S, Hiraki M, Sumi K, Ureshino N, Masuda M, et al. Peritoneal adenomatoid (microcystic) mesothelioma. Pathol Int. 2020;70:876–80.
Acknowledgements
Not applicable.
Funding
There is no financial support to declare associated with this study.
Author information
Authors and Affiliations
Contributions
KKa and HS collected and interpreted the patient data, performed the literature review, and wrote the manuscript. AK executed the pathological analysis and defined the diagnosis. HS, TY, and TFujiwara edited the manuscript. RY, KYa, YU, KYo, TF, KKu, KT, and MK treated and observed the patient. TY and TFujiwara supervised the whole writing process and every clinical conclusion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kawabe, K., Sato, H., Kitano, A. et al. Adenomatoid mesothelioma arising from the diaphragm: a case report and review of the literature. J Med Case Reports 16, 228 (2022). https://doi.org/10.1186/s13256-022-03420-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03420-9