Abstract
Background
Bullous pemphigoid is an uncommon dermatologic manifestation seen in squamous cell lung cancer, and evidence guiding optimal treatment, especially in the elderly population, is limited. We report herein a case of squamous cell lung cancer diagnosed after being investigated for refractory bullous pemphigoid showing marked response to carboplatin-based chemotherapy. This is the first case report that shows carboplatin can be used as an effective alternative in treatment of malignancy-associated bullous pemphigoid.
Case report
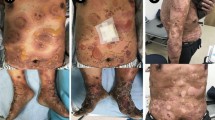
An 80-year-old caucasian man developed extensive vesiculobullous rashes on his trunk, chest, abdomen, and inguinal region associated with significant pruritus causing sleep disturbance. The diagnosis of bullous pemphigoid was confirmed on skin biopsy. The skin lesions continued to worsen even after use of oral and topical steroid in addition to oral doxycycline. Chest computed tomography revealed a spiculated left lung lesion along with mediastinal lymphadenopathy. Fine-needle aspiration from the mediastinal lymph node confirmed metastatic squamous cell lung carcinoma. Carboplatin with gemcitabine was initiated, and significant response was seen within 3 days of chemotherapy. The skin lesions continued to remain in remission even after stopping the chemotherapy.
Conclusion
Although there are still controversies regarding paraneoplastic etiology of bullous pemphigoid, this case presents a temporal association. It is the first case report showing a remarkable response with the use of a carboplatin-based regimen.
Similar content being viewed by others
Introduction
Several case reports have described the association of different types of malignancy with bullous pemphigoid, but a direct paraneoplastic correlation has not been proven. Bullous pemphigoid (BP) is a chronic, autoimmune, subepidermal, blistering skin disease mainly seen in elderly individuals.
Paraneoplastic or malignancy-associated BP differs from typical pemphigus, which is characterized by mucosal involvement and positive Nikolsky sign [2]. Paraneoplastic bullous pemphigoid is most commonly associated with B-cell lymphoproliferative disorders [3]. Bullous pemphigoid can also be associated with diabetes, rheumatoid arthritis, systemic lupus erythematosus, ulcerative colitis, multiple sclerosis, and myasthenia gravis and can be drug induced.1
Bullous pemphigoid is one of the rare paraneoplastic dermatologic manifestations in association with squamous cell lung cancer, and there is no clear evidence of response to carboplatin for its management.
Case report
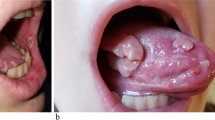
We report herein the case of an 80-year-old caucasian man who developed vesiculobullous rashes involving his trunk, shoulder close to the axilla, and inguinal regions for 2 months before admission on 17 June 2019 (Fig. 1, pictures 1 and 2). These were associated with severe pruritus causing significant sleep disturbance, and Nikolsky sign was negative. Clinical and histological findings were consistent with bullous pemphigoid (BP). His past medical history was only significant for chronic tophaceous gout and hypertension. He was a current smoker with 40 pack-years history of smoking. He lived alone and had a supportive friend who was the only close person in contact with him.
He was commenced on betamethasone cream and oral doxycycline followed by oral prednisone (1 mg/kg/day) daily without any response. Paraneoplastic nature of the disease was suspected, and computed tomography (CT) scan of the chest was performed given the significant history of smoking. CT scan (Fig. 2, image A) revealed a spiculated left lung lesion along with mediastinal lymphadenopathy. PET scan (Fig. 2, image B) showed a hypermetabolic focus on left lung apex mass suspicious of primary malignancy with metastases to the mediastinal nodes and liver lesion. Fine needle aspiration from the mediastinal lymph node confirmed advanced squamous cell lung cancer, which was PDL1 80%.
Although eligible for first-line immunotherapy, because of the likelihood of autoimmune mechanism and association of bullous pemphigoid, the decision was made to treat with chemotherapy. He was treated with carboplatin and gemcitabine [23], based on a previous case report of BP associated with squamous cell carcinoma treated with cisplatin and gemcitabine. The dose was reduced at 75% of the calculated dose due to his age and high risk of toxicity predicted by Cancer and Aging Research Group (CAGR) chemotherapy toxicity calculator. A significant response was seen within 3 days of chemotherapy as depicted in Figs. 3 and 4 (pictures 3–6). But the subsequent dose of chemotherapy had to be withheld after he developed small bowel obstruction secondary to incarcerated inguinal hernia. Even after stopping chemotherapy, the bullous pemphigoid rashes continued to remain in remission after marked clinical improvement (Fig. 5, pictures 7 and 8). On further follow-up, he wanted to pursue best supportive care under palliative care team, hence further scans were not organized.
Discussion and literature review
The paraneoplastic syndrome refers to metabolic or neuromuscular manifestations of certain malignancies, and these paraneoplastic manifestations are not attributable to direct tumor invasion or distant spread of tumor cells [4].
The paraneoplastic cutaneous syndrome is defined by two criteria: (i) the dermatosis must develop only after the development of cancer, and (ii) both dermatosis and cancer follow a parallel course in which complete removal of the cancer results in clearing of the dermatosis, while recurrence of cancer causes relapse of the dermatosis [5]. However, this definition is not without limitation, and exceptions such as pemphigus exist. Robinson et al. described rare forms of pemphigus, including paraneoplastic pemphigus in 1999 [2, 5]. Unlike paraneoplastic pemphigus, the paraneoplastic nature of bullous pemphigoid has not been accepted widely. Hence, some authors prefer to use the term pemphigoid associated with malignancies (PAM) [8].
Despite several published case reports and trials, a definite association remains controversial (Table 1). In their study involving over 1000 Japanese patients, Ogava et al. found a significantly higher incidence of malignancy in BP (5.8%) than that of controls aged over 70 years (0.6%) [13]. Similar associations are cited in Asia [14]. However, studies conducted in Sweden [15] and the USA [16] failed to prove any statistically significant association.
Since 1960, there have been case reports of bullous pemphigoid associated with lung carcinoma, although only a few include details of treatment in the metastatic setting. Data regarding the treatment regimen used for lung cancer in elderly patients with bullous pemphigoid are further limited. A systematic review from Balestri et al. in 2015 reported a total of 33 cases of solid tumors. The most common cancers were breast and colon cancer (n = 4).
Although Bullous pemphigoid associated with malignancies has been reported to be refractory to conventional treatment, most case reports have suggested a good response to cancer treatment, including surgery and chemotherapy [8, 17, 18, 21,22,23]. Rook et al. reported the first case of PAM in 1968 [17]. The patient was a 40-year-old with advanced bronchial carcinoma with partial response to high-dose corticosteroids and who remained in remission after surgery. In 1987, Graham-Brown et al. reported another case of bronchial carcinoma in a 74-year-old man who did not respond to corticosteroid and azathioprine. However, after surgery and radiotherapy, PAM was controlled with low-dose corticosteroid [18]. Shigemori et al. reported an autopsied case of a 78-year-old man with bullous pemphigoid who had undifferentiated large cell carcinoma of the lung in 2001 [19].
Another case of a 52-year-old chronic smoker with squamous cell lung carcinoma was reported in 2014 in the Pan African Medical Journal. The patient was treated with cisplatin and Navelbine, combined with chest and cerebellar radiotherapy along with local corticosteroid therapy, resulting in regression of bullous lesions after two courses of chemotherapy [21]. Das et al. also reported a case of a 76-year-old man with squamous cell carcinoma of the lung, which showed significant improvement after treatment with cisplatin and gemcitabine [23].
Two other case reports were published in Japanese in 1986 and 1996 [2, 6] but did not include details about histopathology. Bullous pemphigoid associated with small cell lung cancer was reported in a French publication by Lakhdar et al. in 2014. The patient was a 44-year-old smoker who had marked regression of the bullous lesions with chemotherapy [20]. Safini et al. also reported a case of bullous pemphigoid associated with small cell lung cancer in a 46-year-old man. He received cisplatin and etoposide, resulting in regression of 75% of cutaneous lesions after two cycles of chemotherapy [22].
We performed a literature search on Embase, Medline, and PubMed from 1996 to 18 August 2020. Table 1 summarizes the cases reported. Our observation and literature review suggest that bullous pemphigoid associated with malignancy may be underreported. The reported association with lung cancer is rare and even more so in case of squamous cell lung cancer. Almost all the reports suggest good response to various chemotherapy regimens that include cisplatin. However, to our knowledge, this is the first case report to show that carboplatin can be used as an effective alternative in treatment of malignancy-associated bullous pemphigoid. This is even more relevant as carboplatin is more commonly used in advanced lung cancer compared with cisplatin, more so in elderly population due to better tolerability.
Conclusion
Many retrospective studies suggest increasing incidence of bullous pemphigoid [9,10,11], but large epidemiological studies have not justified extensive investigations and work-up to find underlying malignancy. We agree with the previously recommended approach of considering more extensive investigations in patients with history of malignancy, in younger age group, and cases with poor response to conventional immunosuppressants. While this case report presents a clear temporal association, and may be anecdotal experience, carboplatin-based chemotherapy in this setting can be considered as an effective alternative in palliative management of PAM.
Availability of data and materials
Not applicable, Additional details regarding the case are available for review in hospital medical records.
Abbreviations
- BP:
-
Bullous pemphigoid
- CAGR:
-
Cancer and Aging Research Group
- FNA:
-
Fine needle aspiration
- PAM:
-
Pemphigoid associated with malignancies
- PET:
-
Positron emission tomography
References
Cakmak O, Seçkin D, Ceken I, Yilmaz I, Akkuzu B, Ozluoglu L. Bullous pemphigoid associated with parotid carcinoma. Otolaryngol Head Neck Surg. 2002;127(4):354–6.
Robinson ND, Hashimoto T, Amagai M, Chan LS. The new pemphigus variants. J Am Acad Dermatol. 1999;40(5):649–71.
Robak E, Robak T. Skin lesions in chronic lymphocytic leukaemia. Leuk Lymphoma. 2007;48(5):855–65.
Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–54.
Chung VQ, Moschella SL, Zembowicz A, Liu V. Clinical and pathologic findings of paraneoplastic dermatoses. J Am Acad Dermatol. 2006;54:745.
Takeuchi M, Okazaki A, Nakajima N, Saito Y, Nozaki M, Niibe H. A case of Lung cancer with bullous pemphigoid. Gan No Rinsho. 1986;32(5):529–33.
Sato Y, Endo K, Ishikawa S, Onizuka M, Mitsui K, Mitsui T. A case of resected lung cancer associated with bullous pemphigoid. Nihon Kyobu Geka Gakkai Zasshi. 1996;44(4):524–8.
Balestri R, La Placa MMM, et al. Malignancies in bullous pemphigoid: a controversial association. J Dermatol. 2015;43:125–33.
Försti AK, Jokelainen J, Timonen M, Tasanen K. Increasing incidence of bullous pemphigoid in Northern Finland: a retrospective database study in Oulu University Hospital. Br J Dermatol. 2014;171(5):1223.
Brick KE, Weaver CH, Lohse CM, Pittelkow MR. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. 2014;71(1):92–9 (Epub 2014 03rd April).
Joly P, Baricault S, Sparsa A. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132(8):1998.
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2018. http://www.healthdata.org/gbd.
Ogawa H, Sakuma M, Morioka S, Kitamura K, Sasai Y, Imamura S, Inaba Y. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995;9(2):136–41.
Chang YT, Liu HN, Wong CK. Bullous pemphigoid—a report of 86 cases from Taiwan. Clin Exp Dermatol. 1996;21(1):20–2.
Lindelöf B, Islam N, Eklund G, Arfors L. Pemphigoid and cancer. Arch Dermatol. 1990;126(1):66–8.
Stone SP, Schroeter AL. Bullous pemphigoid and associated malignant neoplasms. Arch Dermatol. 1975;111(8):991–4.
Rook AJ. A pemphigoid eruption associated with carcinoma of the bronchus. Trans St Johns Hosp Dermatol Soc. 1968;54:152–4.
Graham-Brown RA. Bullous pemphigoid with figurate erythema associated with carcinoma of the bronchus. Br J Dermatol. 1987;117(3):385–8.
Shigemori M, Yoshida S, Azai S, Fujii K, Azuma K, Nishikawa H, Otani Y, Inoue F, Furukawa H, Mizumoto T, Saiga T. An autopsied case of G-CSF producing lung cancer with bullous pemphigoid and hyper-gammaglobulinemia. Nippon Naika Gakkai Zasshi. 2001;90(10):2066–8.
Lakhdar N, ElKhattabi W, Lahroussi M, Afif H, Aichane A. Small cell lung cancer associated with paraneoplastic bullous pemphigoid. Rev Pneumol Clin. 2014;70(3):169–72.
Janah H, Mahhou M, Souhi H, Zegmout A, Naji-Amrani H, Raoufi M, Elouazzani H, Rhorfi IA, Abid A. Pemphigoid bulleuse revelant un carcinome bronchique. Pan Afr Med J. 2014;19:45.
Safini F, Tawfiq N. Paraneoplastic bullous pemphigoid associated with lung cancer. Pan Afr Med J. 2015;21:248.
Das A, Das S, Das SK, Basuthakur S. A case of paraneoplastic bullous pemphigoid in association with squamous cell carcinoma of lung. J Postgrad Med. 2015;61(3):197–9.
Acknowledgements
A/Prof Susan Pendlebury, Dr Roderick Peek.
Funding
Department of Medical Oncology, Northwest Cancer Centre.
Author information
Authors and Affiliations
Contributions
PS and SKR made significant contributions to conception, design, acquisition, interpretation and drafted the work. SB and MKG helped in drafting and revising the manuscript. All authors read and approved the final manuscript. All authors agree to be both personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient’s next of kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
No financial or nonfinancial competing interests from any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shrestha, P., George, M.K., Baidya, S. et al. Bullous pemphigoid associated with squamous cell lung carcinoma showing remarkable response to carboplatin-based chemotherapy: a case report. J Med Case Reports 16, 184 (2022). https://doi.org/10.1186/s13256-022-03323-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03323-9