Abstract
Background
A decrease in bone mineral density is common in patients with Williams syndrome. However, appropriate management for osteoporosis in Williams syndrome patients has not been established. We report the case of a 12-year-old female patient with Williams syndrome, who underwent denosumab treatment for osteoporosis.
Case presentation
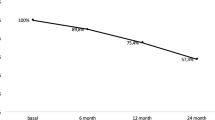
A 12-year-old Japanese female patient with Williams syndrome was shown to have very low bone mineral density. Bone mineral density was evaluated before treatment and at 5, 9, 17, 23, and 29 months of treatment by dual-energy X-ray absorptiometry. After denosumab therapy for 29 months, lumbar and total hip bone mineral density values had increased by 51.6% and 37.6%, respectively. No new fractures occurred during the observation period.
Conclusions
To the best of our knowledge, this is the first experience with denosumab treatment in Williams syndrome patients with osteoporosis. Based on our findings, denosumab may be an effective treatment option for Williams syndrome patients with osteoporosis.
Similar content being viewed by others
Introduction
Williams syndrome is characterized by cardiovascular disease, distinctive facies and personality, mild intellectual disability, connective tissue abnormalities, growth retardation, and endocrine disorders [1,2,3]. Williams syndrome is a genetic multisystem disorder caused by chromosome 7 microdeletion [4]. The prevalence of this syndrome is estimated to be 1 in 10,000–15,000 people [5]. It is common for patients with Williams syndrome to have decreased bone mineral density (BMD), which means they are at higher risk for fractures [6,7,8]. However, the mechanism of BMD reduction in Williams syndrome has not yet been elucidated, and appropriate management therapy for osteoporosis in this syndrome has not been established.
There have been no reports of the use of bone resorption inhibitors, including denosumab, for Williams syndrome complicated by osteoporosis. We have demonstrated the safety and efficacy of denosumab in bisphosphonate-resistant cases in children and young adults [9,10,11,12]. Because it is an antibody preparation and, unlike bisphosphonate, does not accumulate, we used denosumab in this study. Since the use of denosumab in pediatric patients is off-label, consent was obtained from the patient and the patient's parents. We have also obtained approval from our hospital’s ethics committee for the use of denosumab in this case.
In this report, we describe the clinical results of a 12-year-old female patient with Williams syndrome accompanied by osteoporosis. Improvements in BMD and bone metabolic markers were observed over 2 years of denosumab treatment. Written consent for publication was obtained from the patient’s parents prior to the start of this study because she was too young and developmentally delayed.
Case presentation
An 11-year-old Japanese female patient with Williams syndrome was referred to our department for osteoporosis treatment. She has delayed development, characteristic face, and behavioral characteristics such as hypersensitivity to sounds in her infancy period. Collectively, she was diagnosed as Williams syndrome with a genetic deletion of 7q11. at 2 years old by a certified genetic expert at another institution. Her case was complicated by aortic stenosis that did not require surgery. She underwent correction surgery for scoliosis at 12 years old (Fig. 1). Her BMD and laboratory data are presented in Tables 1 and 2, respectively. Her reflexes in her extremities were mildly decreased, but her muscle strength was normal. She was able to walk steadily, and her gait was normal. The patient’s history revealed no previous fractures, and her family had no history of osteoporosis.
Osteoporotic treatment was initiated for the patient’s diminished lumbar and total hip BMD values at 12 years old. Subcutaneous injections of denosumab were given every 6 months since 2 months after surgery (12 years old), and the bone turnover markers and BMD values were examined before treatment and at 5, 9, 17, 23, and 29 months of treatment.
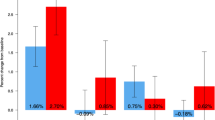
Serum tartrate-resistant acid phosphatase 5b (TRACP-5b) greatly decreased after the first administration (Table 2). Serum bone-specific alkaline phosphatase (BAP), type I procollagen N-terminal propeptide (PINP), whole parathyroid hormone (PTH), and 1-α, 25-dihydroxyvitamin D3 [1,25(OH)2D] decreased as well, while urinary type I collagen amino-terminal telopeptide (NTX) and 25-hydroxyvitamin D3 [25(OH)D] increased (Table 2). During the observation period, no calcium abnormalities such as hypocalcemia were observed (Table 2).
At 29 months of denosumab treatment, her BMD for lumbar and total hip increased by 51.6% and 37.6% at the end of our study, respectively (Fig. 2). There were no fractures or falls during the treatment period. She was able to keep active without getting out of shape through all the follow-up visits.
Discussion
In this report, we evaluated the clinical course of patients treated with denosumab for Williams syndrome complicated by osteoporosis. Twenty-nine months after the start of treatment, the BMD values of the lumbar and total hip increased by 51.6% and 37.6%, respectively. No fractures or adverse effects such as hypocalcemia occurred during the therapy, and her bone metabolism markers were notably improved. To the best of our knowledge, this is the first study to describe the successful treatment of osteoporosis with denosumab in patients with Williams syndrome.
Decreased BMD is common in patients with Williams syndrome [6,7,8]. Stadi et al. reported that nearly 80% of Williams syndrome patients with a history of fractures showed an impaired bone mineral status [7]. However, they did not address treatment for osteoporosis. The causes of decreased BMD in Williams syndrome have not been elucidated yet, but may be multifactorial, including unknown bone characteristics in this syndrome and environmental factors such as lack of physical activity or poor nutrition [13,14,15]. Palmieri et al. showed that reduced renal tubular reabsorption of phosphate results in reduced BMD in adults with Williams syndrome [16]. In this case report, the patient was able to keep active without getting out of shape during the treatment period.
Denosumab is a monoclonal antibody against the anti-receptor activator of nuclear factor-kappa β ligand approved for the treatment of osteoporosis and the prevention of complications of skeletal metastases [17]. Huang et al. reported that denosumab is an available treatment for BMD reduction in pediatric cancer survivors [18]. We have also proven the efficacy and safety of denosumab in children and young adults with refractory osteoporosis [9,10,11,12], although there have been no reports on denosumab for Williams syndrome and osteoporosis. In our patient, BMD values were very low. As a result, we started denosumab treatment to prevent ensuing fractures and improve BMD after a careful discussion with her and her family. At 29 months of denosumab treatment, both lumbar and total hip BMD values increased, and there were no additional fractures.
Markers of bone resorption are frequently used to assess the effectiveness of osteoporosis treatment [19]. BAP decreased to approximately 21% with denosumab, while PINP decreased to approximately 7% with increase in NTX and TRACP-5b. These results suggested that denosumab slightly inhibited bone resorption and increased bone mineral density in patients with Williams syndrome with osteoporosis.
Hypercalcemia occurs in 15–50% of children with Williams syndrome [20, 21]. In our case, calcium abnormality was not observed during the observational period. Vitamin D sensitivity [22] and decreased 1,25(OH)2D [23] were proposed mechanism of hypercalcemia in Williams syndrome. In this case, 1,25(OH)2D was decreased, but hypercalcemia did not occur.
The limitations of this study are that the long-term effects of denosumab on Williams syndrome with osteoporosis have not been confirmed and the sample size is small. Furthermore, further evaluation of this case is needed to validate our results. Because bone metabolism in Williams syndrome itself is not well known, it is difficult to compare our findings with those of previous pediatric patients. Nevertheless, bone density has increased, there have been no fractures during treatment, and patient satisfaction has been high with no adverse events.
Conclusion
After 29 months of treatment with denosumab, BMD levels and bone turnover markers improved in patients with Williams syndrome who had osteoporosis. The drug therefore represents an effective treatment option in such cases and warrants further study.
Availability of data and materials
The clinical and imaging data supporting the analysis and findings of this study will be available from the corresponding author upon reasonable request.
Abbreviations
- BMD:
-
Bone mineral density
- TRACP-5b:
-
Tartrate-resistant acid phosphatase 5b
- BAP:
-
Bone-specific alkaline phosphatase
- PINP:
-
Type I procollagen N-terminal propeptide
- PTH:
-
Parathyroid hormone
- 1,25(OH)2D:
-
1-α, 25-dihydroxyvitamin D3
- NTX:
-
Type I collagen amino terminal telopeptide
- 25(OH)D:
-
5-Hydroxyvitamin D3
References
Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17(4):269–71.
Stagi S, Bindi G, Neri AS, et al. Thyroid function and morphology in patients affected by Williams syndrome. Clin Endocrinol (Oxf). 2005;63(4):456–60.
Stagi S, Lapi E, Cecchi C, et al. Williams-Beuren syndrome is a genetic disorder associated with impaired glucose tolerance and diabetes in childhood and adolescence: new insights from a longitudinal study. Horm Res Paediatr. 2014;82(1):38–43.
Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362(3):239–52.
Kaplan P, Wang PP, Francke U. Williams (Williams Beuren) syndrome: a distinct neurobehavioral disorder. J Child Neurol. 2001;16(3):177–90.
Cherniske EM, Carpenter TO, Klaiman C, et al. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 2004;131(3):255–64.
Stagi S, Manoni C, Scalini P, et al. Bone mineral status and metabolism in patients with Williams-Beuren syndrome. Hormones (Athens). 2016;15(3):404–12.
Waxler JL, Guardino C, Feinn RS, et al. Altered body composition, lipedema, and decreased bone density in individuals with Williams syndrome: a preliminary report. Eur J Med Genet. 2017;60(5):250–6.
Kumaki D, Nakamura Y, Suzuki T, et al. Efficacy of denosumab for osteoporosis in two patients with adult-onset Still’s disease-denosumab efficacy in osteoporotic Still’s disease patients. J Clin Med. 2018;7(4):63.
Uehara M, Nakamura Y, Takahashi J, et al. Efficacy of denosumab therapy for neurofibromatosis type 1 with osteoporosis and history of fractures: a case report. Ther Clin Risk Manag. 2018;14:1243–6.
Isobe F, Nakamura Y, Suzuki T, et al. Effects of denosumab on osteoporosis in three cases with anorexia nervosa and a review of the literature. Mod Rheumatol Case Rep. 2018;2(1):104–6.
Uehara M, Nakamura Y, Takahashi J, et al. Efficacy of denosumab for osteoporosis in three female patients with osteogenesis imperfecta. Tohoku J Exp Med. 2017;242(2):115–20.
Hocking DR, McGinley JL, Moss SA, et al. Effects of external and internal cues on gait function in Williams syndrome. J Neurol Sci. 2010;291(1–2):57–63.
Nordstrøm M, Hansen BH, Paus B, et al. Accelerometer-determined physical activity and walking capacity in persons with Down syndrome, Williams syndrome and Prader-Willi syndrome. Res Dev Disabil. 2013;34(12):4395–403.
Nordstrøm M, Paus B, Andersen LF, et al. Dietary aspects related to health and obesity in Williams syndrome, Down syndrome, and Prader-Willi syndrome. Food Nutr Res. 2015;59:25487.
Palmieri S, Bedeschi MF, Cairoli E, et al. Bone involvement and mineral metabolism in Williams’ syndrome. J Endocrinol Invest. 2019;42(3):337–44.
Diédhiou D, Cuny T, Sarr A, et al. Efficacy and safety of denosumab for the treatment of osteoporosis: a systematic review. Ann Endocrinol (Paris). 2015;76(6):650–7.
Huang TH, Liu HC, Hou JY, et al. Efficacy and safety of denosumab therapy for low bone mineral density in childhood cancer survivors: a report of preliminary experience. Pediatr Blood Cancer. 2019;66(10):e27927.
Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420.
Varma TH, Sahitya DSK, Dusad S, et al. Oral prednisolone for management of persistent hypercalcemia after hypercalcemic crisis in the Williams-Beuren syndrome. Pediatr Endocrinol Diabetes Metab. 2018;24(2):106–9.
Kutilek S, Plasilova I, Chrobok V. Two different causes of paediatric hypercalcaemia. Sultan Qaboos Univ Med J. 2018;18(3):e389–92.
Forfar JO, Balf CL, Maxwell GM, et al. Idiopathic hypercalcaemia of infancy: clinical and metabolic studies with special reference to the aetiological role of vitamin D. Lancet. 1956;270(6930):981–8.
Garabédian M, Jacqz E, Guillozo H, et al. Elevated plasma 1,25-dihydroxyvitamin D concentrations in infants with hypercalcemia and an elfin facies. N Engl J Med. 1985;312(15):948–52.
Acknowledgements
Not applicable
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MU: data curation, formal analysis, writing—original draft, writing—review and editing. YN: conceptualization, formal analysis, writing—review and editing. TS: data curation, writing—review and editing. NS: data curation, writing—review and editing. JT: data curation, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional ethical review board of our hospital prior to its start and was conducted in accordance with the ethical standards set forth in the 2013 Declaration of Helsinki for research involving human subjects.
Consent for publication
Written informed consent was obtained from the patient’s parents for the publication of this case report and any accompanying images. A copy of the written informed consent is available for review by the Editor-in-Chief of this journal.
Competing interests
All authors have declared that they have no potential competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Uehara, M., Nakamura, Y., Suzuki, T. et al. Efficacy of denosumab therapy for a 12-year-old female patient with Williams syndrome with osteoporosis and history of fractures: a case report. J Med Case Reports 15, 594 (2021). https://doi.org/10.1186/s13256-021-03175-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-021-03175-9