Abstract
Background
We report a case of pulmonary edema induced by tocolytic agents that was successfully managed with noninvasive positive-pressure ventilation (NPPV) and resulted in extended gestation.
Case presentation
A 36-year-old Japanese pregnant woman received tocolytic therapy with ritodrine hydrochloride, magnesium sulfate, nifedipine, and betamethasone from 28 weeks of gestation. She developed respiratory failure. and her chest X-ray showed enlarged pulmonary vascular shadows. At 29 weeks and 1 day of gestation, she was diagnosed with pulmonary edema induced by tocolytic agents. Because respiratory failure worsened 2 days after ritodrine hydrochloride and magnesium sulfate were stopped, NPPV was initiated. Her respiratory status improved and she was weaned off of NPPV after 3 days. She underwent cesarean section because of breech presentation at 30 weeks and 0 days of gestation due to initiation of labor pains.
Conclusions
NPPV can be safely administered in cases of tocolytic agent-induced pulmonary edema during pregnancy.
Similar content being viewed by others
Background
Acute pulmonary edema in pregnant women is an uncommon but life-threatening event that requires emergency care [1]. One cause of acute pulmonary edema is administration of tocolytic agents such as β-adrenergic receptor agonists and magnesium sulfate [2]. Therefore, in treatment of threatened preterm labor, development of acute pulmonary edema should be monitored.
Noninvasive positive-pressure ventilation (NPPV) is a form of noninvasive ventilation (NIV) that can be utilized for treatment of pulmonary edema, even in pregnancy [1]. However, few studies have investigated the efficacy of NPPV during pregnancy, and reports describing NPPV management for pulmonary edema due to tocolytic agents are scarce.
We report a case of hypoxemic respiratory failure due to pulmonary edema induced by tocolytic agents that was successfully and safely managed with NPPV and resulted in extended gestation.
Case presentation
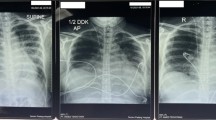
A 36-year-old Japanese pregnant woman (gravida 3, para 1) at 28 weeks and 6 days of gestation was admitted to a hospital for threatened preterm labor. Her past medical history was unremarkable. The patient received tocolytic therapy with intravenous ritodrine hydrochloride. Her uterine contractions increased and she was referred to our hospital at 29 weeks and 1 day of gestation. We administered intravenous magnesium sulfate and oral nifedipine as tocolytic therapy and betamethasone for fetal maturation. Twelve hours after arrival at our hospital, she developed dyspnea and her oxygen saturation (SpO2) fell to 88% on room air; therefore, oxygen administration was initiated. A chest X-ray revealed enlarged pulmonary vascular shadows (Fig. 1), diagnosed as pulmonary edema induced by tocolytic agents. The patient’s blood pressure did not increase; therefore, pulmonary edema associated with preeclampsia was negative. On the other hand, the volume of infusion was approximately 1000 ml per day due to the administration of ritodrine hydrochloride and magnesium sulfate, and thus overhydration was a possibility. Consequently, administration of ritodrine hydrochloride and magnesium sulfate was stopped. After 2 days on 80% oxygen via a non-rebreather mask, the patient’s arterial blood gas results revealed oxygen tension (PO2) of 57 mmHg, pH of 7.46, and carbon dioxide tension (PCO2) of 20 mmHg, and she was diagnosed with type 1 respiratory failure. An echocardiogram showed a normal ejection fraction and a D-dimer test was negative; thus, we excluded cardiac insufficiency and pulmonary embolism. Fetal heart rate monitoring was reassuring, and the fetal biophysical profile score was 8 out of 10 points, indicating normal amniotic fluid volume. Therefore, we decided to continue her pregnancy. She was transferred to the intensive care unit (ICU) for advanced respiratory management. NPPV and furosemide were initiated to treat pulmonary edema. During the next 3 days, her respiratory status dramatically improved; before the introduction of NPPV, the SpO2 level was 93% or less even with oxygen, but after the introduction of NPPV, the SpO2 level was maintained at 97% or more. In addition, her dyspnea was alleviated, and then, even with a reduced oxygen supply, the SpO2 level maintained at 97% or more, and the pulmonary vascular shadows observed by chest X-ray also shrank. Eventually, she was weaned off of NPPV, requiring only nasal cannulation. We monitored her fetal heart rate every day for reassurance while she was in the ICU. She left the ICU at 29 weeks and 6 days of gestation, but the next day her uterine contractions were difficult to control. Finally, she underwent a cesarean section due to breech presentation at 30 weeks and 0 days of gestation. She delivered a healthy, 1292 g female infant whose APGAR score was 7 at 1 minute and 8 at 5 minutes. The patient’s chest X-ray was improved (Fig. 2), and she was discharged on day 7 after delivery. The infant needed continuous positive airway pressure for 4 days. The infant’s development was satisfactory and she was discharged on day 55.
Discussion
To the best of our knowledge, this case is the first report of acute pulmonary edema due to tocolytic agents during pregnancy that was successfully managed with NPPV. In addition, by initiating NPPV before parturition, we succeeded in extending gestation by 5 days. This increase in gestational duration may contribute to better neonatal health outcomes in cases of threatened preterm labor [3, 4].
There are numerous causes of acute pulmonary edema: cardiogenic hydrostatic edema, preeclampsia, acute hemorrhage, sepsis syndrome, pneumonitis, vigorous intravenous fluid therapy, pancreatitis, and tocolytic agents [2]. In addition, diabetes mellitus is a synergistic risk factor with hypertension that can lead to acute heart failure, which may manifest as acute pulmonary edema during pregnancy [5]. Although less commonly utilized worldwide today, tocolytic therapy with β-mimetic drugs was at one time the cause of up to 40% of pulmonary edema [6, 7]. Long-term tocolysis with ritodrine hydrochloride has been implemented in patients and is associated with pulmonary edema [8]. Combination therapy with magnesium sulfate or simultaneous administration of corticosteroids to induce fetal maturation are even more causative of pulmonary edema [2]. A report of pulmonary edema caused by calcium blockers such as nicardipine has also been published [9]. In our case, ritodrine hydrochloride and magnesium sulfate administration increased fluid volume, which may have caused overhydration. Physicians should consider the risk of acute pulmonary edema during treatment for threatened preterm labor.
Several case reports and small series have shown favorable outcomes of NPPV for hypoxemic respiratory failure in pregnancy. In some cases, NPPV during pregnancy improved hypoxemic respiratory failure before delivery, and the baby was delivered at term [10,11,12]. However, the etiologies were not pulmonary edema induced by tocolytic agents, but severe pneumonia [10], acute respiratory distress syndrome (ARDS) induced by transfusion-related acute lung injury [11], and ARDS induced by all-trans retinoic acid syndrome [12]. Another case series demonstrated that NPPV was effective in delaying delivery for patients with preeclampsia-induced pulmonary edema [13].
Two case reports describe pulmonary edema induced by tocolytic agents, and in those cases, NPPV was initiated during vaginal delivery [14] or after cesarean section [15]. In both cases, NPPV was initiated during or after delivery, such that prolongation of gestational duration was not a goal.
In the present case of acute pulmonary edema induced by tocolytic agents, the risk of pulmonary edema was high because ritodrine hydrochloride, magnesium sulfate, and betamethasone were used. NPPV was initiated because respiratory failure worsened even after ritodrine hydrochloride and magnesium sulfate administration was stopped. NPPV improved respiratory status before delivery. Although the patient delivered prematurely because uterine contractions were difficult to control after she left the ICU, we were able to increase gestational duration by 5 days.
After the initiation of NPPV, physicians should pay attention to the timing of intubation or termination. Pregnancy should be terminated when respiratory failure worsens despite NPPV management or fetal well-being deteriorates.
In conclusion, NPPV can be safely administered to pregnant women with hypoxemic respiratory failure due to tocolytic agent-induced pulmonary edema. NPPV may result in increased gestational duration by stabilizing the respiratory condition, thereby delaying delivery.
Availability of data and materials
The data that support the findings of this study are available from the medical records. Data are also available from the authors upon reasonable request.
Abbreviations
- NPPV:
-
Noninvasive positive-pressure ventilation
- NIV:
-
Noninvasive ventilation
- SpO2 :
-
Oxygen saturation
- ICU:
-
Intensive care unit
- ARDS:
-
Acute respiratory distress syndrome
References
Dennis AT, Solnordal CB. Acute pulmonary oedema in pregnant women. Anaesthesia. 2012;67:646–59.
Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, Casey BM, et al editors. Williams obstetrics. 25th ed. New York: McGraw-Hill Education; 2018.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1-147.e8. https://doi.org/10.1016/j.ajog.2006.09.014.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126:443–56.
Lee KW, Ching SM, Hoo FK, Ramachandran V, Chong SC, Tusimin M, et al. Factors associated with poor-to-moderate quality of life among pregnant women with gestational diabetes mellitus: a cross-sectional study in Malaysia. Qual Life Res. 2020;29:2725–36. https://doi.org/10.1007/s11136-020-02532-3.
DiFederico EM, Burlingame JM, Kilpatrick SJ, Harrison M, Matthay MA. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–33.
Jenkins TM, Troiano NH, Graves CR, Baird SM, Boehm FH. Mechanical ventilation in an obstetric population: characteristics and delivery rates. Am J Obstet Gynecol. 2003;188:549–52.
Shigemi D, Aso S, Yasunaga H. Inappropriate use of ritodrine hydrochloride for threatened preterm birth in Japan: a retrospective cohort study using a national inpatient database. BMC Preg Childbirth. 2019. https://doi.org/10.1186/s12884-019-2352-1.
Serena C, Begot E, Cros J, Hodler C, Fedou AL, Nathan-Denizot N, et al. Nicardipine-induced acute pulmonary edema: a rare but severe complication of tocolysis. Case Rep Crit Care. 2014. https://doi.org/10.1155/2014/242703.
Mazlan MZ, Ali S, Abidin HZ, Mokhtar AM, Mukmin LA, Ayub ZN. Non-invasive ventilation in a pregnancy with severe pneumonia. Respir Med Case Rep. 2017;21:161–3.
Gibbs J, Bridges F, Trivedi K, Vullo J. Spontaneous rectus sheath hematoma in pregnancy complicated by the development of transfusion related acute lung injury: a case report and review of the literature. AJP Rep. 2016;6:e325. https://doi.org/10.1055/s-0036-1593353.
Bassani MA, de Oliveira AB, Oliveira Neto AF. Noninvasive ventilation in a pregnant patient with respiratory failure from all-trans-retinoic-acid (ATRA) syndrome. Respir Care. 2009;54:969–72.
Hamada K, Chigusa Y, Kondoh E, Ueda Y, Kawahara S, Mogami H, et al. Noninvasive positive-pressure ventilation for preeclampsia-induced pulmonary edema: 3 case reports and a literature review. Case Rep Obstet Gynecol. 2018. https://doi.org/10.1155/2018/7274597.
Perbet S, Constantin JM, Bolandard F, Vignaud M, Gallot D, Chanseaume S, et al. Non-invasive ventilation for pulmonary edema associated with tocolytic agents during labour for a twin pregnancy. Can J Anesth. 2008;55:769–73.
Fujita N, Tachibana K, Takeuchi M, Kinouchi K. Successful perioperative use of noninvasive positive pressure ventilation in a pregnant woman with acute pulmonary edema. Masui. 2014;63:557–60.
Acknowledgements
We would like to thank Enago (www.enago.com) for English language editing and publication support.
Funding
None.
Author information
Authors and Affiliations
Contributions
KT and KN contributed substantially to the conception or design of the work and the acquisition, analysis, or interpretation of data. KT, KN, MY, KM, and SS were involved in the clinical diagnosis and treatment. MY, KM, and SS critically reviewed the manuscript. TE supervised the writing of the report. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained in comprehensive agreement.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takahashi, K., Nishijima, K., Yamaguchi, M. et al. Noninvasive positive-pressure ventilation in pregnancy to treat acute pulmonary edema induced by tocolytic agents: a case report. J Med Case Reports 15, 126 (2021). https://doi.org/10.1186/s13256-021-02704-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-021-02704-w