Abstract
Background
At the time of diagnosis, giant gastric gastrointestinal stromal tumors are sometimes associated with severe peritoneal dissemination. Unresectable gastrointestinal stromal tumors are considered a systemic disease; therefore, imatinib therapy is currently the primary treatment option in these cases.
Case presentation
A 49-year-old Japanese woman was referred to our hospital with symptoms of anorexia, abdominal discomfort, and a palpable abdominal mass. Contrast-enhanced computed tomography revealed a huge mass with an irregular wall, approximately 22 cm in size, located between the posterior gastric wall and her pancreas. The tumor grew rapidly, and her abdominal symptoms worsened; therefore, a semi-urgent laparotomy was performed. The tumor had arisen from her upper stomach and was removed by wedge resection of her stomach. In addition, widely distributed multiple white nodules were noted, which were resected as far as possible. Immunohistochemical staining of the resected specimen was positive for KIT and CD34. The resected white nodules contained the same cells as the primary tumor. Based on these pathological findings, a final diagnosis of a gastric gastrointestinal stromal tumor with peritoneal dissemination was made. Imatinib was administered at 400 mg per day from 1 month postoperatively. The disease progression of the residual disseminated lesions was favorably controlled, and our patient is now doing well, 12 months after surgery.
Conclusions
Imatinib therapy following debulking surgery can show dramatic effectiveness in giant gastric gastrointestinal stromal tumors with severe peritoneal dissemination.
Similar content being viewed by others
Background
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract [1]. Primary GISTs can arise anywhere along the gastrointestinal tract; however, a majority arises in the stomach (50 to 60 %) [2]. Gastric GISTs are clinically asymptomatic until they reach a significant size; therefore, a majority of GISTs are incidentally discovered either by diagnostic tests or during abdominal surgery. Giant gastric GISTs are infrequently associated with symptoms such as abdominal pain, digestive bleeding, and a palpable mass [3–5]. Such GISTs are aggressive and have the potential to cause distant metastases. Severe peritoneal dissemination is sometimes observed at the time of diagnosis of giant gastric GISTs and is associated with an extremely poor prognosis.
Recent progress in understanding the origin and molecular oncology of GISTs has contributed to a rapid improvement in their management [6]. Imatinib, which inhibits the KIT signal transduction pathway, is considered a promising therapeutic agent [7]. Unresectable GISTs are considered a systemic disease; therefore, imatinib therapy is currently the primary treatment option in these cases [8, 9]. However, in clinical practice, a precedent surgery is performed for various reasons. Surgery is beneficial to control tumor-associated complications such as abdominal pain and digestive bleeding; therefore, a precedent surgery for unresectable GISTs is sometimes necessary.
Prior to the imatinib era, surgery traditionally played only a palliative role in patients with unresectable GISTs; however, with the introduction of imatinib, it may be necessary to reassess the role of surgery in these cases. Here we report the case of a patient with a giant gastric GIST with severe peritoneal dissemination, showing favorable progression control by postoperative imatinib therapy following debulking surgery. This case demonstrated that surgery can possibly play a role even in unresectable GISTs.
Case presentation
A 49-year-old Japanese woman was referred to our hospital with symptoms of anorexia, abdominal discomfort, and a palpable abdominal mass. She reported a 7 kg weight loss in a few months and had a medical history of hypertension and hyperlipidemia as well as a maternal history of gastric cancer. On performing a physical examination, a palpable mass was detected in her left upper abdominal quadrant. She had regular bowel movements with normal stools.
A complete blood count test revealed only mild microcytic hypochromic anemia (hemoglobin, 11.0 g/dL). A blood biochemical test revealed no abnormalities, except for an elevated lactate dehydrogenase level of 401 U/L. Her levels of tumor markers were assessed, including carcinoembryonic antigen, carbohydrate antigen 19-9, and α-fetoprotein, all of which were within normal ranges.
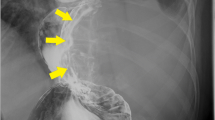
An abdominal X-ray revealed a diffuse opaque area in her upper abdomen without a gastrointestinal gas shadow; accompanying the finding, her transverse colon was dislocated downward (Fig. 1). Endoscopy showed compression of the posterior wall of her gastric body along with mucosal redness. Several biopsy specimens were taken, which showed evidence of erosive atrophic gastritis. Contrast-enhanced computed tomography (CT) revealed a huge mass with an irregular wall, approximately 22 cm in size, located between the posterior gastric wall and her pancreas (Fig. 2). The mass demonstrated heterogeneous contrast and internal low-density components, suggestive of necrosis. The mass was mainly supplied by her left gastric artery branches. Moreover, many small nodules around her stomach were observed, suggestive of enlarged lymph nodes or disseminated lesions. There were no signs of hepatic masses. Based on these findings, a mesenchymal tumor, such as a GIST, leiomyoma, leiomyosarcoma, or a neurogenic tumor, was suspected.
a, b Contrast-enhanced computed tomography showing a huge mass with an irregular wall, approximately 22 cm in size, located between the posterior gastric wall and the pancreas. The mass demonstrates heterogeneous contrast and internal low-density components, suggestive of necrosis. Moreover, many small nodules around the stomach (arrows) are observed, suggestive of enlarged lymph nodes or disseminated lesions
The tumor grew rapidly, and her abdominal symptoms worsened; therefore, a semi-urgent laparotomy was performed, although a definitive diagnosis could not be preoperatively made. After gastrocolic ligament division, a giant extraluminal tumor arising from the posterior wall of her upper stomach on the lesser curvature was noted. Furthermore, multiple white nodules, suspected of being disseminated lesions, were widely distributed in her greater omentum, lesser omentum, transverse mesocolon, and retroperitoneum. Serous ascites was also seen in the pouch of Douglas. The giant tumor adhered to her pancreas, transverse mesocolon, and transverse colon; however, the tumor was removed by wedge resection of her stomach following ligation of the vessels supplying the mass, without resecting other organs. The radical resection of the multiple white nodules was impossible; therefore, the nodules were resected as far as possible.
The resected specimen was a well-circumscribed tumor (Fig. 3a). The cut surface of the tumor revealed a white–gray solid mass with coagulative necrosis, accompanying a large central cavity (Fig. 3b).
The microscopic findings of hematoxylin–eosin staining revealed a bundle-like growth of spindle-shaped tumor cells, with acidophilic cytoplasm and enlarged nuclei accompanying increased chromatin levels (Fig. 4a). Nuclear atypia was prominent, and the mitotic count was over 150 per 50 high-power fields (Fig. 4b). Tumor cells grew externally from the proper muscle layer of her stomach (Fig. 4c). The resection margins were free of tumor cells. Immunohistochemical staining revealed that the tumor was positive for KIT and CD34 and negative for desmin and S-100 protein (Fig. 5). The MIB-1 labeling index of the tumor cells was 40 to 50 %. On histologic examination, the resected white nodules had the same appearance as the primary tumor and contained no components of lymph nodes (Fig. 6). Based on these pathological findings, a final diagnosis of gastric GIST with peritoneal dissemination was made. According to the modified Fletcher’s classification, our patient was classified in the high-risk category [10].
a The microscopic findings of hematoxylin–eosin staining showing a bundle-like growth of spindle-shaped tumor cells. b Nuclear atypia is prominent, and the mitotic count is over 150 per 50 high-power fields. The red arrow shows mitosis. c Tumor cells growing externally from the proper muscle layer of the stomach
Our patient had an uneventful postoperative course and was discharged from our hospital on postoperative day 15. Postoperative CT showed several recognizable disseminated lesions in her abdominal cavity (Fig. 7a, b). Treatment with imatinib at 400 mg per day was started from 1 month postoperatively. The treatment was well tolerated, with grade 1 eyelid swelling and grade 1 skin rash, which improved immediately. A follow-up CT, 9 months after imatinib administration, revealed ambiguous residual disseminated lesions (Fig. 7c, d). She is now doing well, 12 months after surgery. Imatinib administration at 400 mg per day is to be continued until disease progression is favorably controlled, with follow-up CT planned once every 3 months.
Discussion
Complete tumor resection with negative margins is the principle treatment for resectable GISTs, whereas imatinib therapy is the principle treatment for unresectable GISTs [11, 12]. Recent studies have demonstrated that the prognosis of unresectable GISTs can be improved by surgery following their conversion to resectable tumors by imatinib therapy [13, 14]. Du et al. reported that the 2-year progression-free survival in patients with advanced GISTs who underwent surgery for residual lesions after having responded to imatinib therapy was higher than that in patients with advanced GISTs who received imatinib therapy alone (88.4 % versus 57.7 %) [13]. Furthermore, Rubió-Casadevall et al. reported that the median survival in patients with advanced GISTs who underwent surgery for residual lesions after having responded to imatinib therapy was longer than that in patients with advanced GISTs who received imatinib therapy alone (87.6 months versus 59.9 months) [14]. However, the effectiveness of imatinib therapy following a precedent surgery for unresectable GISTs remains unclear. For controlling tumor-associated complications, we performed a precedent surgery that was non-radical and subsequently administered postoperative imatinib therapy for the residual lesions. As a consequence of administering imatinib therapy following debulking surgery, the disease progression of the residual disseminated lesions was favorably controlled. The present case suggests that this treatment strategy for giant gastric GISTs with severe peritoneal dissemination can show dramatic effectiveness.
In our case, a precedent surgery was performed prior to imatinib administration for an unresectable giant GIST. As previously reported, serious adverse events, such as gastrointestinal or intra-abdominal bleeding due to tumor degeneration, occur in approximately 5 % of giant GISTs treated with imatinib [7]. Therefore, the application of imatinib must be carefully considered, particularly for giant GISTs. Furthermore, it is commonly assumed that GISTs with severe peritoneal dissemination showing conversion to resectable tumors by imatinib therapy are relatively rare. Thus, we propose that a precedent surgery followed by imatinib therapy is an attractive treatment option for treating giant GISTs with severe peritoneal dissemination. As previously reported, the development of secondary resistant lesions was related to the amount of residual tumor, which is consistent with the hypothesis that a larger number of tumor cells are quantitatively proportional to a greater likelihood of harboring more resistant clones [15, 16]. Therefore, tumor debulking, as far as possible, is desirable for delaying secondary resistance to imatinib.
Our case demonstrated that surgery can possibly play a role even in unresectable GISTs. Prior to the introduction of imatinib, outcomes for patients with unresectable GISTs were exceedingly poor, with median survival times ranging from 10 to 20 months and a 5-year survival of <10 % [17, 18]. With the introduction of tyrosine kinase inhibitors, including imatinib, sunitinib, and regorafenib, the prognosis for unresectable GISTs has tremendously improved, and the treatment strategy for these GISTs has considerably changed [7, 19, 20]. A combination of surgery and tyrosine kinase inhibitor therapy may be a promising strategy, with a large role for surgery in unresectable GISTs in addition to resectable GISTs.
At present, 11 months following imatinib administration, the disease progression of the severe peritoneal dissemination in our patient has been favorably controlled. However, the appearance of resistant lesions may become a problem later as the median progression-free survival has been shown to be approximately 2 years for patients treated with imatinib [6, 12]. For the early detection of disease progression, frequent follow-up CT at 3-month intervals has been planned [21]. Imatinib has been planned to be continuously administered as its interruption may result in rapid disease progression [22].
As this was a single-patient case report, these findings need to be confirmed by the accumulation of prospective evidence from more patients in multiple institutions. Gastric GISTs are relatively rare; therefore, the number of patients treated in a single institution is limited. However, the current findings provide important information that can contribute to the development of a treatment strategy for giant gastric GISTs with severe peritoneal dissemination.
Conclusions
We reported the case of a patient with a giant gastric GIST with severe peritoneal dissemination showing favorable progression control by imatinib therapy following debulking surgery. The present case suggests that imatinib therapy following debulking surgery for giant gastric GISTs with severe peritoneal dissemination can show dramatic effectiveness.
Abbreviations
- CT:
-
Computed tomography
- GIST:
-
Gastrointestinal stromal tumor
References
Kitamura Y, Hirota S, Nishida T. Gastrointestinal stromal tumors (GIST): a model for molecule-based diagnosis and treatment of solid tumors. Cancer Sci. 2003;94:315–20.
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–65.
Cappellani A, Piccolo G, Cardi D, Cavallaro A, Lo Menzo E, Cavallaro V, et al. Giant gastrointestinal stromal tumor (GIST) of the stomach cause of high bowel obstruction: surgical management. World J Surg Oncol. 2013;11:172.
Cruz Jr RJ, Vincenzi R, Ketzer BM, Cecilio AL, Cepeda LA. Spontaneous intratumoral bleeding and rupture of giant gastric stromal tumor (>30 cm) in a young patient. World J Surg Oncol. 2008;6:76.
Zhou L, Liu C, Bai JG, Wei JC, Qu K, Tian F, et al. A rare giant gastrointestinal stromal tumor of the stomach traversing the upper abdomen: a case report and literature review. World J Surg Oncol. 2012;10:66.
Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–78.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80.
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1–41.
ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49–55.
Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9.
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–8.
Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–83.
Du CY, Zhou Y, Song C, Wang YP, Jie ZG, He YL, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a retrospective randomised trial in China. Eur J Cancer. 2014;50:1772–8.
Rubió-Casadevall J, Martinez-Trufero J, Garcia-Albeniz X, Calabuig S, Lopez-Pousa A, Del Muro JG, Spanish Group for Research on Sarcoma (GEIS), et al. Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol. 2015;22:2948–57.
Van Glabbeke M, Verweij J, Casali PG, Le Cesne A, Hohenberger P, Ray-Coquard I, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group Study. J Clin Oncol. 2005;23:5795–804.
Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–5.
Tielen R, Verhoef C, van Coevorden F, Gelderblom H, Sleijfer S, Hartgrink HH, et al. Surgery after treatment with imatinib and/or sunitinib in patients with metastasized gastrointestinal stromal tumors: is it worthwhile? World J Surg Oncol. 2012;10:111.
Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655–64.
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38.
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, GRID study investigators, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302.
Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, GIST Guideline Subcommittee, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–30.
Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, French Sarcoma Group, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942–9.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
The authors declare that they did not receive any funding for this study.
Availability of data and materials
Not applicable.
Authors’ contributions
SF designed the study and drafted the manuscript. SF and YF performed the operation. TW and YO performed the histopathological examination. KK, MT, MY, and MI participated in the manuscript revision process. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
The need for institutional ethics committee approval was waived because the content of this case report did not require ethics approval.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fukuda, S., Fujiwara, Y., Wakasa, T. et al. Giant gastric gastrointestinal stromal tumor with severe peritoneal dissemination controlled by imatinib therapy following debulking surgery: a case report. J Med Case Reports 11, 33 (2017). https://doi.org/10.1186/s13256-017-1215-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-017-1215-5