Abstract
Objective
To evaluate the diagnostic accuracy of supplemental 3D automated breast ultrasound (ABUS) in the diagnostic work-up of BI-RADS 0 recalls. We hypothesized that 3D ABUS may reduce the benign biopsy rate.
Materials and methods
In this prospective multicenter diagnostic study, screening participants recalled after a BI-RADS 0 result underwent bilateral 3D ABUS supplemental to usual care: digital breast tomosynthesis (DBT) and targeted hand-held ultrasound (HHUS). Sensitivity, specificity, positive predictive value, and negative predictive value of 3D ABUS, and DBT plus HHUS, were calculated. New 3D ABUS findings and changes of management (biopsy or additional imaging) were recorded.
Results
A total of 501 women (median age 55 years, IQR [51–64]) with 525 BI-RADS 0 lesions were included between April 2018 and March 2020. Cancer was diagnosed in 45 patients. 3D ABUS sensitivity was 72.1% (95% CI [57.2–83.4%]), specificity 84.4% (95% CI [80.8–87.4%]), PPV 29.2% (95% CI [21.4–38.5%]), and NPV 97.1% 95.0–98.4%). Sensitivity of DBT plus HHUS was 100% (95% CI [90.2–100%]), specificity 71.4% (95% CI [67.2–75.2%]), PPV 23.8% (95% CI [18.1–30.5%]) and NPV 100% (95% CI [98.7–100%]). Twelve out of 43 (27.9%) malignancies in BI-RADS 0 lesions were missed on 3D ABUS, despite being detected on DBT and/or HHUS. Supplemental 3D ABUS resulted in the detection of 57 new lesions and six extra biopsy procedures, all were benign.
Conclusion
3D ABUS in the diagnostic work-up of BI-RADS 0 recalls may miss over a quarter of cancers detected with HHUS and/or DBT and should not be used to omit biopsy. Supplemental 3D ABUS increases the benign biopsy rate.
Trial registration
Dutch Trial Register, available via https://www.onderzoekmetmensen.nl/en/trial/29659
Critical relevance statement
Supplemental 3D automated breast ultrasound in the work-up of BI-RADS 0 recalls may miss over a quarter of cancers detected with other methods and should not be used to omit biopsy; ABUS findings did increase benign biopsy rate.
Key Points
-
Automated breast ultrasound (ABUS) may miss over 25% of cancers detectable by alternative methods.
-
Don’t rely solely on 3D ABUS to assess indication for biopsy.
-
New findings with supplemental 3D ABUS increase the benign biopsy rate.
Graphical Abstract

Similar content being viewed by others
Introduction
In the Netherlands, women between the ages of 50 and 75 years are invited for biennial breast cancer screening using full-field digital mammography. Each year approximately one million women participate and ~23 per 1000 participants are recalled and referred to a hospital for diagnostic work-up of inconclusive (BI-RADS 0) or suspicious findings (BI-RADS 4/5) [1]. The BI-RADS 0 recalls account for the largest share of all recalls with approximately 11.000/year. Of these women, 25–30% undergo breast biopsy and 10–12% are diagnosed with breast cancer [1]. Therefore, the BI-RADS 0 recalls have an important impact on the false-positive recall rate and benign biopsy rate. We hypothesized that supplemental three-dimensional automated breast ultrasound (3D ABUS) in the diagnostic work-up of BI-RADS 0 recalls may reduce the benign biopsy rate.
3D ABUS was originally designed as an adjunct screening tool for women with dense breasts to overcome the limitations of hand-held ultrasound (HHUS), which is operator-dependent, lacks standardization and reproducibility, and imposes a considerable workload on radiologists [2]. 3D ABUS enables standardized acquisition of volumetric images of the whole breast, facilitating double reading and objective comparison with previous imaging. The diagnostic accuracy of 3D ABUS has been reported to be comparable to HHUS [3,4,5,6,7,8,9]. A meta-analysis by Meng et al showed a pooled sensitivity of ABUS of 92% (range: 89.9–93.8%) and specificity of 84.9% (range 82.4–87%), with no significant difference in diagnostic accuracy between HHUS and ABUS. Three-dimensional reconstruction of the ABUS imaging enables evaluation in the coronal plane, which has been reported to significantly aid in the differentiation between benign and malignant breast lesions [10]. Spiculation of malignant lesions and the retraction phenomenon caused by invasive growth and surrounding desmoplastic reaction lead to architectural distortions, which may be appreciated best in the coronal plane [7, 11, 12]. Using the coronal view, benign lesions seen in the transverse plane may be downgraded in up to 18% of benign cases, potentially avoiding benign biopsy [10]. False positive findings necessitate further imaging, biopsy, or additional follow-up examinations which often cause anxiety in the patient, increase healthcare costs, and sometimes lead to biopsy-related morbidity. 3D ABUS in the diagnostic work-up of BI-RADS 0 recalls may reduce the benign biopsy rate. The objective of this study was to evaluate the diagnostic accuracy of supplemental 3D ABUS in the diagnostic work-up of Dutch breast cancer screening participants recalled with a BI-RADS 0 screening mammography result.
Materials and methods
This prospective multicenter diagnostic study was approved by the Medical Research Ethics Committee of the University Medical Center Utrecht. Written informed consent was obtained from all participants prior to the 3D ABUS examination. The study protocol is available via Dutch Trial Register (https://www.onderzoekmetmensen.nl/en/trial/29659).
Between April 2018 and March 2020, all Dutch breast cancer screening participants (aged 50–75 years), recalled for diagnostic work-up after a BI-RADS 0 screening mammography result and referred to one of three participating non-academic hospitals, were considered eligible for participation (Fig. 1). Women who were unable or unwilling to provide informed consent were excluded. Reasons for non-participation were recorded. The conventional diagnostic work-up (usual care) consisted of mediolateral oblique (MLO) and craniocaudal (CC) digital breast tomosynthesis (DBT) of both breasts and targeted hand-held ultrasound (HHUS) of the BI-RADS 0 lesion. DBT was performed on Hologic Dimensions, Hologic Dimensions 3D, or Hologic Selenia Dimensions. HHUS was performed on a Philips Epiq 7 G or Toshiba Aplio 500 system. In line with clinical practice, DBT and HHUS were evaluated together leading to a final BI-RADS classification. In addition to the usual care work-up, all study participants received supplemental bilateral 3D ABUS. 3D ABUS imaging was evaluated directly after the evaluation of DBT and HHUS by the same radiologist, who was not blinded for the results of previous imaging.
All breast imaging was interpreted according to the ACR BI-RADS Atlas (Fifth Edition) by one of 15 radiologists (including C.M., W.S.P., and A.V.), all dedicated breast radiologists whose experience ranged from 1.5 to 29 years. BI-RADS classification of DBT plus HHUS and 3D ABUS, additional findings with 3D ABUS, changes in management after 3D ABUS (biopsy or additional imaging), and adverse events were recorded.
Breast density was visually assessed on reconstructed DBT and classified into four categories according to the ACR BI-RADS Atlas (Fifth Edition): almost entirely fatty breasts (A), scattered areas of fibroglandular density (B), heterogeneously dense breasts (C), and extremely dense breasts (D) [13]. Malignant lesion size, measured as the largest diameter on any imaging modality, was recorded.
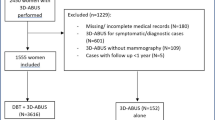
Study flowchart. A total of 1059 women, recalled for diagnostic work-up after a BI-RADS 0 screening mammography result and referred to one of three participating hospitals between April 2018 and March 2020 were considered eligible. A total of 501 women signed informed consent and received supplemental 3D ABUS, in addition to usual care diagnostic work-up with DBT and HHUS. All breast imaging (DBT, HHUS and 3D ABUS) was evaluated to assign each patient a BI-RADS score. 341 participants were classified BI-RADS 1 or 2 (benign); these participants were referred back to the breast cancer screening programme. 76 participants were classified BI-RADS 3; of these women, 43 underwent breast biopsy and 33 were invited for follow-up imaging. Six participants were lost to follow up. After a minimum of six months, follow-up imaging was performed in 27 participants, after which 23 were referred back to the screening programme and four underwent breast biopsy. 84 patients were classified BIRADS 4/5; all underwent breast biopsy. In total, 45/501 participants were diagnosed with cancer; 44 had breast cancer and one participant was diagnosed with non-Hodgkin lymphoma after detection of an intramammary lymph node on breast imaging
3D ABUS imaging
3D ABUS imaging was obtained by trained technicians using an ABUS system (InveniaTM ABUS, Automated Breast Ultrasound System, GE Healthcare, Sunnyvale, CA, USA). This ABUS system consists of a scan station, equipped with a 6–15 MHz wide transducer attached to a flexible arm, a touch-screen monitor, and a dedicated workstation for image evaluation. All technicians received a three-day training in 3D ABUS examination from a GE application specialist before the start of the study and a refresher course after approximately six months. Patients were scanned in supine position with the ipsilateral arm raised above the head. A lotion was applied to the breast to establish good skin contact. In each scan a volume data set up to 16.9 × 15.3 × 5.0 cm was obtained with slice intervals of 2 mm. Of each breast, anterior-posterior, lateral, and medial views were obtained. Additional superior and inferior anterior-posterior views were obtained in large breasts (Fig. 2).
3D ABUS imaging was sent to the dedicated workstation for multiplanar reconstruction and review in the transverse, sagittal, and coronal plane. All radiologists received five hours of peer-to-peer training in 3D ABUS interpretation from an expert radiologist and had access to a digital learning environment to practice 3D ABUS readings.
Breast cancer diagnosis was defined as a histopathological diagnosis of DCIS or invasive malignancy, all other histopathological findings were considered benign. Histopathology was indicated for all lesions classified BI-RADS 4 or 5. In cases with no indication for histopathological diagnosis, the final conclusion after assessment of all available breast imaging was used. A final BI-RADS 1 or 2 category was considered benign. All women with a final BI-RADS 1 or 2 result were referred back to the breast cancer screening programme to get reinvited for screening mammography in the next round after two years. In case of a BI-RADS 3 result after diagnostic work-up, the Dutch guideline recommends tissue diagnosis or short-interval (i.e., six months) follow-up. In all participants with a BI-RADS 3 result without histopathological diagnosis, the results of follow-up imaging (and histopathology if available) of at least six months after inclusion were recorded.
Statistical analysis
Data was analyzed using SPSS (IBM® SPSS Statistics® version 25) and RStudio (R version 3.6.1). Diagnostic accuracy was calculated for 3D ABUS and for DBT plus HHUS. Imaging examinations were considered positive if the BI-RADS 0 lesion of referral was categorized as BI-RADS ¾/5 and negative if categorized as BI-RADS ½. Sensitivity was calculated by dividing the number of true-positive examinations by the total number of breast cancer cases. Specificity was calculated by dividing the number of true-negative examinations by the total number of benign cases. Positive predictive value was calculated by dividing the number of true-positive examinations by the total number of positive examinations. The negative predictive value was calculated by dividing the number of true-negative examinations by the total number of negative examinations. 95% confidence intervals were calculated using the Agresti-Coull method [14].
Results
Study population
A total of 1059 women, recalled for diagnostic work-up after a BI-RADS 0 screening mammography result and referred to one of three participating hospitals between April 2018 and March 2020 were considered eligible. A total of 501 women (median age 55 years, IQR [51–64]) signed informed consent (Fig. 1). Recorded reasons for patient non-participation were: no interest in research participation (n = 33), breast cancer anxiety related to the diagnostic work-up (n = 20), no time for the ABUS examination (n = 11) and three women could not participate because of physical impairments that hampered correct positioning for 3D ABUS scanning. Most women (n = 491) were excluded because there was no study technician, study radiologist, or ultrasound room available at the time of diagnostic work-up.
From the total of 501 study participants, ten women were recalled for a BI-RADS 0 result of both breasts, twelve had two BI-RADS 0 lesions in one breast, and one woman was recalled with three BI-RADS 0 lesions, resulting in a total number of 525 screen-detected BI-RADS 0 lesions. The majority of the lesions were described as masses (327/525) on screening mammography, followed by asymmetries (159/525) and architectural distortions in one direction (24/525). There were 15 lesions categorized as BI-RADS 0 that, in hindsight, should have been categorized differently based on the lesion description in the recall letter: eleven architectural distortions in two directions, two masses with architectural distortion, one calcifications-only lesion and one mass with calcifications. According to the Dutch screening guidelines the mass with calcifications should have been classified as BI-RADS 5, and the other lesions as BI-RADS 4. However, as they were BI-RADS 0 recalls and met all inclusion criteria, these cases were included in our analysis.
Out of 501 participants, 78 women (15.6%) had almost entirely fatty breasts, 320 (63.9%) had scattered areas of fibroglandular density, 93 (18.6%) had heterogeneously dense breasts and ten (2%) had extremely dense breasts.
Diagnostic work-up results and histopathology
After diagnostic work-up, including DBT, HHUS and 3D ABUS, the final clinical BI-RADS classification was BI-RADS 1 or 2 in 341 (68.1%) participants; these participants were referred back to the breast cancer screening programme. 12 (2.4%) participants with a final BI-RADS 5 and 72 (14.4%) with a BI-RADS 4 result underwent breast biopsy for histopathological diagnosis, yielding breast cancer in 12/12 (100%) and in 29/72 (40.3%) participants respectively. Out of 76 (15.2%) participants with a BI-RADS 3 result, 43 women underwent breast biopsy and 33 were invited for follow-up imaging. After a minimum of six months, follow-up imaging was performed in 27 participants and six patients were lost to follow-up. Based on all available breast imaging for these six patients they were considered benign in further analysis. After follow-up imaging 23/27 were referred back to the screening programme and four underwent breast biopsy. Out of 76 participants with a final BI-RADS 3 result, three (3.9%) were diagnosed with breast cancer. In total, 44/501 (8.8%) participants were diagnosed with breast cancer (median lesion size 9.5 mm, range 4–69 mm). In addition, one participant was diagnosed with non-Hodgkin lymphoma after the detection of an intramammary lymph node on breast imaging. Out of all 45 detected cancers, 43 resulted from a BI-RADS 0 lesion, and two resulted from a new lesion detected during diagnostic work-up on HHUS and DBT. Histopathological results are shown in Table 1.
Diagnostic accuracy of 3D ABUS
Out of the 525 BI-RADS 0 lesions, 42 yielded breast cancer and one resulted in the diagnosis of non-Hodgkin lymphoma. Complete 3D ABUS examination was available for 523/525 BI-RADS 0 lesions (Table 2), two examinations were terminated before completion due to adverse events. 3D ABUS sensitivity was 72.1% (95% CI [57.2–83.4%]), specificity was 84.4% (95% CI [80.8–87.4%]), FPR was 15.6% (95% CI [12.6–19.2%]), FNR was 27.9% (16.6–42.8%), PPV was 29.2% (95% CI [21.4–38.5%]) and NPV was 97.1% (95.0–98.4%) (Table 2). Twelve lesions recalled from screening with a BI-RADS 0 result that contained cancer were missed on 3D ABUS despite being detected on DBT and/or HHUS. Sensitivity of DBT plus HHUS was 100% (95% CI [90.2–100%]), specificity was 71.4% (95% CI [67.2–75.2%]), PPV was 23.8% (95% CI [18.1–30.5%]), and NPV was 100% (95% CI [98.7–100%]) (Table 2). The cancers missed with 3D ABUS included eight cases of grade I invasive carcinoma (size range 2–18 mm), one grade II invasive carcinoma (4 mm), one grade II invasive lobular carcinoma (9 mm), one grade II invasive micropapillary carcinoma (5 mm) and one grade I DCIS (5 mm). An imaging example of a breast cancer missed on 3D ABUS is provided in Fig. 3.
In addition, there were two new cancers detected on both DBT and HHUS during diagnostic work-up that was missed on 3D ABUS. Cancers that were missed on 3D ABUS had a mean lesion size of 9.0 mm, versus 14.6 mm in cancers detected on 3D ABUS (p-value 0.06). A detailed overview of all 3D ABUS missed breast cancers is presented in Table 3.
Example of a breast cancer missed on 3D ABUS. A 60-year old woman was recalled after detection of a new mass in the superior lateral quadrant of the left breast, classified as BI-RADS 0 on screening mammography (A: CC view, B: MLO view). The lesion was classified as BI-RADS 4 on digital breast tomosynthesis (C: CC view, D: MLO view). On hand-held ultrasound the lesion was difficult to visualize and described as a mass of 4 mm in diameter, classified as BI-RADS 3 (E: ultrasound). The lesion was missed on 3D ABUS imaging. In hindsight, the exact location of the lesion was reevaluated on 3D ABUS and a 3 mm lesion was noted on the anterior-posterior view (F: AP view, G: lateral view). The patient underwent biopsy (PA: invasive carcinoma NST), followed by a radioactive seed-guided lumpectomy. Histopathology showed a grade I invasive carcinoma NST, ER+/PR+/HER2− with a diameter of 4 mm, TNM stage (8th edition): pT1aN0(i-)(sn)
New 3D ABUS findings and changes of management
Supplemental 3D ABUS resulted in the detection of 57 new lesions, which were all benign (Table 4). There was one new lesion classified BI-RADS 4 on 3D ABUS for which an extra target HHUS was performed, at which the lesion was classified as benign. There were six new BI-RADS 3 lesions; in five a target HHUS and biopsy were performed, yielding benign histopathological diagnoses in all. In the remaining BI-RADS 3 lesion a follow-up HHUS was performed and the lesion was classified as BI-RADS 2.
Furthermore, one BI-RADS 0 lesion was classified BI-RADS 2 on DBT and HHUS, but BI-RADS 3 on 3D ABUS, leading to an additional target HHUS and biopsy procedure. In total, 3D ABUS resulted in six extra benign biopsy procedures.
Adverse events
In two participants an erythematous skin reaction occurred after application of the lotion. In one participant the 3D ABUS examination was not completed for this reason. In both patients, the skin redness resolved spontaneously within 48 h. One participant experienced pain due to breast compression during the 3D ABUS examination and the examination was prematurely terminated.
Discussion
This multicenter diagnostic study evaluated the diagnostic accuracy of 3D ABUS, supplemental to DBT and HHUS, in 501 women recalled after a BI-RADS 0 screening mammography. 3D ABUS sensitivity was 72.1% (95% CI [57.2–83.4%]), and specificity 84.4% (95% CI [80.8–87.4%]). On 3D ABUS 12 out of 43 malignancies in BI-RADS 0 lesions were missed, despite being detected on DBT or HHUS. In contrast to our hypothesis that 3D ABUS may reduce the benign biopsy rate, these findings indicate that 3D ABUS should not be used to omit biopsy. Supplemental 3D ABUS in addition to HHUS and DBT resulted in the detection of 57 new lesions and six extra biopsy procedures, all of which were benign. As such, supplemental 3D ABUS increased the benign biopsy rate.
Previous studies yielded a better diagnostic performance of 3D ABUS compared to our study, although differences in study populations limit comparability. Hellgren et al found a sensitivity of 88% and specificity of 89.2% of 3D ABUS in 113 women recalled because of a suspicious mammographic finding in screening [15]. However, this population was not limited to BI-RADS 0 recalls and included lesions with a higher suspicion of malignancy (further progressed state of disease), which may explain the higher sensitivity compared to our study. Several other studies, performed in even more heterogeneous study populations, found a 3D ABUS sensitivity ranging from 74% to 100%, and specificity of 68–95% [4, 7, 8, 11, 16, 17].
In our study, cancers that were missed on 3D ABUS had a mean lesion size of 9.0 mm versus 14.6 mm in cancers that were detected on 3D ABUS. Radiologists commented that the resolution of 3D ABUS was lower compared to HHUS, which complicated lesion detection and characterization, specifically of smaller lesions. In line with this, Shin et al described that lesion detection was reliable only when the mean lesion diameter was > 1.2 cm and Jeh et al mentioned that smaller lesions were statistically significantly less frequently detected on 3D ABUS [18, 19]. In our study, there were nine cancers > 1.2 cm, of which one, lesion size 2.2 cm, was missed on 3D ABUS.
When interpreting the measures of diagnostic accuracy it is important to recognize the learning curve of 3D ABUS readings. Many studies do not report the experience of 3D ABUS readers and in the literature, there is no consensus on the number of examinations that a radiologist must have interpreted to obtain the right skill level. As with other imaging modalities, experience improves the performance of radiologists. In our study 15 study radiologists participated, resulting in an average of ~33 ABUS examinations per radiologist. Although all radiologists received training and were encouraged to practice 3D ABUS readings in a digital learning environment, the limited experience with 3D ABUS and lack of continuity, the number of examinations may have been insufficient to reach the optimal skill level. In addition, for technicians, there is a learning curve to obtain state-of-the-art imaging with adequate breast compression and full breast coverage [20]. In our study, all technicians and radiologists were equally (in)experienced in obtaining/evaluating 3D ABUS imaging and the potential impact of the level of experience on the lesions missed in 3D ABUS could not be evaluated.
An important limitation of our study was that radiologists assessed the 3D ABUS imaging directly after evaluation of the DBT and HHUS. With unblinded sequential evaluation, the radiologist may be more inclined to score the 3D ABUS in line with the results of previous imaging. However, despite the detection of a suspicious lesion on DBT and/or HHUS, in over a quarter of cancers in BI-RADS 0 recalls the lesion was missed on 3D ABUS. This further underlines that 3D ABUS should not be used to omit biopsy.
Follow-up imaging was lacking in 6 out of 33 patients scheduled for follow-up after a BI-RADS 3 result. The six lesions were considered breast cancer negative based on all available imaging and included in our analysis. Exclusion of these six lesions does not affect the reported 3D ABUS sensitivity (31/43, 72.1%) nor the increase in benign biopsies.
Looking to the future of 3D ABUS, development should focus on further improvement of 3D ABUS resolution. Future research may look into stratified analysis for breast density and different applications of 3D ABUS, such as operative planning and follow-up of benign lesions [20, 21]. In addition, prototypes are developed that combine ABUS and DBT in a single device without decompression of the breast, offering practical and logistic advantages [22]. Furthermore, researchers and clinicians adopting 3D ABUS should be aware of the learning curve regarding image interpretation and acquisition.
In conclusion, 3D ABUS in the diagnostic work-up of breast cancer screening participants recalled with a BI-RADS 0 result may miss over a quarter of cancers detected on HHUS and/or DBT and should not be used to omit biopsy. Supplemental 3D ABUS after DBT and HHUS increases the benign biopsy rate.
Data availability
Authors agree to make data and materials supporting the results or analyses presented in this manuscript available upon reasonable request.
Abbreviations
- CC:
-
Craniocaudal
- DBT:
-
Digital breast tomosynthesis
- HHUS:
-
Handheld ultrasound
- MLO:
-
Mediolateral oblique
- 3D ABUS:
-
Three-dimensional automated breast ultrasound
References
Monitor bevolkingsonderzoek borstkanker 2016. Integraal Kankercentrum Nederland 2018. http://www.rivm.nl/documenten/monitor-bevolkingsonderzoek-borstkanker-2016. Accessed 14 May 2023
Evans A, Trimboli RM, Athanasiou A et al (2018) Breast ultrasound: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 9:449–461
Lin X, Jia M, Zhou X et al (2021) The diagnostic performance of automated versus handheld breast ultrasound and mammography in symptomatic outpatient women: a multicenter, cross-sectional study in China. Eur Radiol 31:947–957
Wang HY, Jiang YX, Zhu QL et al (2012) Differentiation of benign and malignant breast lesions: a comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur J Radiol 81:3190–3200
Xiao YM, Chen ZH, Zhou QC, Wang Z (2015) The efficacy of automated breast volume scanning over conventional ultrasonography among patients with breast lesions. Int J Gynaecol Obstet 131:293–296
Wang ZL, Xu JH, Li JL, Huang Y, Tang J (2012) Comparison of automated breast volume scanning to hand-held ultrasound and mammography. Radiol Med 117:1287–1293
Lin X, Wang J, Han F, Fu J, Li A (2012) Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur J Radiol 81:873–878
Kim SH, Kang BJ, Choi BG et al (2013) Radiologists’ performance for detecting lesions and the interobserver variability of automated whole breast ultrasound. Korean J Radiol 14:154–163
Zhang Q, Hu B, Li WB (2012) Detection of breast lesions using an automated breast volume scanner system. J Int Med Res 40:300–306
Van Zelst JC, Platel B, Karssemeijer N, Mann RM (2015) Multiplanar reconstructions of 3D automated breast ultrasound improve lesion differentiation by radiologists. Acad Radiol 22:1489–1496
Kotsianos-Hermle D, Hiltawsky KM, Wirth S, Fischer T, Friese K, Reiser M (2009) Analysis of 107 breast lesions with automated 3D ultrasound and comparison with mammography and manual ultrasound. Eur J Radiol 71:109–115
Chen L, Chen Y, Diao XH et al (2013) Comparative study of automated breast 3-D ultrasound and handheld B-mode ultrasound for differentiation of benign and malignant breast masses. Ultrasound Med Biol 39:1735–1742
American College of Radiology (ACR) (2013) Breast Imaging Reporting and Data System (BI-RADS) Atlas: breast imaging reporting and data system. 5th ed. Virginia, Reston
Agresti A, Coull BA (1998) Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52:119–126
Hellgren R, Dickman P, Leifland K, Saracco A, Hall P, Celebioglu F (2017) Comparison of handheld ultrasound and automated breast ultrasound in women recalled after mammography screening. Acta Radiol 58:515–520
Golatta M, Baggs C, Schweitzer-Martin M et al (2015) Evaluation of an automated breast 3D-ultrasound system by comparing it with hand-held ultrasound (HHUS) and mammography. Arch Gynecol Obstet 291:889–895
Golatta M, Franz D, Harcos A et al (2013) Interobserver reliability of automated breast volume scanner (ABVS) interpretation and agreement of ABVS findings with hand held breast ultrasound (HHUS), mammography and pathology results. Eur J Radiol 82:e332–e336
Jeh SK, Kim SH, Choi JJ et al (2016) Comparison of automated breast ultrasonography to handheld ultrasonography in detecting and diagnosing breast lesions. Acta Radio 57:162–169
Shin HJ, Kim HH, Cha JH, Park JH, Lee KE, Kim JH (2011) Automated ultrasound of the breast for diagnosis: interobserver agreement on lesion detection and characterization. AJR Am J Roentgenol 197:747–754
Boca Bene I, Ciurea AI, Ciortea CA, Dudea SM (2021) Pros and cons for automated breast ultrasound (ABUS): a narrative review. J Pers Med 11;703
Zanotel M, Bednarova I, Londero V et al (2018) Automated breast ultrasound: basic principles and emerging clinical applications. Radiol Med 123:1–12
Schafgen B, Juskic M, Radicke M et al (2021) Evaluation of the FUSION-X-US-II prototype to combine automated breast ultrasound and tomosynthesis. Eur Radiol 31:3712–3720
Acknowledgements
The authors acknowledge the study participants for their contributions and thank prof. dr. H.M. Verkooijen, the study radiologists, technicians and project support personnel for their valuable contribution to this work.
Funding
Research funding was received from General Electric (GE). GE had no role in the design of the study, in collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Principal investigator, conceptualization of the study: RP Data collection: BD, CM, WS, AV Clinical coordination: BD, CM, WS, AV Data analysis: BD, RP, CG, MB Drafting the manuscript: BD, MB, CG, RP Critical review and editing: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective multicenter diagnostic study was approved by the Medical Research Ethics Committee of the University Medical Center Utrecht. Written informed consent was obtained from all participants.
Consent for publication
Written informed consent was obtained.
Competing interests
The authors of this manuscript report financial support from General Electric (GE). GE had no role in the design of the study, in collection, analysis, and interpretation of data and in writing the manuscript. CG reports support for research work related to the DENSE trial, including support from Bayer Pharmaceuticals (project number, BSP-DENSE) and Volpara Health Technologies. The DENSE trial is not related to this research work and publication.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
den Dekker, B.M., Broeders, M.J.M., Meeuwis, C. et al. Diagnostic accuracy of supplemental three-dimensional breast ultrasound in the work-up of BI-RADS 0 screening recalls. Insights Imaging 15, 131 (2024). https://doi.org/10.1186/s13244-024-01714-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01714-8