Abstract
Objectives
To longitudinally assess and correlate the prevalence of superolateral Hoffa fat pad (SHFP) edema with changes in features of the knee extensor mechanism in adolescent competitive alpine skiers over 48 months.
Methods
Competitive alpine skiers were prospectively enrolled in 2018 and underwent bilateral knee MRI at baseline and after 48 months. MRI was assessed for the prevalence of SHFP edema. Features of the knee extensor mechanism were assessed by measuring the trochlear sulcus angle and depth, lateral and medial trochlear inclination, trochlear angle, patella tilt, Insall‒Salvati ratio (ISR), and patellar ligament to lateral trochlear facet (PL-T) distance. Separate logistic regression models were used to calculate the odds ratios between each measurement and the presence of SHFP edema at both time points.
Results
Sixty-three athletes were included in the study (mean age 15.3 ± 1.3 years, 25 women). At baseline, 23 knees had SHFP edema, increasing to 34 knees at the 48-month follow-up. At baseline, knees with measurements in the highest quartile for ISR and lowest quartile for trochlear depth and PL-T were 9.3, 5.1, and 7.7 times more likely to show SHFP edema, respectively. At follow-up, these correlations were confirmed and additionally, knees with measurements in the highest quartile for trochlear sulcus angle and the lowest quartile for lateral trochlear inclination were 4.1 and 3.4 times more likely to show SHFP edema.
Conclusion
An increased prevalence of SHFP edema in competitive alpine skiers during adolescence was associated with persistent high-riding patella, reduced patellar ligament to trochlear distance, and flattened lateral trochlear facet.
Critical relevance statement
In clinical routine, assessment of the mechanical properties of the knee extensor mechanism, together with anatomical developments during adolescence, may improve the understanding and management of patellofemoral instability.
Key points
• Superolateral Hoffa fat pad (SHFP) edema is a frequent cause of anterolateral knee pain but the role of predisposing factors is still debated.
• A higher prevalence of SHFP edema was associated with high-riding patella, reduced patellar ligament to trochlear distance, and flattened lateral trochlear facet.
• Understanding of the mechanical interaction and the anatomical development of the knee during adolescence provides further insight into the development of SHFP edema.
Graphical Abstract

Similar content being viewed by others
Introduction
Superolateral Hoffa fat pad (SHFP) edema is a common finding on MRI in patients with pain in the anterior or anterolateral knee, which is commonly exacerbated by hyperextension of the knee or focal pressure on the inferior pole of the patella [1]. SHFP edema is characterized by an increased signal intensity on T2-weighted MR images in the superolateral Hoffa fat pad, which lies inferior to the patella, posterior to the patellar tendon, and anterior to the infrapatellar synovium [1, 2]. SHFP edemas are often associated with “patellar tendon-lateral femoral condyle friction syndrome,” which is caused by compression of the Hoffa fat pad between the patellar tendon and lateral femoral condyle due to overuse [2, 3]. As possible underlying causes, features of the knee extensor mechanism were identified, including the misalignment of the patellofemoral joint (laterally displaced patella and high riding patella) as well as the trochlear morphology (more anterior trochlear facet, larger trochlear sulcus angle, smaller lateral trochlear inclination angle, and increased tibial tuberosity-trochlear groove distance) [4,5,6,7]. Campagna et al. identified a short distance between the patellar ligament and the lateral trochlear facet as well as a high-riding patella to be associated with an increased prevalence of SHFP edemas [8]. Matcuk et al. successfully used the lateral patellar displacement, Insall‒Salvati ratio, and lateral trochlear inclination to create a predictive model for SHFP edema [9].
An increased prevalence of SHFP edema was found in professional athletes with higher demands for squatting and kneeling, such as competitive volleyball and beach-volleyball players [4, 10, 11]. Likewise, in competitive alpine skiing, loads of up to 1.75 body weight are exerted on one leg with the knees strongly flexed and pushed forward, resulting in extensive muscular activation of the knee extensors [12, 13]. Overuse-related knee pain is also especially frequent in young athletes, and it was shown that highly specialized young athletes are more than twice as likely to have some form of overuse complaint [14]. Growth spurts have been identified as another predisposing factor for overuse-related knee pain in young athletes, most likely due to the changed proportions and leverage caused by the rapidly growing bones and the thus altered joint loadings [15, 16]. However, to date, the longitudinal changes in the features of the knee extensor mechanism and in the patellofemoral joint alignment and their association to the prevalence of SHFP edemas have not been assessed in athletes during adolescence.
Therefore, the aim of this study was to longitudinally assess and correlate the prevalence of SHFP edema with sports-related changes in the features of the knee extensor mechanism in adolescent competitive alpine skiers over 48 months using MRI.
Materials and methods
Participant selection
In this prospective study, adolescent competitive alpine skiers were enrolled at baseline between November 2018 and February 2019 and received bilateral 3 T MRI of the knee (n = 108, Fig. 1). The criteria for being characterized as a competitive alpine skier at baseline were participation in a certified regional performance center, five or more training units per week, and eight or more consecutive years of participation in competitive alpine sports. Participants with previous knee surgery or acute sports-related knee injury were excluded (n = 3). Between baseline and follow-up, 42 skiers withdrew their participation. Follow-up MRI of both knees was performed after 48 months in 63 participants. Participants ranged in age from 14.4 to 16.8 years at baseline and from 18.4 to 20.8 years at follow-up. Written informed consent was obtained from all study participants prior to inclusion. The underlying study protocol was approved by our institutional review board (Cantonal Ethics Committee Zurich (BASEC Nr. 2017–01395)).
MR imaging
At baseline and 48-month follow-up, all participants were scanned with a 3 T MRI scanner (MAGNETOM Prisma; Siemens Healthcare) using a dedicated 15-channel knee coil (Tx/Rx 15-Channel Knee Coil, Siemens Healthcare). At both timepoints, a noncontrast isotropic fat-suppressed T2-weighted three-dimensional SPACE sequence was acquired for each knee. The slice position was aimed at the center of the femorotibial joint. Scan parameters: repetition time 1000 ms, echo time 108 ms, parallel imaging acceleration factor 4, acquired slice thickness 0.63 mm; field of view 160 × 160; matrix 256 × 256; receiver bandwidth, 415 Hz per pixel, leading to an acquisition time of 4 min 42 s [17].
Image analysis
All images were assessed by two radiologists (A.M. and G.F.) in random order, independently, separately, and blinded to all clinical information. The frequency and location of SHFP edemas were recorded for each knee, and SHFP edema was defined as focal high signal intensity on the fat-suppressed T2-weighted images located in the superolateral corner of the Hoffa fat pad (Fig. 2). Assessment of the knee extensor mechanisms was performed on axial images. The trochlear sulcus angle was measured as the angle between the medial and lateral trochlear facets. For further measurements, a reference line (G) was drawn parallel to the posterior aspect of the femoral condyles (Fig. 3) [18]. Medial and lateral trochlear inclinations were measured as the angles from the medial and lateral trochlear facets, respectively, to the reference line (G) [19, 20]. Trochlear sulcus depth was measured by drawing lines perpendicular to the reference line (G) indicating the largest anterior to posterior diameter of the lateral (A) and medial (C) trochlear facets as well as the deepest point of the sulcus (B). Finally, trochlear depth was calculated as follows: trochlear depth in mm = ([A + C]/2) − B [8]. The trochlear angle was defined as the angle between the reference line (G) and a line drawn along the most anterior points of the medial and lateral trochlear facets [21]. The patellar tilt angle was measured between the reference line (G) and a line joining the medial and lateral margins of the patella (Fig. 4) [22]. To measure the shortest patellar ligament to the lateral trochlear facet (PL-T) distance, a line was drawn perpendicular to the reference line (G), which projected anteriorly through the most anterior point of the lateral trochlear facet. Afterwards, the distance between the dorsal aspect of the ligament and the ventral aspect of the lateral trochlear facet cartilage was measured on this line [8]. The Insall‒Salvati ratio (ISR) was measured on sagittal reconstructions [23, 24], and the tibial tubercle to trochlear groove (TT-TG) distance was measured as previously reported (Fig. 5) [8, 25,26,27]. Image analysis was performed on a picture archiving and communication system (PACS) workstation certified for clinical use (MERLIN 7.1.22, Phönix-PACS GmbH).
Multiplanar MRI of bilateral superolateral Hoffa fat pad (SHFP) edemas (arrows) of an 18-year-old youth competitive alpine skier (a–c axial, sagittal, and coronal images of the right knee; d–f axial, sagittal, and coronal images of the left knee). SHFP edema was defined as focal high signal intensity in the superolateral corner of the Hoffa fat pad on the fat-suppressed T2-weighted images
Measurements of the trochlear morphology shown on axial MR images of the left knee: The trochlear sulcus angle \(\alpha\) (a) was defined as the angle between the medial and lateral trochlear facets. The lateral (\(\beta ) and\) medial (\(\gamma )\) trochlear inclination (b) were defined as the angles between the lateral and medial trochlear facets and the reference line (G), respectively. The trochlear sulcus depth (c) was measured by drawing lines perpendicular to the reference line (G) indicating the largest anterior-to-posterior diameter of the lateral (A) and medial (C) trochlear facets as well as the deepest point of the sulcus (B). Finally, trochlear depth was calculated as follows: trochlear depth in mm = ([A + C]/2) − B. The trochlear angle (d) was defined as the angle between the reference line (G) and a line (H) drawn along the most anterior points of the medial and lateral trochlear facets
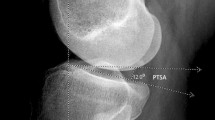
The patella tilt angle \(\delta\) (a) was measured between the reference line (G) at the posterior femoral condyles and a line going through the medial and lateral edges of the patella at the level of the maximal width. The PL-T distance (b) was measured by drawing a line perpendicular to the reference line (G) which projects anteriorly through the most anterior point of the lateral trochlear facet. The distance between the dorsal aspect of the ligament and the ventral aspect of the lateral trochlear facet cartilage was measured. The patellar height is assessed with the ISR (c) which is defined by the ratio of the patellar tendon length (A) to the length of the patella (B). ISR, Insall‒Salvati ratio; PL-T, patella ligament to trochlear distance
Statistics
Comparison of measurements between participants with and without SHFP edema was performed using the Mann‒Whitney U test, and comparison between the baseline and 48-month follow-up was performed with the Wilcoxon signed-rank test. Separate logistic regression models were used to assess the relationship between each measurement of trochlear morphology and patellofemoral alignment and the presence or absence of SHFP edema at each time point. The variables were divided into quartiles and adjusted for age and sex. To assess the linear relationship of each variable to SHFP edema, they were entered as continuous variables in separate models [5]. For interreader agreement, the interclass correlation coefficient (ICC) and Cohen’s weighted \(\kappa\) were used. Statistics were performed in SPSS (v. 28.0 IBM Corp.) by G. F. under supervision of an experienced biostatistician.
Results
In total, 63 adolescent competitive alpine skiers were included in the study (mean age at baseline 15.6 ± 1.2 years and at follow-up 19.6 ± 1.2 years, 25 women, Table 1). At baseline, 23 knees with SHFP edema were detected (10 male and 6 female), increasing to 34 knees with SHFP edema at the 48-month follow-up (10 male, 8 female) (Fig. 6). A significant increase in bilateral affection was observed at the 48-month follow-up (baseline: 44% vs 89% follow-up, p = 0.03).
Correlation of trochlear morphology and patellofemoral joint alignment with SHFP edema
At baseline, knees within the lowest quartile for trochlear depth and PL-T distance were 5.1 (95% CI: 1.2–9.2) and 7.7 (95% CI: 3.2–9.8) times more likely to show SHFP edema compared to knees within the highest quartile (p = 0.002 and p < 0.001 for linear trend, respectively). Compared to knees with the lowest quartile, knees within the highest quartile for ISR were 9.3 times (95% confidence interval (CI): 4.0–11.2) more likely to have SHFP edema (p < 0.001 for linear trend). No statistically significant associations of the trochlear sulcus angle, trochlear angle, lateral and medial trochlear inclination, TT-TG distance, and patella tilt with SHFP edema were found at baseline (Table 2).
At follow-up again, higher odds were detected within the lowest quartile for trochlear depth (odds: 5.95 (95% CI: 1.51–9.45), p = 0.042 for linear trend) and PL-T distance (odds: 6.50 (95% CI: 1.96–9.60), p < 0.001 for linear trend) as well as for knees within the highest quartile for ISR (odds: 9.34 (95% CI: 4.02–11.23), p < 0.001 for linear trend, Table 3). Additionally, knees within the highest quartile for trochlear sulcus angle as well as the lowest quartile for lateral trochlear inclination were 4.1 (95% confidence interval (CI): 1.2–9.2) and 3.4 (95% CI: 0.9–11.7) times more likely to show SHFP edema (p = 0.035 and p = 0.018 for linear trend, respectively). No increased odds ratios were found for the other measurements at the 48-month follow-up.
Comparison of measurements between athletes with and without SHFP edema
At baseline and 48-month follow-up, a significantly larger trochlear depth was detected for athletes without SHFP edema compared to athletes with SHFP edema (baseline: p = 0.002, follow-up: p = 0.014, Table 4). In athletes without SHFP edema, a significantly larger PL-T distance was measured at baseline and follow-up (baseline: p < 0.001, follow-up: p < 0.001). At follow-up, significantly higher trochlear sulcus angles were found in athletes with SHFP edema compared than in athletes without edema (follow-up: p = 0.03), indicating a flatter trochlear surface. At baseline and follow-up athletes with SHFP edema showed higher values for ISR than in athletes without SHFP edema (baseline: p < 0.001, follow-up: p = 0.002). No significant differences between athletes with and without SHFP edema were found for the other measurements (Table 4).
Comparison of measurements between baseline and follow-up in athletes with SHFP edema
Comparing athletes with SHFP edema at baseline and 48-month follow-up, a significant increase was seen in the trochlear sulcus depth, lateral trochlear sulcus angle, medial trochlear sulcus angle, TT-TG distance, patella tilt angle and PL-T (p < 0.05, Table 5). A significant decrease was detected in the trochlear sulcus angle (baseline: 136.65 ± 6.53°, follow-up: 123.09 ± 10.98°, p < 0.001). No significant difference was found for the trochlear angle and ISR (p \(\ge\) 0.05).
Interrater agreement
The agreement for the detection of SHFP edemas was perfect with κ 1.00 (95% CI 1.00–1.00) and with the same number of edemas detected by both raters. The interrater agreement for the measurements of the knee extensor mechanisms and measurements of the patellofemoral joint alignment was substantial to almost perfect (trochlear depth: ICC 0.91 (95% CI 0.82–0.96) trochlear sulcus angle ICC 0.94 (95% CI 0.86–0.97), lateral trochlear inclination ICC 0.87 (95% CI 0.79–0.93) medial trochlear inclination ICC 0.86 (95% CI 0.76–0.95), trochlear angle ICC 0.92 (95% CI 0.85–0.98), patella tilt ICC 0.83 (95% CI 0.72–0.91), ISR ICC 0.89 (95% CI 0.81–0.93), PL-T distance ICC 0.81 (95% CI 0.71–0.89), and TT-TG distance ICC 0.85 (95% CI 0.79–0.95)).
Discussion
In this study, we longitudinally assessed and correlated the prevalence of SHFP edema with sports-related changes in features of the knee extensor mechanism in a cohort of 63 adolescent competitive alpine skiers over 48 months. Over the last decade, the relationship between SHFP edema and abnormalities in the knee extensor mechanism was investigated by examining the malalignment of the patellofemoral joint with different underlying pathologies or at different levels of physical activity. However, there is only partial consensus about the underlying anatomical variations and angles associated with SHFP edema, and thus far, no longitudinal analysis has been performed [4, 5, 8, 9].
Our results revealed a SHFP edema prevalence of 25% at baseline, increasing to 29% at the 48-month follow-up. At baseline, athletes in the lowest quartile for trochlear depth and PL-T distance as well as the highest quartile for ISR demonstrated greater odds of developing a SHFP edema. In addition, athletes in the lowest quartile for lateral trochlear inclination angles and the highest quartile for trochlear sulcus angles had greater odds of developing SHFP edema at follow-up. This is in line with a previous study by Campagna et al., in which the authors noted an association of high ISR (p = 0.023), short PL-T distance (p < 0.001), and short TT-TG (p = 0.046) with SHFP in a cohort of 90 patients [8]. They suggested that the lateral displacement of the patella and narrowing between the patellar ligament and bone predispose an impingement of the SHFP due to the increased pressure on the SHFP during motion. Additionally, younger participants were more likely to present with SHFP edema, and the authors assumed this was due to increased physical activity [8]. High physical activity might also explain the high prevalence of 29% of athletes reported in our study, in which only competitive athletes with five or more training units per week were included. Widjajahakim et al. reported a significantly lower prevalence of 13.4% for SHFP edema in a cohort of 1134 patients aged 50 to 79 years with risk factors for osteoarthritis of the knee [5]. Similar to our study, participants with larger trochlear angles and high ISR were 1.5 and 8.9 times more likely to show SHFP edema. However, no associations were demonstrated for the lateral trochlear inclination and sulcus angle, which might be due to the changed level of physical activity or the changes associated with beginning osteoarthritis. Mehta et al. assessed the prevalence of SHFP edema and knee extensor mechanisms in a cohort of 16 competitive collegiate volleyball players aged 18–22 years and found a prevalence of 50% [4]. Similar to our study, the authors found significant differences in TT-TG distance in SHFP edema-positive athletes. The high prevalence was explained by the excessive kneeling and squatting performed during volleyball playing inducing overuse and pressure in the anterolateral knee, loading patterns that are also present in competitive alpine skiing [12, 13]. This assumption is further supported by a study by Jarraya et al., who found a prevalence of 52.1% for SHFP edema in a sample cohort of summer sports athletes participating in the 2016 Olympic games [11].
The high prevalence of SHFP edema in adolescent athletes might be better understood when assessing the development of trochlear morphology and patellofemoral joint alignment during adolescence. It was demonstrated that the lateral trochlear slope has a significant effect on the patella alignment during knee flexion and that the anterolateral femoral condyle has more contact with the patella than the medial facet, even during contraction of the quadriceps muscle [28, 29]. Furthermore, the lateral trochlea was found to be one of the most consistent anatomical determinants of trochlear morphology during maturation and it is able to compensate for changes in the slope of the medial trochlear facet, keeping the trochlear sulcus angle consistent [29, 30]. In this study, athletes with SHFP edema showed consistently lower values for lateral trochlear inclination and trochlear sulcus depth at baseline and at the 48-month follow-up than athletes without SHFP edema. The more flattened anterolateral trochlear facet predisposes the lateral displacement of the patella and increases the pressure on the SHFP during knee flexion, which favors impingement. Additionally, the consistently lower values might indicate that the development of SHFP edemas is not exclusively caused by overuse but also due to altered or delayed trochlear development, which affects patellofemoral alignment from adolescence. Therefore, the assessment of the mechanical relationship between the patella and lateral trochlea together with anatomical development is key to understanding patellofemoral instability.
Our study has limitations: First, only competitive alpine skiers were included in the study, which might introduce a selection bias. Second, only imaging of the knee was performed, and the relationship between the muscle volume and strength of the knee extensor muscles with SHFP edema could not be investigated. Additionally, functional aspects of the patellofemoral tracking during, e.g., kneeling could not be assessed due to the nature of the study.
In summary, an increased prevalence of SHFP edema in adolescent competitive alpine skiers was associated with a high-riding patella and a more anterolateral flattened trochlear facet. The altered anatomical proportions did not change over the course of 48 months, which highlights the importance of taking anatomical development into consideration when assessing the mechanical relationship of the patellofemoral alignment.
Availability of data and materials
The data that support the findings of this study are not publicly available. Data are however available from the authors upon reasonable request.
Abbreviations
- CI:
-
Confidence interval
- ICC:
-
Interclass correlation coefficient
- ISR:
-
Insall‒Salvati ratio
- MRI:
-
Magnetic resonance imaging
- PL-T:
-
Patella ligament to trochlear distance
- SHFP:
-
Superolateral Hoffa fat pad
- TT-TG:
-
Tibial tuberosity to trochlear groove distance
References
Chung CB, Skaf A, Roger B et al (2001) Patellar tendon-lateral femoral condyle friction syndrome: MR imaging in 42 patients. Skeletal Radiol 30(12):694–697
Mills MK, Allen H (2022) Knee plical pathology and impingement syndromes. Magn Reson Imaging Clin N Am 30(2):293–305
Dragoo JL, Johnson C, McConnell J (2012) Evaluation and treatment of disorders of the infrapatellar fat pad. Sports Med 42(1):51–67
Mehta K, Wissman R, England E et al (2015) Superolateral Hoffa’s fat pad edema in collegiate volleyball players. J Comput Assist Tomogr 39(6):945–950
Widjajahakim R, Roux M, Jarraya M et al (2017) Relationship of trochlear morphology and patellofemoral joint alignment to superolateral Hoffa fat pad edema on MR images in individuals with or at risk for osteoarthritis of the knee: the MOST study. Radiology 284(3):806–814
Li J, Sheng B, Liu X et al (2020) Sharp margin of antero-inferior lateral femoral condyle as a risk factor for patellar tendon-lateral femoral condyle friction syndrome. Eur Radiol 30(4):2261–2269
Subhawong TK, Eng J, Carrino JA et al (2010) Superolateral Hoffa’s fat pad edema: association with patellofemoral maltracking and impingement. AJR Am J Roentgenol 195(6):1367–1373
Campagna R, Pessis E, Biau DJ et al (2012) Is superolateral Hoffa fat pad edema a consequence of impingement between lateral femoral condyle and patellar ligament? Radiology 263(2):469–474
Matcuk GR Jr, Cen SY, Keyfes V et al (2014) Superolateral hoffa fat-pad edema and patellofemoral maltracking: predictive modeling. AJR Am J Roentgenol 203(2):W207–W212
Amin S, Goggins J, Niu J et al (2008) Occupation-related squatting, kneeling, and heavy lifting and the knee joint: a magnetic resonance imaging-based study in men. J Rheumatol 35(8):1645–1649
Jarraya M, Roemer FW, Engebretsen L et al (2021) Association of markers of patellofemoral maltracking to cartilage damage and bone marrow lesions on MRI: data from the 2016 olympic games of Rio De Janeiro. Eur J Radiol Open 8:100381
Kröll J, Spörri J, Gilgien M et al (2016) Effect of ski geometry on aggressive ski behaviour and visual aesthetics: equipment designed to reduce risk of severe traumatic knee injuries in alpine giant slalom ski racing. Br J Sports Med 50(1):20–25
Spörri J, Müller E, Kröll J (2022) “When you’re down, stay down”: a lesson for all competitive alpine skiers supported by an ACL rupture measured in vivo. J Sport Health Sci 11(1):14–20
Jayanthi NA, LaBella CR, Fischer D et al (2015) Sports-specialized intensive training and the risk of injury in young athletes: a clinical case-control study. Am J Sports Med 43(4):794–801
Patel DR, Villalobos A (2017) Evaluation and management of knee pain in young athletes: overuse injuries of the knee. Transl Pediatr 6(3):190–198
Hewett TE, Myer GD, Kiefer AW et al (2015) Longitudinal increases in knee abduction moments in females during adolescent growth. Med Sci Sports Exerc 47(12):2579–2585
Stern C, Galley J, Fröhlich S et al (2020) Distal femoral cortical irregularity at knee MRI: increased prevalence in youth competitive Alpine skiers. Radiology 296(2):411–419
Brossmann J, Muhle C, Schröder C et al (1993) Patellar tracking patterns during active and passive knee extension: evaluation with motion-triggered cine MR imaging. Radiology 187(1):205–212
Ali SA, Helmer R, Terk MR (2010) Analysis of the patellofemoral region on MRI: association of abnormal trochlear morphology with severe cartilage defects. AJR Am J Roentgenol 194(3):721–727
Carrillon Y, Abidi H, Dejour D et al (2000) Patellar instability: assessment on MR images by measuring the lateral trochlear inclination-initial experience. Radiology 216(2):582–585
Stefanik JJ, Roemer FW, Zumwalt AC et al (2012) Association between measures of trochlear morphology and structural features of patellofemoral joint osteoarthritis on MRI: the MOST study. J Orthop Res 30(1):1–8
Grelsamer RP, Weinstein CH, Gould J et al (2008) Patellar tilt: the physical examination correlates with MR imaging. Knee 15(1):3–8
Insall J, Salvati E (1971) Patella position in the normal knee joint. Radiology 101(1):101–104
Miller TT, Staron RB, Feldman F (1996) Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol 167(2):339–341
Diederichs G, Issever AS, Scheffler S (2010) MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics 30(4):961–981
Schoettle PB, Zanetti M, Seifert B et al (2006) The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee 13(1):26–31
Wittstein JR, Bartlett EC, Easterbrook J et al (2006) Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy 22(6):643–649
Teng HL, Chen YJ, Powers CM (2014) Predictors of patellar alignment during weight bearing: an examination of patellar height and trochlear geometry. Knee 21(1):142–146
Salsich GB, Ward SR, Terk MR et al (2003) In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. https://doi.org/10.1097/01.blo.0000093024.56370.79(417):p.277-84
Trivellas M, Kelley B, West N et al (2021) Trochlear morphology development: study of Normal pediatric knee MRIs. J Pediatr Orthopaedics 41(2):77–82
Acknowledgements
We would like to thank Daniel Fitze, Jonas Hanimann, and Daniela Meyer for conducting the MR imaging. We would like to thank Sabine Schrimpf for proofreading and editing. Special thanks go to the Swiss Centre for Musculoskeletal Imaging, SCMI, Balgrist Campus AG, Zurich, where the study was performed.
Funding
This study was generously supported by the Balgrist Foundation.
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of the entire study, G.F., R.S.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of the final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, G.F., J.Sp.; clinical studies, G.F., A.M.; experimental studies, J.Sp.; statistical analysis, G.F.; and manuscript editing, all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by our institutional review board (Cantonal Ethics Committee Zurich (BASEC Nr. 2017–01395)).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feuerriegel, G.C., Marth, A.A., Fröhlich, S. et al. Superolateral Hoffa fat pad edema in adolescent competitive alpine skiers: temporal evolution over 4 years and risk factors. Insights Imaging 15, 52 (2024). https://doi.org/10.1186/s13244-024-01633-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01633-8