Abstract
Introduction
The main generator of delayed onset muscle soreness (DOMS) is still unknown. This study aimed to clarify the main generator of DOMS.
Methods
Twelve participants performed eccentric exercise (EE) on lower legs. MRI and ultrasound were used to assess changes of calf muscle and deep fascia before and after EE. These results were then compared to the muscle pain level.
Results
Compared to baseline, muscle pain peaked at 24–48 h after EE (downstairs 22.25 ± 6.196, 57.917 ± 9.298, F = 291.168, p < 0.01; resting 5.833 ± 1.899, 5.083 ± 2.429, F = 51.678, p < 0.01). Shear wave speed (SWE) of the deep fascia and T2 values of the gastrocnemius muscle and deep fascia all increased and peaked at 48 h after EE (1.960 ± 0.130, F = 22.293; 50.237 ± 2.963, F = 73.172; 66.328 ± 2.968, F = 231.719, respectively, p < 0.01). These measurements were positively correlated with DOMS (downstairs: r = 0.46, 0.76, 0.87, respectively, p < 0.001; resting: r = 0.42, 0.70, 0.77, respectively, p < 0.001). There was a significant positive correlation between SWE and T2 values of deep fascia (r = 0.54, p < 0.01).
Conclusion
DOMS is a common result of muscle and fascia injuries. Deep fascia edema and stiffness play a crucial role in DOMS, which can be effectively evaluated MR-T2 and SWE.
Critical relevance statement
Delayed-onset muscle soreness is a common result of muscle and deep fascia injuries, in which the edema and stiffness of the deep fascia play a crucial role. Both MRI and shear wave elastography can be effectively used to evaluate soft tissue injuries.
Key points
• The deep fascia is the major pain generator of delayed-onset muscle soreness.
• There is a significant correlation between fascia injury and delayed-onset muscle soreness.
• MRI and shear wave elastography are preferred methods for assessing fascia injuries.
Graphical Abstract

Similar content being viewed by others
Introduction
Eccentric exercises (EE) can result in delayed-onset muscle soreness (DOMS) [1,2,3]. In the “Munich consensus statement” [4], DOMS was classified as “muscle-related neuromuscular muscle disorder (type 1B)”. Many hypotheses to explain the mechanism of DOMS have been proposed and most studies have focused on the skeletal muscles [5,6,7,8,9,10]. However, there is increasing evidence to indicate that injuries to the fascial system could play a potential role in the development of DOMS. Thus, fascial tissues deserve more detailed attention [11,12,13].
The fascial system is a three-dimensional structure permeating the body that enables all body systems to operate in an integrated manner [13]. Pathological changes in the biomechanical properties of fascial tissues have been hypothesized to play a crucial role in musculoskeletal diseases [14]. The muscle fibers are closely fused with the endomysium in vivo, and the collagenous connective tissue is inextricably linked to the skeletal muscle [15]. Muscle fibers can transmit cross layer shear force through the fascia [16], and the fascia is very likely to be damaged during this transmission of force [17, 18].
The fascia comprises dense innervation, free nerve endings, and pain receptors, which make it more sensitive to pain [19,20,21,22,23]. By comparing the pain sensitivity of different structures to hypertonic saline, electrical stimulation, and local inflammation, some researchers were able to confirm that the fascia induced a stronger pain response than the muscle [24,25,26]. Thus, it can be speculated that the fascia, rather than the muscle, is the main generator of DOMS.
Imaging methods, such as high-field magnetic resonance imaging (MRI) and ultrasound shear-wave elastography (SWE), are promising tools for quantifying edema and hardness of the soft tissues under in vivo conditions [27, 28]. Especially, transverse relaxation time-weighted imaging (T2WI) is the most common approach [29]. As a parameter associated with osmotic changes, T2WI can provide information on the changes in water content of the muscle tissue, which can be quantified by T2-mapping technology. Due to the sensitivity of MR-T2 mapping to acute changes in the activity states of muscle, the method shows great potential for identifying muscle activation patterns in a variety of normal and pathological conditions [28, 30]. The results of previous studies have confirmed that T2 mapping can effectively and accurately evaluate the muscle damage caused by EE [28, 31, 32]. Second, the high-frequency ultrasonic probe produces an acoustic radiofrequency force impulse that generates transversely oriented shear waves that propagate throughout the surrounding tissues. The velocity of the propagating shear waves is measured on the qualitative color maps. Theoretically, the tissue elasticity can be calculated from the shear wave speed (SWS), providing important biomechanical information on tissue quality [33, 34]. However, due to its heterogeneity and anisotropic characteristics, SWE measurements in the musculoskeletal system should be presented in terms of the SWS in m/s [35, 36].
In the present study, MRI T2-mapping technology and SWE were applied to evaluate the edema and stiffness of the gastrocnemius muscle (GM) and deep fascia after EE, and to identify the main pain generator in DOMS.
Methods
Participants
Twelve healthy participants (6 men and 6 women; 18–30 years of age; body mass index = 19–24 kg/m2) were invited to participate in the prospective study. The inclusion criteria were as follows: no or little experience with weight-lifting exercises during the 90-day period before study participation, no basic diseases (cardiovascular, musculoskeletal, respiratory, or neurological diseases) that might affect the results. All participants were asked to avoid performing any sports activities for 1 week prior to the test and during the investigation period.
Eccentric exercise
All participants performed a standardized EE of the calf muscles to induce DOMS. A specifically manufactured slant plate was set between the parallel bars, on which the participants performed EE after the warm-up. At the starting position, the participants raised their heels as high as possible, held this position for 1 s, and then lowered their heels slowly over 3 s until the sole touched the bottom of the slant plate. Participants pushed themselves up the parallel bars to move back to the starting position, allowing relief of the calf muscles. To increase the load, each participant wore a weighted backpack weighing approximately 20% of their body weight during exercise. All participants performed 4 sets of 12 repetitions, with a 30-s rest period between each set; however, the last three sets were performed until muscle fatigue occurred. Muscle fatigue was defined as obvious shaking of the leg and inability to control the speed of descent. In order to obtain accurate data, examinations were performed at approximately the same time of the day for each participant.
Muscle soreness assessment
The level of muscle soreness was evaluated using a visual analog scale (VAS), with scores ranging from 0 (no pain) to 100 (worst pain). Muscle soreness was assessed before and at 0, 24, 48, 72, 120, and 168 h after exercise. The participants were asked to mark their muscle pain levels on the VAS scale at rest and while walking downstairs.
Serum creatine kinase (CK) level
Blood (5 mL) was collected from the antecubital vein of each participant and centrifuged at 1000 g for 10 min. The serum CK levels were measured before and 24 and 48 h after the EE using the BECKMAN Au5800 automatic biochemical analyzer (Beckman Coulter, Inc, Bria, CA, USA).
Magnetic resonance imaging
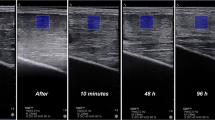
MRI scans were performed on a 3.0 T whole-body MR scanner (Ingenia CX, Philips Healthcare, Best, Netherlands) using a dedicated sensitivity encoding knee coil. Participants were positioned in the supine position. Both ends of the lower limb were raised to avoid compressing the calf muscles. Fat suppressed T2WI and T2 mapping were performed for the entire GM belly. T2-mapping scans were acquired as multi-shot SE sequences, echoes = 6; TE = 7.9, 15.8, 23.7, 31.6, 39.5, 47.4 ms; TR = 1006 ms; NAX = 1; flip angle 90°; FOV 160 × 160 × 75 mm; voxel 0.9 × 1.12; slice thickness 5 mm; interslice gap 0 mm. The original images were entered into the IntelliSpace Workstation (Philips), and the T2 values were automatically computed from T2 maps. The regions of interest (ROI) were drawn manually, and the borders of the ROI were traced to exclude the bone and large blood vessels. The ROIs were reconfirmed on the corresponding fat suppressed T2WI images. Three continual typical images of the GM belly were selected from the transaxial MR images. The final result was determined as the mean of the measured values in the above three images (Fig. 1). T2 values were assessed before and at 0, 24, 48, 72, 120, and 168 h after exercise.
Ultrasound
SWE was performed by one examiner educated in musculoskeletal ultrasound imaging with 10 years of work experience. The SWS (m/s) values of the GM and deep fascia were measured using a high-resolution ultrasound device with an 8.0 × 1.5 cm linear array transducer (frequency range of 10–2 MHz, display depth of 3.0 cm, dynamic range of 60 dB, and image gain of 50). To avoid false measurements caused by muscle tension, all participants were positioned in the prone position. A cushion was placed under the foot to keep the calf muscles relaxed, as muscle tension can significantly change SWS. To avoid compressing the tissue, the doctor stabilized the probe on the skin with minimal pressure. A sufficient amount of ultrasound gel was applied between the skin and the probe. To ensure identical positioning during the entire study, the probe location was marked on the calf skin with a semi-permanent marker. The transducer was positioned at the belly of the medial head of GM, perpendicular to the skin, avoiding interfering signals caused by the large vessels. A sagittal examination plane was chosen parallel to the muscle fibers to make the shear waves propagate better [37, 38]. To measure the SWS of the GM and deep fascia, six ROIs (diameter = 5 mm) were manually located at the middle of GM, while five ROIs (diameter = 1 mm) were manually located equidistantly along the deep fascia. The final results were determined as the mean of the measurements (Fig. 2). SWS values were assessed before and at 0, 24, 48, 72, 120, and 168 h after exercise.
a and b separately show the shear wave elastography qualitative color maps of the lower leg soft tissue before and 48 h after exercise. Six regions of interest (diameter = 5 mm) were manually located at the medial head of the gastrocnemius muscle, while five regions of interest (diameter = 1 mm) were manually located equidistantly along the deep fascia
Statistical analysis
All data were tested for normal distribution, and inference statistics were applied as appropriate. For continuous variables that conform to a normal distribution, the mean ± standard was used for statistical description, while for those that do not conform to a normal distribution, the median (quartile) was used. Repeated measurement analysis of variance was used to compare CK levels at baseline and 24 h and 48 h. Before applying analysis of variance (ANOVA) of single factor repeated measurement data to check the trends in the changes of the degree of muscle soreness, T2 values, and deep fascia thickness, the Mauchly test was applied to verify sphericity. If p values > 0.05, it was considered that the data satisfies the sphericity assumption; one-way ANOVA was performed directly. Otherwise, the Greenhouse–Geisser correction was applied. Bonferroni’s multiple comparison was used for post hoc comparison.
Linear correlation analysis was performed to analyze the relationship between muscle injury, fascia injury, and DOMS, with T2 and SWS values as the independent variables and muscle pain level as the dependent variable. Linear correlation analysis was performed to analyze the relationship between edema and the hardness of soft tissue, with T2 values as the independent variables and SWS values as the dependent variable. Data were considered significant for p values < 0.05.
Results
Delayed-onset muscle soreness
DOMS was successfully induced in each participant, and the changes in pain levels of the calf muscles are shown in Fig. 3. The results of one-way repeated measures ANOVA showed that, compared with the baseline value (0 ± 0), there was a significant difference in muscle soreness indicators of participants both when walking downstairs (0–168 h, 7.917 ± 4.316, 22.250 ± 6.196, 57.917 ± 9.298, 11.000 ± 2.828, 3.583 ± 2.021, 0.250 ± 0.452, F = 291.168, p < 0.001) and at rest (0–168 h, 1.25 ± 0.866, 5.833 ± 1.899, 5.083 ± 2.429, 0.667 ± 0.651, 0.333 ± 0.492, 0 ± 0, F = 51.678, p < 0.001). The results of Bonferroni’s multiple comparison showed that when walking downstairs, the muscle pain level at 0, 24, 48, 72, and 120 h after EE was significantly higher than the baseline level (separately, p < 0.01), reaching a peak at 48 h. The muscle pain had disappeared in all participants by 168 h after EE, there was no significant difference compared to baseline (p > 0.05). Conversely, in the resting state, all participants could feel a slight muscle soreness only at 0, 24, and 48 h after EE (separately, p < 0.01).
Serum CK level
The results of one-way repeated measures ANOVA showed that, compared with the baseline value, there were statistical differences in serum CK level after EE (F = 10.88, p = 0.007). The results of Bonferroni’s multiple comparison showed that the serum CK level at 24 and 48 h after EE were significantly higher than the baseline level before exercise (separately, p = 0.024 and p = 0.018) (Table 1).
Magnetic resonance imaging
The temporal changes in GM and deep fascia edema in all participants are shown in Figs. 1 and 4. Under physiological conditions, no signs of edema were observed in any part of the calf muscles or deep fascia. The T2 values of the GM increased immediately following EE, peaking at 48 h. Subsequently, the signal intensity and range decreased over time. During this period, the results demonstrated muscle edema only after EE, with no architectural disorganization. The results of one-way repeated measures ANOVA showed that there were statistical differences in T2 values of GM after EE (0–168 h, 46.538 ± 3.832, 46.666 ± 1.153, 50.237 ± 2.963, 42.586 ± 1.283, 39.811 ± 0.757, 37.694 ± 1.436, F = 73.172, p < 0.001) compared with the baseline value (37.441 ± 1.138). The results of Bonferroni’s multiple comparison showed that the T2 values of GM at 0, 24, 48, 72, and 120 h after EE were significantly higher than the baseline level before exercise (separately, p < 0.01).
The results show that the increase in the T2 value of the deep fascia is obviously lower than that of the GM during the first 24 h. Representative fascial edema peaked 24 h after EE. The results of one-way repeated measures ANOVA showed significant increases in the T2 values of the deep fascia after EE (0–168 h, 47.679 ± 2.216, 59.114 ± 2.790, 66.328 ± 2.968, 53.058 ± 2.057, 47.519 ± 1.831, 45.219 ± 1.528, F = 231.719, p < 0.001) compared with the baseline value (44.573 ± 1.636). The results of Bonferroni’s multiple comparison showed that the T2 values of the deep fascia at 0, 24, 48, 72, and 120 h after EE were significantly higher than the baseline level (separately, p < 0.01).
At 168 h after EE, there was no significant difference in T2 values of GM and deep fascia compared to baseline (p > 0.05).
Ultrasound
The temporal changes of the SWS of the GM and deep fascia in all participants are shown in Figs. 2 and 5, with no architectural disorganization. SWS detected by ultrasound showed that the hardness of the GM rapidly increased after EE. The results of one-way repeated measures ANOVA showed that, compared with the baseline value (1.818 ± 0.177), there were significant increases in the SWS of the GM after EE (0–168 h, 1.967 ± 0.185, 1.860 ± 0.187, 1.842 ± 0.195, 1.862 ± 0.204, 1.868 ± 0.199, 1.818 ± 0.166, F = 9.947, p < 0.001). The results of Bonferroni’s multiple comparison showed that the SWS of the GM was higher than the baseline level at 0 h after EE (p = 0.024).
The SWS of the deep fascia increased after EE, peaking at 48 h. The results of one-way repeated measures ANOVA showed that, compared with the baseline value (1.740 ± 0.100), there were statistical differences in the SWS of the deep fascia after EE (0–168 h, 1.870 ± 0.098, 1.896 ± 0.120, 1.961 ± 0.130, 1.878 ± 0.138, 1.798 ± 0.113, 1.775 ± 0.092, F = 22.293, p < 0.001). The results of Bonferroni’s multiple comparison showed that the SWS of the deep fascia at 0, 24, 48, and 72 h after EE were significantly higher than the baseline level before EE (separately, p < 0.05). At 120, 168 h after EE, there was no significant difference in SWS of deep fascia compared to baseline (p > 0.05).
Correlation
The linear correlation analyses results are shown in Fig. 6. Linear correlation analysis showed that muscle pain level (downstairs and at rest separately) correlated strongly with T2 values of GM (r = 0.76, p < 0.001 and r = 0.70, p < 0.001) and T2 values of deep fascia (r = 0.87, p < 0.001 and r = 0.77, p < 0.001) and correlated with SWS of deep fascia (r = 0.46, p < 0.001 and r = 0.42, p < 0.001) following EE. There was no significant correlation between muscle pain level (downstairs and at rest separately) with SWS of GM (r = 0.03, p = 0.78 and r = 0.02, p = 0.88). In addition, T2 values of deep fascia was positively correlated with SWS of deep fascia (r = 0.54, p < 0.01). There was no significant correlation between T2 value and SWS of GM (r = 0.12, p = 0.28).
a, b, e, and f show the correlation between the pain level (when walking downstairs and at rest) and the T2 values of the gastrocnemius muscle and deep fascia; c, d, g, and h show the correlation between the pain level (when walking downstairs and at rest) and the shear wave speed of the gastrocnemius muscle and deep fascia; i and j show the correlation between the T2 values and SWS
Discussion
In this study, all participants successfully induced DOMS, with pain peaking at 48 h after EE, both when walking downstairs and resting. The muscle injuries of all participants were confirmed as type 1B [4]. The SWS of the deep fascia and the T2 values of both the GM and deep fascia showed positive associations with DOMS. Meanwhile, there was a significant correlation between T2 values and the SWS of the deep fascia. These results suggested that edema of both the muscle and deep fascia may be involved in the development of DOMS. Due to the structural characteristics of the fascia and its high sensitivity to injury and inflammatory stimulation [13, 19,20,21], we speculated that the deep fascia would play a crucial role in DOMS. In this study, we produced strong evidence to support this hypothesis, but there are still several details that need to be discussed.
Before EE
According to previous studies, damage to the GM after EE is more obvious than in other calf muscles [38, 39]. In addition, due to limitations in the SWE detector, it is difficult to detect deep muscles and accurately measure changes in their hardness [40, 41]. Therefore, GM and its adjacent fascia were selected as observation objects. The serum CK level was used as an indicator of muscle injury in this study. Many factors, including training mode, age, and gender may significantly influence CK levels [42, 43]. Furthermore, serum CK activity after exercise is poorly related to muscle soreness level, strength, and range of motion [44]. Therefore, CK appears to be of more use as a qualitative marker, rather than a quantitative indicator, of muscle damage [42].
Immediately post-EE
The results showed that the T2 values of the posterior and lateral groups of calf muscles increased significantly. The T2 signal is determined through the metabolism of water molecules in the muscle, while an increased relaxation time of skeletal muscles reflects tissue activity-related changes [28]. T2 values from T2 maps can be used as a quantitative index of changes in tissue composition, water metabolism, and other biochemical changes, implying a significant increase in calf muscle perfusion following EE [45], but without obvious architectural disorganization. In contrast, the increase in the T2 values of the deep fascia is obviously lower than that of the GM, indicating that no apparent edema or inflammatory exudation occurred in the deep fascia at this stage. In addition, representative fascial edema peaked 24 h after EE. These results are similar to previous conclusion [1, 3, 6, 46] and may provide a reasonable explanation as to why DOMS typically presents 24–72 h after EE. In the present study, many of the participants felt only discomfort, rather than pain immediately following EE. This may be due to the fact that regular and acute exercise can increase water content and muscle volume [47], leading to an increase in fascia tension [6, 10, 45, 46, 48]. Therefore, we speculate that this is the main reason for discomfort at this stage.
The relative increase in deep fascia hardness at 0 h after EE was observed from the SWE results, confirming the above hypothesis [34, 36]. However, this hypothesis still requires further verification. In the future, more tests should be conducted 0–24 h after EE to study what happens to the muscles and fascia on the first day.
Degeneration processes
The CK results at 24 and 48 h after EE showed that all of the participants successfully induced delayed injury to GM.
The MRI results demonstrated that obvious injury and inflammatory exudation occurred in GM based on uneven signals [31]. With reference to MRI, bound water shows a shorter relaxation time than free water [49], and free water in the intermuscular space is rare. Therefore, normal muscle tissues exhibit an even shorter T2 signal intensity [50]. According to the previous study [31], the distributions and states of intracellular and extracellular water were altered by the inflammatory reactions following EE. After EE, the muscle fibers were arranged irregularly, and the extracellular spaces were enlarged, with inflammatory cells gradually infiltrating and gathering. The content of bound water in the intercellular space increased. The permeability of skeletal muscle cells increased, and large molecules in cells entered the extracellular fluid, resulting in a decrease in the bound water on the cellular surface. Meanwhile, free water from the interstitial space entered muscle cells, leading to cellular swelling. These changes prolonged the relaxation time and affected the uniformity of the image.
The SWS and T2 values of the deep fascia increased significantly from 24 to 48 h after EE. Recent results showed that both the thickness and hardness of deep fascia increases after EE [18, 51]. These findings indicate that fascial micro-injury and edema may be potential causes. Earlier studies have shown that edema is associated with increased stiffness [6, 52, 53]. Our findings confirmed a moderate positive correlation between fascial edema and stiffness, providing some support for their hypothesis.
In clinical practice, muscle injury rarely occurs alone, and injury to the collagen connective tissue is very common in exercise-induced muscle strain [17, 54]. Muscle fibers are tightly fused with the endomysium in the body, and collagen connective tissue is inseparable from skeletal muscle [15, 55]. They can transmit force between both ends of the muscle through the endomysium [16]. EE generates high shear force, which may damage the fascia and cause edema due to the mechanical interaction between adjacent muscle fibers [17, 18]. This overstretch could cause micro damage in the fascia, which may contribute to the development of fascia edema post-exercise. Our results show that, compared with the muscle, deep fascia injury has a higher correlation with DOMS. According to the high sensitivity of the deep fascia to edema and inflammation [19,20,21,22,23,24,25,26], we believed that both muscle and fascial edema contribute to DOMS; however, the deep fascia seems to be the main generator of DOMS at this stage.
In our study, SWE was used to evaluate the hardness of soft tissue. Compared to the baseline values, the hardness of the GM is significantly higher than that immediately after EE. There was no significant correlation between the hardness of GM and DOMS. These results largely, but not completely, agree with those of previous studies [56, 57]. We speculated that one reason to explain our finding may be that different muscles have different responses to EE, and the influence of muscle tissue viscoelasticity, heterogeneity, and anisotropy cannot be ignored [35, 36]. Furthermore, muscle quality and structure may further affect the recovery rate after resistance training [58, 59]. In addition, the daily use rate of the leg muscles is significantly higher than that of the arms, which may promote quicker recovery [60]. To sum up, we confirmed that a single bout of EE does not affect muscle stiffness constantly, and there is no significant correlation between muscle stiffness and DOMS. We therefore believe that muscle stiffness cannot be used to explain DOMS yet.
Regeneration processes
The magnitude of DOMS decreased gradually after peaking at 48 h post-EE, and the pain had disappeared in all participants by 168 h after EE. The MRI results showed that the edema of the GM and deep fascia declined gradually and recovered to baseline at 168 h after EE. With the disappearance of edema, the hardness of the deep fascia gradually recovered, returning to the baseline level at 120 h.
Limitations
Firstly, the sample size in this study was small. This study was only prospective; in future research, we will consider expanding the sample size and diversity of research objects. Secondly, due to the small sample size of this study, it is not possible to establish cut-off values to differentiate normal muscle and fascia from DOMS. In addition, multiple examinations within 0–24 h should be added, which will help to understand the details of the muscle and fascia evolution in the early stage of injury. Furthermore, more muscles will be included in future research.
Conclusion
In conclusion, we confirmed that the deep fascia, rather than the muscle, is the predominant pain generator of the complicated process of DOMS. The changes in MRI T2 values and ultrasound SWS values can accurately display the edema and hardness of the deep fascia. Due to non-invasive and quantitative characteristics, T2 mapping and SWE could become preferred methods for assessing soft tissue injuries in DOMS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DOMS:
-
Delayed-onset muscle soreness
- EE:
-
Eccentric exercise
- MRI:
-
Magnetic resonance imaging
- SWE:
-
Shear-wave elastography
- SWS:
-
Shear wave speed
References
Armstrong RB (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc 16:529–538
Córdova-Martínez A, Caballero-García A, Bello HJ et al (2022) Concentric sports on blood muscular damage markers in male professional players. Biology (Basel) 11:343. https://doi.org/10.3390/biology11030343
Hotfiel T, Freiwald J, Hoppe MW et al (2018) Advances in delayed-onset muscle soreness (DOMS): part I: pathogenesis and diagnostics. Sportverletz Sportschaden 32:243–250. https://doi.org/10.1055/a-0753-1884
Mueller-Wohlfahrt HW, Haensel L, Mithoefer K et al (2013) Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med 47:342–350. https://doi.org/10.1136/bjsports-2012-091448
Touron J, Costes F, Coudeyre E et al (2021) Aerobic metabolic adaptations in endurance eccentric exercise and training: from whole body to mitochondria. Front Physiol 11:596351. https://doi.org/10.3389/fphys.2020.596351
Borghi SM, Bussulo SKD, Pinho-Ribeiro FA et al (2022) intense acute swimming induces delayed-onset muscle soreness dependent on spinal cord neuroinflammation. Front Pharmacol 12:734091. https://doi.org/10.3389/fphar.2021.734091
Dupuy O, Douzi W, Theurot D et al (2018) An evidence-based approach for choosing post-exercise recovery techniques to reduce markers of muscle damage, soreness, fatigue, and inflammation: a systematic review with meta-analysis. Front Physiol 9:403. https://doi.org/10.3389/fphys.2018.00403
Peterson RA, König C, Zimmermann K et al (2020) Effects of lactate on one class of group III (CT3) muscle afferents. Front Cell Neurosci 14:215. https://doi.org/10.3389/fncel.2020.00215
Amann M, Wan HY, Thurston TS et al (2020) On the influence of group III/IV muscle afferent feedback on endurance exercise performance. Exerc Sport Sci Rev 48:209–216. https://doi.org/10.1249/JES.0000000000000233
Sonkodi B (2022) Should we void lactate in the pathophysiology of delayed onset muscle soreness? Not so fast! Let’s see a neurocentric view! Metabolites 12:857. https://doi.org/10.3390/metabo12090857
Wilke J, Behringer M (2021) Is “delayed onset muscle soreness” a false friend? The potential implication of the fascial connective tissue in post-exercise discomfort. Int J Mol Sci 22:9482. https://doi.org/10.3390/ijms22179482
Krause F, Wilke J, Niederer D et al (2019) Acute effects of foam rolling on passive stiffness, stretch sensation and fascial sliding: a randomized controlled trial. Hum Mov Sci 67:102514. https://doi.org/10.1016/j.humov.2019.102514
Zügel M, Maganaris CN, Wilke J et al (2018) Fascial tissue research in sports medicine: from molecules to tissue adaptation, injury and diagnostics: consensus statement. Br J Sports Med. 52:1497. https://doi.org/10.1136/bjsports-2018-099308
Langevin HM, Sherman KJ (2007) Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses 68:74–80. https://doi.org/10.1016/j.mehy.2006.06.033
Berthier C, Blaineau S (1997) Supramolecular organization of the subsarcolemmal cytoskeleton of adult skeletal muscle fibers. A review. Biol Cell 89:413–434. https://doi.org/10.1016/s0248-4900(97)89313-6
Patel TJ, Lieber RL (1997) Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev 25:321–363
Wilke J, Vogt L, Pfarr T et al (2018) Reliability and validity of a semi-electronic tissue compliance meter to assess muscle stiffness. J Back Musculoskelet Rehabil 31:991–997. https://doi.org/10.3233/BMR-170871
Tenberg S, Nosaka K, Wilke J (2022) The relationship between acute exercise-induced changes in extramuscular connective tissue thickness and delayed onset muscle soreness in healthy participants: a randomized controlled crossover trial. Sports Med Open 8:57. https://doi.org/10.1186/s40798-022-00446-7
Suarez-Rodriguez V, Fede C, Pirri C et al (2022) Fascial innervation: a systematic review of the literature. Int J Mol Sci 23:5674. https://doi.org/10.3390/ijms23105674
Mense S (2019) Innervation of the thoracolumbar fascia. Eur J Transl Myol 29:8297. https://doi.org/10.4081/ejtm.2019.8297
Cozacov R, Minerbi A, Haddad M et al (2022) Differential sensitization of muscle versus fascia in individuals with low back pain. Bioengineering (Basel) 9:440. https://doi.org/10.3390/bioengineering9090440
Lau WY, Blazevich AJ, Newton MJ et al (2015) Changes in electrical pain threshold of fascia and muscle after initial and secondary bouts of elbow flexor eccentric exercise. Eur J Appl Physiol 115:959–968. https://doi.org/10.1007/s00421-014-3077-5
Stecco C, Stern R, Porzionato A et al (2011) Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat 33:891–896. https://doi.org/10.1007/s00276-011-0876-9
Schilder A, Hoheisel U, Magerl W et al (2014) Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 155:222–231. https://doi.org/10.1016/j.pain.2013.09.025
Ryskalin L, Morucci G, Natale G et al (2022) Molecular mechanisms underlying the pain-relieving effects of extracorporeal shock wave therapy: a focus on fascia nociceptors. Life (Basel) 12:743. https://doi.org/10.3390/life12050743
Schilder A, Magerl W, Hoheisel U et al (2016) Electrical high-frequency stimulation of the human thoracolumbar fascia evokes long-term potentiation-like pain amplification. Pain 157:2309–2317. https://doi.org/10.1097/j.pain.0000000000000649
Finnoff JT, Hall MM, Adams E et al (2015) American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med 49:145–150. https://doi.org/10.1136/bjsports-2014-094219
Patten C, Meyer RA, Fleckenstein JL (2003) T2 mapping of muscle. Semin Musculoskelet Radiol 7:297–305. https://doi.org/10.1055/s-2004-815677
Shellock FG, Fukunaga T, Mink JH et al (1991) Exertional muscle injury: evaluation of concentric versus eccentric actions with serial MR imaging. Radiology 179:659–664. https://doi.org/10.1148/radiology.179.3.2027970
Fleckenstein JL (2000) MRI of neuromuscular disease: the basics. Semin Musculoskelet Radiol 4:393–419. https://doi.org/10.1055/s-2000-13162
Fu C, Xia Y, Meng F et al (2020) MRI quantitative analysis of eccentric exercise-induced skeletal muscle injury in rats. Acad Radiol 27:e72–e79. https://doi.org/10.1016/j.acra.2019.05.011
Gao Y, Lu Z, Lyu X et al (2021) A longitudinal study of T2 mapping combined with diffusion tensor imaging to quantitatively evaluate tissue repair of rat skeletal muscle after frostbite. Front Physiol 11:597638. https://doi.org/10.3389/fphys.2020.597638
Kane D, Grassi W, Sturrock R et al (2004) A brief history of musculoskeletal ultrasound: ‘from bats and ships to babies and hips.’ Rheumatology (Oxford) 43:931–933. https://doi.org/10.1093/rheumatology/keh004
Jeong WK, Lim HK, Lee HK et al (2014) Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography 33:149–160. https://doi.org/10.14366/usg.14003
Ryu J, Jeong WK (2017) Current status of musculoskeletal application of shear wave elastography. Ultrasonography 36:185–197. https://doi.org/10.14366/usg.16053
Davis LC, Baumer TG, Bey MJ et al (2019) Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 38:2–12. https://doi.org/10.14366/usg.18039
Liu J, Qian Z, Wang K et al (2019) Non-invasive quantitative assessment of muscle force based on ultrasonic shear wave elastography. Ultrasound Med Biol 45:440–451. https://doi.org/10.1016/j.ultrasmedbio.2018.07.009
Kellermann M, Heiss R, Swoboda B et al (2017) Intramuscular perfusion response in delayed onset muscle soreness (DOMS): a quantitative analysis with contrast-enhanced ultrasound (CEUS). Int J Sports Med 38:833–841. https://doi.org/10.1055/s-0043-112501
Hotfiel T, Kellermann M, Swoboda B et al (2018) Application of acoustic radiation force impulse elastography in imaging of delayed onset muscle soreness: a comparative analysis with 3T MRI. J Sport Rehabil 27:348–356. https://doi.org/10.1123/jsr.2017-0003
Taljanovic MS, Gimber LH, Becker GW et al (2017) Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics 37:855–870. https://doi.org/10.1148/rg.2017160116
Klauser AS, Miyamoto H, Bellmann-Weiler R et al (2014) Sonoelastography: musculoskeletal applications. Radiology 272:622–633. https://doi.org/10.1148/radiol.14121765
Koch AJ, Pereira R, Machado M et al (2014) The creatine kinase response to resistance exercise. J Musculoskelet Neuronal Interact 14:68–77
Damas F, Nosaka K, Libardi CA et al (2016) Susceptibility to exercise-induced muscle damage: a cluster analysis with a large sample. Int J Sports Med 37:633–640. https://doi.org/10.1055/s-0042-100281
Morawetz D, Blank C, Koller A et al (2020) Sex-related differences after a single bout of maximal eccentric exercise in response to acute effects: a systematic review and meta-analysis. J Strength Cond Res 34:2697–2707. https://doi.org/10.1519/JSC.0000000000002867
Huang YL, Zhou JL, Jiang YM et al (2020) Assessment of lumbar paraspinal muscle activation using fMRI BOLD imaging and T2 mapping. Quant Imaging Med Surg 10:106–115. https://doi.org/10.21037/qims.2019.10.20
Cheung K, Hume P, Maxwell L et al (2003) Delayed onset muscle soreness : treatment strategies and performance factors. Sports Med 33:145–164. https://doi.org/10.2165/00007256-200333020-00005
Mukaimoto T, Semba S, Inoue Y et al (2014) Changes in transverse relaxation time of quadriceps femoris muscles after active recovery exercises with different intensities. J Sports Sci 32:766–775. https://doi.org/10.1080/02640414.2013.855803
Wroblewski AP, Amati F, Smiley MA et al (2011) Chronic exercise preserves lean muscle mass in masters athletes. Phys Sportsmed 39:172–178. https://doi.org/10.3810/psm.2011.09.1933
Fleckenstein JL, Weatherall PT, Bertocci LA et al (1991) Locomotor system assessment by muscle magnetic resonance imaging. Magn Reson Q 7:79–103
Fullerton GD, Potter JL, Dornbluth NC et al (1982) NMR relaxation of protons in tissues and other macromolecular water solutions. Magn Reson Imaging 1:209–226. https://doi.org/10.1016/0730-725x(82)90172-2
Wilke J, Schwiete C, Behringer M et al (2022) Effects of maximal eccentric exercise on deep fascia stiffness of the knee flexors: a pilot study using shear-wave elastography. J Sports Sci Med 21:419–425. https://doi.org/10.52082/jssm.2022.419
Mridha M, Odman S (1985) Characterization of subcutaneous edema by mechanical impedance measurements. J Invest Dermatol 85:575–578. https://doi.org/10.1111/1523-1747.ep12283588
Vexler A, Polyansky I, Gorodetsky R et al (1999) Evaluation of skin viscoelasticity and anisotropy by measurement of speed of shear wave propagation with viscoelasticity skin analyzer. J Invest Dermatol 113:732–739. https://doi.org/10.1046/j.1523-1747.1999.00751.x
Wilke J, Hespanhol L, Behrens M et al (2019) Is it all about the fascia? A systematic review and meta-analysis of the prevalence of extramuscular connective tissue lesions in muscle strain injury. Orthop J Sports Med 7:2325967119888500. https://doi.org/10.1177/2325967119888500
Pamuk U, Cankaya AO, Yucesoy CA et al (2020) Principles of the mechanism for epimuscular myofascial loads leading to non-uniform strain distributions along muscle fiber direction: finite element modeling. Front Physiol 11:789. https://doi.org/10.3389/fphys.2020.00789
Agten CA, Buck FM, Dyer L et al (2017) Delayed-onset muscle soreness: temporal assessment with quantitative MRI and shear-wave ultrasound elastography. AJR Am J Roentgenol 208:402–412. https://doi.org/10.2214/AJR.16.16617
Niitsu M, Michizaki A, Endo A et al (2011) Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol 52:99–105. https://doi.org/10.1258/ar.2010.100190
Nosaka K, Newton M, Sacco P et al (2002) Muscle damage and soreness after endurance exercise of the elbow flexors. Med Sci Sports Exerc 34:920–927. https://doi.org/10.1097/00005768-200206000-00003
LaRoche DP, Connolly DA (2006) Effects of stretching on passive muscle tension and response to eccentric exercise. Am J Sports Med 34:1000–1007. https://doi.org/10.1177/0363546505284238
Trecroci A, Perri E, Lombardi G et al (2021) Perceptual and biochemical responses in relation to different match-day +2 training interventions in soccer players. Front Physiol 12:685804. https://doi.org/10.3389/fphys.2021.685804
Acknowledgements
To all the participants, administrative staff, and support teams at China Medical University and Xiamen University, we acknowledge, and thank, your hard work, dedication, and valuable input.
Funding
The authors acknowledge support by the National Natural Science Foundation of China (grant number 81271538); Basic Scientific Research Project, Department of Education of Liaoning province (No. LJKMZ20221163); and from the Natural Science Foundation of Xiamen, China (No. 3502Z20227353).
The design, manuscript writing, and language editing of this study were funded by the National Natural Science Foundation of China (grant number 81271538). The collection, analysis, and interpretation of data were funded by the Basic Scientific Research Project, Department of Education of Liaoning province (No. LJKMZ20221163), and the Natural Science Foundation of Xiamen, China (No. 3502Z20227353).
Author information
Authors and Affiliations
Contributions
Congcong Fu and Shinong Pan conceived the idea for the article; Congcong Fu and Yu Xia performed the literature search and the data analysis; Bingshan Wang collected the magnetic resonance imaging date; Yu Xia collected the ultrasonic data; Congcong Fu, Yu Xia, Qiang Zeng, and Shinong Pan drafted and critically revised the work. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Research Committee of Zhongshan Hospital of XiaMen University (Ethical Approval, Protocol number: 2022–193). All participants signed an informed consent form prior to their participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, C., Xia, Y., Wang, B. et al. MRI T2 mapping and shear wave elastography for identifying main pain generator in delayed-onset muscle soreness: muscle or fascia?. Insights Imaging 15, 67 (2024). https://doi.org/10.1186/s13244-024-01619-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01619-6