Abstract
Objectives
The purpose of this study is to assess the equivalency of MRI-based synthetic CT (sCT) to conventional CT for sacroiliac joint bony morphology assessment in children.
Methods
A prospective study was performed. Children who had (PET-)CT-scan underwent additional MRI. sCT-CT image quality was analyzed by two readers subjectively overall, semi-quantitatively in terms of cortical delineation, joint facet defects, growth plate fusion, ossified nuclei, lumbosacral transitional anomaly, and bony bridges, and quantitatively for disc space height, spinal canal width, and sacral vertebrae width and height. Cohen’s kappa and equivalence analyses with Bland–Altman plots were calculated for categorical and continuous measures respectively.
Results
Ten patients were included (6 boys; aged 9–16 years; mean age 14 years). Overall sCT image quality was rated good. Semi-quantitative assessment of cortical delineation of sacroiliac joints, bony bridges, and joint facet defects on the right iliac and sacral sides showed perfect agreement. Correlation was good to excellent (kappa 0.615–1) for the presence of lumbosacral transitional anomaly, fusion of sacral growth plates, joint facet defect, and presence of ossified nuclei. sCT-CT measurements were statistically equivalent and within the equivalence margins (–1–1 mm) for intervertebral disc space height and spinal canal width.
Intra- and inter-reader reliability was excellent for quantitative assessment (0.806 < ICC < 0.998). For categorical scoring, kappa ranged from substantial to excellent (0.615–1).
Conclusion
sCT appears to be visually equivalent to CT for the assessment of pediatric sacroiliac joints. sCT may aid in visualizing sacroiliac joints compared to conventional MRI, with the benefit that no ionizing radiation is used, especially important in children.
Critical relevance statement
MRI-based synthetic CT, a new technique that generates CT-like images without ionizing radiation, appears to be visually equivalent to CT for assessment of normal pediatric sacroiliac joints and can potentially assess structural damage as it clearly depicts bony cortex.
Key points
• MRI-based sCT is a new image technique that can generate CT-like images.
• We found that sCT performs similarly to CT in displaying bony structures of pediatric sacroiliac joints.
• sCT has already been clinically validated in the sacroiliac joints in adults.
• sCT can potentially assess structural damage from erosions or ankylosis as it clearly depicts bony cortex.
Graphical Abstract

Similar content being viewed by others
Introduction
Imaging of the sacroiliac (SI) joints in children is increasingly used for diagnosis and classification of juvenile spondyloarthritis (JSpA) [1]. Early diagnosis of sacroiliitis in children with JspA is important for therapy, especially with new biologic treatment options now available to delay progression and treat sacroiliitis in JspA [2, 3]. Historically the diagnosis of sacroiliitis was based on radiography. Radiography is not sensitive in early-stage disease [4] and has been replaced by MRI as the first line of investigation. MRI is excellent to detect active lesions of sacroiliitis and can also assess structural damage [2]. T1-weighted MRI images are the standard in evaluating structural lesions of sacroiliitis [5]. However, structural lesions including erosions, can be difficult to reliably assess on routine T1-weighted MRI scans in children [6]. The SI joint in adults is delineated as a sharply defined low signal black line on T1-weighted MRI images, which is rarely the case in children. Normal growth-related variations such as the absence of this black line representing the subchondral bone plate, blurring, and irregularity can mimic erosions, making this diagnosis more difficult in children [6, 7]. New ways to visualize and assess the bony structures of the SI joints on MRI would be helpful.
In most parts of the body, computed tomography (CT) is the method of choice to demonstrate bony anatomy [8]. In adults, CT has proven to be superior to MRI for assessing bone sclerosis and ankylosis in sacroiliitis [8]. Unfortunately, single-energy CT is not able to detect active lesions of sacroiliitis, which are key to diagnosing sacroiliitis according to the Assessment of SpondyloArthritis International Society (ASAS) criteria [9, 10]. CT can be used as a standard in adults in the evaluation of chronic sacroiliitis [1, 5, 11,12,13], but it cannot routinely be used in children due to radiation exposure [2, 8].
Synthetic CT (sCT), a new MRI technique, is a deep learning-based technology performing a 3-dimensional MRI-to-CT mapping, which is learned from paired MRI and CT data, generating ‘CT-like’ images without ionizing radiation. The sCT is generated from an axial 3D T1-weighted radiofrequency spoiled multiple gradient-echo sequence (T1MGE) [14]. This technology has previously been clinically validated for the SI joints in adults but has not yet been validated for use in children [9, 14, 15].
The purpose of this study is to assess the equivalence of MRI-based sCT to conventional CT in normal SI joints in children in a semi-quantitative and quantitative assessment of bony morphology.
Materials and methods
This prospective study was approved by the local ethics committee and written informed consent was obtained from all patients and their parents. Authors without conflicts of interest had full control of the inclusion of any data and information submitted for publication. None of the data from study participants has been reported previously.
Study patients
From May 2021 until June 2022, patients aged 6 to 18 years who underwent clinical CT of the pelvis or abdomen or whole-body PET-CT for any reason except for lower back pain, were asked to participate in our study. Patients admitted to the intensive care unit, very ill patients, patients who needed sedation for MRI, and immunocompromised patients were excluded. An additional MRI scan was done within approximately 1 month after the CT or PET-CT scan.
CT protocol
CT and PET-CT images were acquired during clinical practice. CT scans were performed on Somatom Definition Edge Siemens Healthineers and Somatom Definition FLASH Siemens Healthineers. PET-CT was performed on Siemens Biograph mCT flow 20 PET/CT scanner (Siemens Healthcare, Erlangen, Germany) and GE Discovery MI 3ring scanner (GE Healthcare, Waukesha, WI, USA).
MRI protocol
All MRI scans were performed on a 3.0-Tesla MRI unit (Prisma, Siemens Healthineers, Erlangen, Germany). An axial 3-dimensional T1-weighted radio-frequency-spoiled multiple gradient echo (3DT1MGE) sequence was performed: 2 echoes: repetition time/echo time 1/echo time 2: 7/2/3.5 ms, field of view 360 × 360 mm, acquisition matrix: 352 × 352, voxel size: 0.5 × 0.5 × 0.8 mm, acquisition time: 5 min 12 s.
Synthetic CT reconstruction
sCT images were reconstructed with a commercially available software (BoneMRI Pelvic Region, version 1.4, MRIguidance BV). The software runs on-site and is connected to the hospital picture and archiving and communication system (PACS). The PACS automatically forwards the source MRI images to the sCT software, which reconstructs sCT images with a processing time of around 30 min. No manual input is required. The software reconstructed sCT images from two 3DT1MGE images derived from two different echoes using a deep learning method based on the U-net architecture. This method exploits local spatial contextual information in the multi-echo data to reconstruct the latent bone structures, which was learned using paired MRI and CT data. The resulting sCT image expresses radiodensity contrast in Hounsfield units (HU) values [14].
Analysis/image assessment/definitions
CT and sCT were reconstructed in a paracoronal plane (parallel to the dorsal cortex of the S2 vertebral body) a true axial and a true sagittal plane, all with a slice thickness of 1 mm. Two radiologists (N.H. and E.S.) with 18 and 10 years of experience respectively, independently reviewed sCT and CT images. For the measurements, sCT and CT images were mixed and displayed in random order. Readers were blinded to clinical and demographic findings. Definitions were first defined in consensus on 5 other sCT and 5 other CT scans of pediatric patients, not included in the study because they did not have paired data.
Overall image quality, presence of lumbosacral transitional anomaly, and fusion of sacral vertebral growth plates at levels S1/S2 and S2/S3 were scored. Cortical delineation of the joint space at both iliac and sacral sides, presence of joint facet defects, ossified nuclei, and bony bridges were scored (Table 1). Quantitative analysis was performed by measuring the maximal diagonal width and height of the S1 and S2 vertebral bodies and the maximum height of the disc space at levels L5–S1, both on a midsagittal plane. The maximum width of the spinal canal at levels L5–S1 was measured in the axial plane (Table 2). Prior to quantitative analysis, the two readers reached a consensus on measurement methodology.
In order to investigate the intra-reader reliability, 1 reader (E.S.) repeated the measurements 1 month later (a delay added to avoid recall bias).
Statistical analysis
An agreement between sCT and CT for the categorical measures was determined by Cohen’s kappa. Confusion matrices were generated for CT as the gold standard. For the continuous measures, differences between methods were analyzed with Bland–Altman plots and equivalence analyses. An equivalency design was chosen to prove whether the outcomes did not differ by more than a clinically or scientifically meaningful threshold [16]. For the equivalence analyses, the equivalence margin was set to 1 mm, which represents 1 voxel of a typical high-resolution CT scan.
Inter- and intra-reader reliability
For the categorical measures, Cohen’s kappa was calculated, and confusion matrices were constructed. For the continuous measures, inter- and intra-rater reliability measures were obtained by calculating the intra-class correlation coefficient (ICC), using a two-way and one-way random model, respectively, for absolute agreement and a single measure.
All statistical analyses were performed using R 4.2.2.
Results
In all patients, MRI was obtained within a maximum period of just over one month (range 5–33 days) after performing clinical CT or PET-CT scan. Six boys and 4 girls were included aged between 9 and 16 years (mean age 14 years), resulting in a total of 20 SI joints. There was no reported SI joint pathology in these patients.
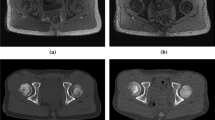
In addition to the features that were scored semi-quantitatively and quantitatively, some obvious other incidental findings were depicted on CT as well as on sCT, such as spina bifida occulta (2 cases), ossification centers of the apophysis of the iliac crest (4 cases) and an enostoma (1 case) (Fig. 1).
Some incidental findings were seen on both MRI-based synthetic CT (sCT) (left images a, c, and e) and CT (right images b, d, and f). a–b Spina bifida occulta of S1 (arrowhead) in a 15-year-old girl in an axial plane. Also note the ossification centers of the apophysis of the iliac crest that can be seen as well (arrows). c–d Lumbosacral transitional anomaly without bony fusion on the left side (asterisk) in a 12-year-old boy in a paracoronal plane. Also note the white line in the right SI joint (black arrowhead), consistent with an artifact on sCT (this was not seen on CT). e–f Enostoma in a 16-year-old female on the left side of S1 in a paracoronal plane (black arrows)
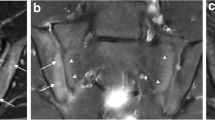
One patient had a joint facet defect meeting the criterion of ≥ 3 mm, seen on both sCT and CT (Fig. 2). There were multiple irregularities seen on sCT and CT on the iliac and sacral side of the SI joint that did not meet the criterion of ≥ 3 mm (Fig. 3).
Comparison between MRI-based synthetic CT (sCT) (a, c) and CT (b, d) in a 15-year-old girl in a paracoronal plane. Arrows indicate ossified nuclei. Also note the irregularities seen on sCT and CT (arrowheads) on the iliac side of the sacroiliac (SI) joints, these did not meet the criterion of ≥ 3 mm for joint facet defect
Semi-quantitative assessment
Excellent image quality was scored on all sCT and CT images except for 1 sCT that was scored as good due to motion artifacts.
Agreement on the presence of features in categorical scoring was high. Kappa ranged from substantial (0.672) to excellent (1) (median = 0.672) [17], except for bony bridges on the left side, (kappa = 0. Table 3). However, this is due to low variability and small sample size, as the methods agreed on 9 out of 10 times for this measure (Table S1) [17]. Because of a lack of variability, we were unable to calculate a kappa for 8 measures. For these measures, there was a 100% agreement between sCT and CT.
Quantitative assessment
In the Bland–Altman plots the differences between sCT and CT were plotted against the mean score for each measurement, showing that the deviations were independent of the size of the measurements (Fig. S1). For the 6 continuous measures, we could not find statistically significant differences in means in this sample. sCT was equivalent to CT for the geometric measurement of intervertebral disc space height and maximum spinal canal width. The measurements of S1 and S2 vertebral bodies were not within the equivalency margin of 1 mm. The equivalency plot of sCT to CT is shown in Fig. 4, indicating equivalency when the entire confidential interval (CI) is within the two margins (–1–1 mm). For the 6 continuous measures, equivalence was calculated (in mm): for diagonal width S1 (− 1.7–0.62), diagonal height S1 (− 1.13–0.21), diagonal width S2 (− 1.75–0.23), diagonal height S2 (− 1.16–1.18), intervertebral disc space L5–S1 (− 0.25–0.65), and spinal canal width (− 0.74–0.38).
Equivalency plot of synthetic CT (sCT) to CT depicting the outcome difference in the geometrical measurements on sCT and CT. The plot indicates the equivalency of sCT to CT when the entire confidential interval (CI) is within the two margins (− 1 and 1 corresponding with an equivalency margin of − 1 mm and 1 mm, respectively). Shaded area, equivalency range; L, lumbar; S, sacral
Inter- and intra-reader reliability
Semi-quantitative assessment
Inter- and intra-reader agreement on categorical scoring was generally high. Kappa ranged from substantial (0.615) to excellent (1), except for inter-reader reliability for bony bridges of the right SI joint and cortical delineation of the joint space of the left sacral side on sCT (both 0, again due to low variability and small sample size, as the readers agreed in 9 out of 10; Table 4). One reader scored cortical delineation as insufficient one-time due to motion artifacts on sCT. Because of a lack of variability, we were unable to calculate a kappa for inter-reader reliability in 15 measures and for intra-reader reliability in 16 measures (Table 4). For all these measures there was a 100% agreement between the readers.
Quantitative assessment
ICCs were excellent for inter- and intra-reader reliability for all quantitative measurements [18]. The overall inter-reader ICC was 0.945 to 0.996 on sCT and 0.909 to 0.994 on CT. The intra-reader ICC was 0.806 to 0.998 on sCT and 0.946 to 0.955 on CT. In Table 5, the ICCs obtained for the combined measurements for the intra- and inter-reader reliability are displayed.
Discussion
This prospective study was performed to test the equivalency of MRI-based sCT images with conventional CT images of (normal) SI joints in children. The sCT images were generated from the 3DT1MGE sequence using a deep learning-based method aiming at specific visualization of the osseous morphology by HU estimation [14].
We found that overall image quality was good for all bony structures on sCT and CT. Osseous morphology was correctly visualized on sCT for detection and visualization of lumbosacral transitional anomalies, fusion of sacral growth plates, cortical delineation of the SI joint, presence of ossified nuclei of the SI joint, and joint facet defects of the SI joint. Cortical delineation of the joint space was equally well seen on sCT and CT.
Subjectively, in some cases, the sCT images even demonstrated sharper cortical delineation than the CT and PET-CT images (Fig. S2). This made ossified nuclei and growth plates better visualized and more sharply delineated on sCT than on CT (Figs. 3, 4, and 5), explaining partly the variability in measurement of S1 and S2 vertebral bodies between sCT and CT. Suboptimal CT scan images may be due to the use of low-dose protocols conforming to the ALARA principle (as low as reasonably achievable), in which the lowest possible radiation dose to achieve the clinical diagnosis is used, which does not necessarily provide excellent images for evaluation of bony structures [19, 20]. This may explain why measurements of S1 and S2 vertebral bodies were not within the 1 mm equivalency margin, especially the endplate of the S2 vertebral body which was sometimes difficult to see on a midsagittal plane.
In this study, we set a very strict 1-mm equivalence margin; all measures would have been within an equivalence margin of 2 mm.
The ongoing ossification process in children can result in unsharp cortical margins, making cortical assessment difficult. Multiple irregularities of the SI joints were seen in our study that did not meet the prespecified size definition of ≥ 3 mm (Fig. 3). Only 1 cortical irregularity met the definition and was observed on sCT as well as on CT by both readers (Fig. 2).
This is the first study using MRI-based sCT in children. In adults, several studies were published comparing sCT and CT in the SI joints, spine, and pelvis [9, 15, 21,22,23,24,25,26]. In a study of the lumbar spine and hips, geometric analysis of MRI-based sCT showed similar measurements in comparison to CT [15, 25]. Jans et al. showed that the diagnostic accuracy of sCT was higher than that of T1-weighted MRI sequences for detecting erosions, sclerosis, and ankylosis in adult patients suspected of having sacroiliitis [9]. Using sCT in children can be of great benefit in the assessment of sacroiliitis as assessing pediatric SI joints is very challenging on MRI, mainly due to normal variability. In children, blurring and irregularity of the SI joint line is frequently seen on classical T1-weighted sequences, making it difficult to detect erosions. sCT could definitely improve the assessment of joint delineation and bone erosions in pediatric SI joints [6]. Cortical delineation of the SI joint was excellent in all but one patient (who had motion artifacts) in our study on both sCT and CT.
sCT has a key advantage in that it can be scanned in one examination together with the classical MRI sequences, allowing assessment of active and structural lesions concomitantly and thus possibly better diagnostic interpretation of sacroiliitis in children with JSpA. Scan time is just 5 min 12 s longer when including the sCT sequence. Importantly, sCT comes with no ionizing radiation, which is essential in the younger population as the radiosensitive reproductive organs are in close proximity to the SI joints. Even though low-dose CT shows promising results in adults with SpA, in pediatric patients it is particularly preferable to perform an examination without ionizing radiation such as sCT [11,12,13].
sCT is also a 3D modality, thus allowing for images to be reconstructed in all planes, which could be especially beneficial to evaluate the rather complex SI joints [21].
Ankylosis and bony bridges of the SI joint are known structural lesions in sacroiliitis [3, 4, 10], however they were not expected in this normal population. In our study, bony bridges were scored in only 1 patient on sCT on the left side, not on CT, resulting in a kappa value of 0 (Table 3) (Table S1). Bony bridge-like features are a known artifact on sCT and a potential pitfall that has already been described in adults when using sCT for the diagnosis of sacroiliitis. Morbée et al. have shown that the vacuum phenomenon can be falsely seen as the bony bridge on sCT in SI joints [21]. In our case too, this rather thin white “artifactual” line crossing the SI joint on sCT (Fig. 1b.) did not mimic true ankylosis as can be seen in sacroiliitis, also no other features of sacroiliitis were present. In our normal study group, we also detected an incidental enostoma (Fig. 1e–f.) near the SI joint, which was equally seen on CT and sCT. This is promising as sCT might be used as well for assessing sclerosis as a structural lesion of sacroiliitis [21].
Our study has some limitations. The main limitation is that we did not image children with sacroiliitis, hence our study is limited to assessing image quality for normal appearances and normal variation. CT scans are not commonly performed in children with sacroiliitis due to radiation dose. Also, CT and MRI did not take place at the same day. The additional MRI scan was obtained within approximately 1 month after the CT scan. Also, different kinds of CT, including PET-CT were used, depending on the clinical question.
Another limitation is the low number of examined patients in this study. CT is in most children replaced by ultrasound or MRI in abdominal and pelvic pathology. Moreover, if an abdominal or pelvic CT was nevertheless performed, critical illness prevented these children from undergoing an additional MRI examination within one month. In this study, the youngest patient was 9 years old. In even younger age groups sedation might even be necessary, which was not approved by the ethics committee.
Future studies in larger groups of children, also including pathological scans of the SI joint, are required to further examine the added value of sCT for assessment of structural lesions (erosions, ankylosis, and sclerosis) of sacroiliitis on sCT in pediatric SI joints in comparison with the classically used MRI sequences and CT as gold standard where available.
Conclusion
By all our measures in a group of normal children, sCT was visually equivalent to CT for assessment of the bony morphology of pediatric sacroiliac joints. In a clinical setting, adding sCT to the MRI protocol may have additional value as it allows for easily visually interpretable visualization of the bony structures of the sacroiliac joints in children without the use of ionizing radiation.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3DT1MGE:
-
3-Dimensional T1-weighted radio-frequency-spoiled multiple gradient echo
- AI:
-
Artificial intelligence
- ALARA:
-
As low as reasonable achievable
- ASAS:
-
Assessment of SpondyloArthritis International Society
- CT:
-
Computer tomography
- HU:
-
Hounsfield units
- ICC:
-
Intra-class correlation coefficient
- JSpA:
-
Juvenile spondyloarthritis
- MRI:
-
Magnetic resonance imaging
- PACS:
-
Picture and archiving and communication system
- PET:
-
Positron emission tomography
- sCT:
-
Synthetic CT
- SI:
-
Sacroiliac
- STIR:
-
Short tau inversion recovery
References
Baraliakos X, Hoffmann F, Deng X, Wang YY, Huang F, Braun J (2019) Detection of erosions in sacroiliac joints of patients with axial spondyloarthritis using the magnetic resonance imaging volumetric interpolated breath-hold examination. J Rheumatol 46:1445–1449
Algin O, Gokalp G, Ocakoglu G (2010) Evaluation of bone cortex and cartilage of spondyloarthropathic sacroiliac joint: efficiency of different fat-saturated MRI sequences (T1-weighted, 3D-FLASH, and 3D-DESS). Acad Radiol 17:1292–1298
Herregods N, Dehoorne J, Joos R et al (2015) Diagnostic value of MRI features of sacroiliitis in juvenile spondyloarthritis. Clin Radiol 70:1428–1438
Jaremko JL, Liu L, Winn NJ, Ellsworth JE, Lambert RGW (2014) Diagnostic utility of magnetic resonance imaging and radiography in juvenile spondyloarthritis: evaluation of the sacroiliac joints in controls and affected subjects. J Rheumatol 41:963–970
Xie R, Sun D, Morelli JN, Yin C, Xiong Y, Li X (2020) Recognition of sacroiliac joint structural lesions: comparison of volumetric interpolated breath-hold examination (VIBE) sequences with different slice thicknesses to T1-weighted turbo-echo. Eur J Radiol 124:108849
Herregods N, Lambert RGW, Schiettecatte E et al (2021) Blurring and irregularity of the subchondral cortex in pediatric sacroiliac joints on T1 images: incidence of normal findings that can mimic erosions. Arthritis Care Res 75:190–197
Zejden A, Jurik AG (2017) Anatomy of the sacroiliac joints in children and adolescents by computed tomography. Ped Rheumatol 15:82
Montandon C, Costa MAB, Carvalho TN, Montadon Junior ME, Teixeira KS (2007) Sacroiliitis: imaging evaluation. Radiol Bras 40(1):53–60
Jans LBO, Chen M, Elewaut D et al (2020) MRI-based Synthetic CT in the Detection of Structural Lesions in Patients with Suspected Sacroiliitis: Comparison with MRI. Radiology 00:1–7
Maksymowych WP, Lambert RGW, Østergaard M et al (2019) MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis 78:1550–1558
Lambert RG, Hermann KGA, Diekhoff T (2021) Low-dose computed tomography for axial spondyloarthritis: update on use and limitations. Curr Opin Rheumatol 33(4):326–332
Diekhoff T, Hermann KGA, Lambert RG (2022) Future of low-dose computed tomography and duel-energy computed tomography in axial spondyloarthritis. Curr Rheumatol Rep 24(6):198–205
Maksymowych WP, Lambert RG (2018) Spondyloarthritis: low-dose CT for spondyloarthritis - a brilliant new chapter? Nat Rev Rheumatol 14(3):130–131
Florkow MC, Zijlstra F, Willemsen K et al (2020) Deep learning-based MR-to-CT synthesis: the influence of varying gradient echo-based MR images as input channels. Magn Reson Med 83(4):1429–1441
Morbée L, Chen M, Van Den Berghe T, et al. (2022) MRI-based synthetic CT of the hip: can it be an alternative to conventional CT in the evaluation of osseous morphology? Eur Radiol 32:3112–3120
Ahn S, Park SH, Lee KH (2013) How to demonstrate similarity by using noninferiority and equivalence statistical testing in radiology research. Radiology 267(2):328–338
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficient for reliability research. J Chiropr Med 15(2):155–163
Miglioretti DL, Johnson E, Willams A et al (2013) The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 167(8):700–707
Slovis TL (2013) Children, computed tomography radiation dose, and the As Low As Reasonably Achievable (ALARA) concept. Pediatrics 112(4):971–972
Morbée L, Vereecke E, Laloo F, Chen M, Herregods N, Jans L (2022) MR imaging of the pelvic bones: the current and cutting-edge techniques. J Belgian Soc Radiol 106(1):123
Florkow MC, Willemsen K, Zijlstra F, et al (2021) MRI‐based synthetic CT shows equivalence to conventional CT for the morphological assessment of the hip joint. J Orthop Res 40(4):954–964
van der Kolk BY, van Stralen M, Podlogar M et al (2019) Reconstruction of osseous structures in MRI scans of the cervical spine with bone MRI: a quantitative analysis. ASNR 57th Annual Meeting, Boston, the USA, May 18–23.
van Stralen M, van der Kolk BY, Zijlstra F, et al (2019) Bone MRI of the cervical spine: deep learning-based radiodensity contrast generation for selective visualization of osseous structures. ISMRM 27th Annual Meeting, Montreal, Canada, 11–16.
Morbée L, Chen M, Herregods N, Pullens P, Jans L (2021) MRI-based synthetic CT of the lumbar spine: geometric measurements for surgery planning in comparison with CT. Eur J Radiol 144:109999
Van der Kolk BYM, Slotman DJ, Nijholt IM et al (2022) Bone visualization of the cervical spine with deep learning-based synthetic CT compared to conventional CT: a single-center noninferiority study on image quality. Eur J Radiol 154:110414
Acknowledgements
Ghent University statistical department (SW) kindly provided statistical advice for this manuscript.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
ES has drafted the work and did the initial analysis and interpretation of the data. EV made substantial contributions for the analysis and interpretation of the data and has substantively revised it. JLJ made substantial contributions for the analysis and interpretation of the data and has substantively revised it. The revision of the English grammar was also provided by this coauthor. LM made substantial contributions to the conception and has substantively revised it. CVW has substantively revised it. LJ made substantial contributions to the conception and design of the work and has substantively revised it. NH made substantial contributions to the conception and design of the work and has substantively revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board approval was obtained (University Hospital Ghent).
Consent for publication
Written informed consent was obtained from all subjects (patients and parents) in this study.
Competing interests
Dr. Jacob L. Jaremko is supported by a Canada CIFAR AI Chair and by Medical Imaging Consultants.
The remaining authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Contingency table for categorical scoring of bony bridges of the SI joint (left side) on sCT and CT. Fig. S1. Bland–Altman plots for maximum diagonal width (left) and height (right) of S1; maximum diagonal width (left) and height (right) of S2; maximum height of the intervertebral disc space L5–S1 and maximum spinal canal width. Fig. S2. Comparison of MRI-based synthetic CT (sCT) (left images a.- c.- e.) and CT (right images b.- d.- f.) in a 15-year-old girl (a.- b.), a 12-year-old boy (c.- d.) and a 15-year-old boy (e.- f.) in a paracoronal plane.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiettecatte, E., Vereecke, E., Jaremko, J.L. et al. MRI-based synthetic CT for assessment of the bony elements of the sacroiliac joints in children. Insights Imaging 15, 53 (2024). https://doi.org/10.1186/s13244-023-01603-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01603-6