Abstract
Objective
To test whether preoperative pain sensitivity is associated with the postoperative axial pain (PAP) in degenerative cervical myelopathy (DCM) and to explore its underlying brain mechanism.
Methods
Clinical data and resting-state fMRI data of 62 DCM patients along with 60 age/gender matched healthy participants were collected and analysed. Voxel-wise amplitude of low frequency fluctuation (ALFF) was computed and compared between DCM patients and healthy controls. Correlation analyses were performed to reveal the association between the clinical metrics and brain alterations. Clinical data and ALFF were also compared between DCM patients with PAP and without PAP.
Results
(1) Relative to healthy participants, DCM patients exhibited significantly lower preoperative pain threshold which is associated with the PAP intensity; (2) Relative to patients without PAP, PAP patients exhibited increased ALFF in mid-cingulate cortex (MCC) and lower preoperative pain threshold; (3) Further, multivariate pattern analysis revealed that MCC ALFF provide additional value for PAP vs. non-PAP classification.
Conclusion
In conclusion, our findings suggest that preoperative pain hypersensitivity may be associated with postoperative axial pain in degenerative cervical myelopathy patients. This finding may inspire new therapeutic ideas for patients with preoperative axial pain.
Key points
-
DCM patients exhibited pain hypersensitivity preoperatively compared to healthy participants.
-
The pain hypersensitivity is associated with postoperative axial pain intensity in DCM.
-
MCC ALFF could be used to predict occurrence of PAP in DCM.
Similar content being viewed by others
Introduction
Degenerative cervical myelopathy (DCM), which characterised by degenerative changes in the cervical spine, is the most common cause of non-traumatic spinal cord injuries in adults, and requires timely surgical decompression to prevent progressive neurological deficits [1,2,3]. Until now, surgery remains the foremost treatment option for patients with DCM, and a corrective surgery at an early stage of DCM may effectively change the unfavorable prognosis for patients [4]. Despite that surgical strategy for DCM has been controversial (e.g., anterior approach or posterior approach), posterior laminoplasty and laminectomy still are the standard treatment for effective decompression of multi-level lesions, and their clinical efficacy remain satisfactory while the surgery-related complications are significantly fewer than anterior approaches [5, 6]. However, a major resulting complication—postoperative axial pain (PAP, i.e., pain from the nuchal to the periscapular region), has been largely overlooked and related factors remained controversial [7, 8]. Currently, there is no effective perioperative management to prevent or reduce this vexing complication and thus needs further investigation for its potential mechanism [8]. Recently Zheng et al. investigated the pressure pain thresholds, temporal summation and conditioned pain modulation in DCM patients and found that preoperative endogenous pain modulation deficiency may be associated with axial pain after posterior decompression surgery indicating preoperative pain hypersensitivity in DCM might contributed to the prevalence of PAP [9]. However, the brain mechanism underlying such phenomenon is still unknown.
In the past decade, resting-state fMRI (rs-fMRI) has been widely applied for investigating neural mechanism of pain. Researchers have highlighted the potential use of rs-fMRI data in interpreting the neuropathology and developing prognostic biomarkers for chronic pain [10,11,12]. Increasing evidence has uncovered structural and functional brain changes in regions associated with pain modulation, and such changes have been associated with pain intensity, disability, and pain sensitivity in patients with chronic pain [13,14,15,16]. In these studies, Amplitude of Low Frequency Fluctuation (ALFF), which is a widely used rs-fMRI metric, has gained much attention for its simplicity, interpretability and replicability among commonly used rs-fMRI metrics [17,18,19]. Moreover, recent studies have shown that ALFF was tightly associated with cerebral blood flow [20, 21] and task-evoked activation [22, 23] and could serve as a biomarker for predicting the analgesic-response in cervical spondylosis patients with chronic neck pain [17]. Therefore, ALFF is ideally suited for investigating PAP in DCM patients, considering the current lack of the knowledge for the underlying brain mechanism. Understanding such mechanism may be beneficial in enabling stratification in the perioperative period of DCM, and developing new analgetic strategy for reduce the PAP in DCM patients.
Therefore, in our current study, we conducted rs-fMRI to test whether preoperative pain sensitivity is associated with PAP in DCM patients and its association with brain alterations measured by ALFF; and to explore the utility of brain imaging markers based on ALFF for predicting the occurrence of PAP in DCM patients.
Materials and methods
Subjects
A local institutional review board approved this study, and all participants signed written informed consents. The detailed inclusion and exclusion criteria can also be found in Additional files (Subjects’ inclusion criteria). A total of 62 DCM patients and 60 Healthy Controls (HC) were recruited between 2015 and 2020.
fMRI data acquisition and preprocessing
The detailed information of data acquisition and preprocessing steps can be found in Additional files (fMRI data acquisition and preprocessing).
Clinical assessment
Preoperative
Each DCM patient was evaluated using the Japanese Orthopaedic Association (JOA) score, which is the most widely used scale for determining the severity of DCM in clinical practice [24]. The Pain Vigilance and Awareness Questionnaire (PVAQ) was assessed in both DCM patients and healthy controls. The PVAQ is ranged from 0 (minimal attention to pain) to 80 with a higher score indicating more attention to pain [25]. Electrical stimulation was used preoperatively to determine the pain threshold in both DCM patients and healthy controls. Electric stimulation (0.2-ms square wave pulse; Digi-timer DS-7A, Hertfordshire, England) of the posterior neck area was then performed using a bipolar probe with the anode placed distally (20 mm inter-electrode distance). This stimulation method reduces the risk of peripheral sensitization and receptor fatigue. Stimulus intensities corresponding to the sensory detection and pain detection thresholds (pricking sensation) were registered using the method of ascending limits in 4 series (the first was discarded). Both DCM patients and healthy controls were instructed to immediately respond verbally when each level was felt. The perception of a pricking sensation is thought to correspond to Aδ-fiber activation. No rating scale was administered because only detection thresholds were assessed. Preoperative neck pain intensity was assessed using a standardised numerical rating scale (NRS) ranging from 0 to 100 (10 = warm (no pain); 20 = threshold pain; 100 = intolerable pain). The patients were instructed to rate the average intensity of axial neck pain in the last month.
Postoperative
Postoperative neck pain intensity was also assessed using NRS from 0 to 100 at the 1-year follow-up telephonically. The patients were instructed to rate the average intensity of axial neck pain in the last month.
ALFF calculation
For the ALFF analysis, a fast-Fourier transform was performed to convert the time series to the frequency domain. Subsequently, the square root of the power spectrum was calculated and averaged across 0.01–0.08 Hz to obtain the ALFF, and the resultant ALFF values were subsequently Z-scored. Therefore, we used zALFF in our current analyses.
Analysis 1: Clinical data
We first Pearson correlation was performed to identify pairwise relationship(s) between all measured clinical features. Second, two-sample t tests were performed to reveal the differences in PVAQ score and pain threshold between DCM patient and HC, two-sample t tests were performed. Further, despite JOA score and preoperative/postoperative pain intensity were not investigated in healthy participants, the mean ± SD for both metrics were also illustrated. Third, we divided the DCM patients into postoperative axial pain (PAP) group and non-postoperative axial pain (nPAP) group based on the postoperative axial pain intensity using a cut-off value of 4 or more for NRS same as previous reports (Patients with postoperative pain intensity > 4 were included in PAP group) [7]. Furthermore, to rule out the possible confound of differences in severity of myelopathy, we also optimally match the JOA score between two group to avoid the possibility that differences we observed between nPAP and PAP group were due to the difference in severity of myelopathy using following procedures: (1) One target PAP patient was randomly selected, and the absolute differences for this target patient’s JOA score and the rest of the nPAP group were calculated; (2) This target patient was then matched with a patient whose JOA score was the closest to the target patient. If there were several nPAP patients whose JOA scores were the same as the target patient, one nPAP patient was then randomly selected; (3) These procedures were repeated until all PAP patients were paired. The un-paired nPAP patients were excluded for further analyses. Paired-t tests were performed to reveal the differences in clinical metrics (e.g., JOA score, PVAQ score, Pain threshold, Preoperative and postoperative pain intensity) between PAP group and nPAP group.
Analysis 2: ALFF differences between DCM patients and HC
To reveal the differences in ALFF between DCM patients and healthy controls,voxel-wise two-sample t test was performed within a grey matter mask using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) to explore the ALFF differences between DCM patients and healthy controls with age, gender, education years as covariates. Voxel-level p value ≤ 0.001 (significance threshold) was corrected for multiple comparisons using family-wise error correction at the cluster level, resulting in a corrected p ≤ 0.05. Subsequently, the resultant clusters were selected as masks to extract the mean ALFF for each cluster in DCM patients. Correlation analyses were performed to detect the association between ALFF alterations and clinical measurements in DCM patients, and Bonferroni correction was performed for multiple comparison correction.
Analysis 3: ALFF differences between PAP and nPAP DCM patients
To investigate the possible neural mechanism for postoperative neck pain following posterior decompression surgery. Therefore, voxel-wise paired-t test was performed within a grey matter mask using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) to explore the ALFF differences between PAP group and nPAP group (i.e., same as analysis 1) with age, gender, education years as covariates. Voxel-level p value ≤ 0.001 (significance threshold) was corrected for multiple comparisons using family-wise error correction at the cluster level, resulting in a corrected p ≤ 0.05.
Analysis 4: Multi-variate classification—PAP vs. nPAP
To further test the utility of the ALFF (i.e., the mean within clusters obtained in analysis 3) for identifying PAP patients from nPAP patient, multi-variate pattern analysis (MVPA) was performed via support vector machine (SVM) using both clinical metrics and ALFF as features. Classification accuracy was assessed by a leave-one-out cross-validation procedure (LOOCV). The detailed procedure of LOOCV can be found in Additional files (Leave-one-out-cross-validation procedure).
A control analysis was also performed using only clinical metrics as features for PAP vs. nPAP classification. In this way, we can investigate whether ALFF could provide additional information for predicting the occurrence of postoperative neck pain in DCM patients. LOOCV and permutation test were also performed using the same procedures as described above. Furthermore, to test whether these two classification accuracies (e.g., using both clinical metrics and ALFF, using clinical metrics alone) were differ significantly, a permutation test was performed. The detailed procedure of permutation can be found in Additional files (Permutation test).
Validation analysis
To further rule out the influence of head-motion as a potential confound, we conducted a validation analysis for revealing the differences in head-motion between DCM patients and HC; between PAP and nPAP DCM patients. Framewise displacement (FD) values, that quantifiably estimate head motion during scan, were calculated, averaged across all timepoints in all participants, and compared between groups. The FD value was calculated using 3 robust methods, Jenkinson method, Power method, and VanDijk method.
Moreover, to further make sure that any detected differences for ALFF between PAP and nPAP was determined by preoperative pain intensity, we also conducted a validation analysis to reveal the differences in clinical metrics and ALFF between PAP and nPAP by optimally matching the preoperative neck pain intensity between these two groups. The same approach as in analysis 1 and 3 (i.e., the same procedures as matching the JOA score between two groups) was conducted and paired-t tests were performed to reveal the differences in clinical metrics (e.g., JOA score, PVAQ score, Pain threshold) between PAP group and nPAP group with age, gender, education years as covariates. In this way, there would be no significant difference for preoperative pain intensity between two groups, thus the observed ALFF differences between PAP and nPAP group would most likely to be associated with PAP rather than the reflection of the preoperative pain intensity.
Results
Demographic data
The demographic data of all participants are summarised in Table 1. There were no significant inter-group differences with regards to age, gender, or years of education (p ≤ 0.05).
Analysis 1: Relative to healthy controls (HC), degenerative cervical myelopathy (DCM) patients were more sensitive to pain
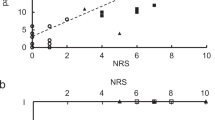
In DCM patients, we observed significant correlation between preoperative pain intensity and Pain Vigilance and Awareness Questionnaire (PVAQ) (R = 0.32, p = 0.005), between postoperative pain intensity and PVAQ score (R = 0.33, p = 0.004), between postoperative pain intensity and preoperative pain intensity (R = 0.54, p < 0.001), between preoperative pain intensity and Pain Threshold (PT) (R = −0.39, p = 0.001), between postoperative pain intensity and PT (R = −0.66, p < 0.001) Fig. 1a. In healthy participants, we observed a significant negative correlation between PVAQ score and PT (R = −0.37, p = 0.002) Fig. 1b. Further, compared with healthy participants, DCM patients exhibited significant decreased Pain Threshold (i.e., more sensitive to pain) Fig. 1c.
Analyses of clinical parameters. a The association among clinical metrics in Degenerative Cervical Myelopathy (DCM) patients; b The association between Pain Threshold (PT) and Pain Vigilance and Awareness Questionnaire (PVAQ) score; c The differences in PT between DCM patients and HC; d The differences in PVAQ between DCM patients and HC; e The mean±SD for Japanese Orthopedic Association (JOA) score, preoperative pain intensity and postoperative pain intensity in DCM patients. NRS: numerical rating scale
To further investigate the factors for postoperative axial pain in DCM patients following posterior decompression surgery, we divided the DCM patients into postoperative axial pain (PAP) group and non-postoperative axial pain (nPAP) group while controlling the effect of JOA score. Relative to nPAP group, PAP group exhibited significantly higher preoperative pain intensity along with lower pain threshold (i.e., more sensitive to pain) Fig. 2.
Analysis 2: Compared to HC, DCM patients exhibited increased ALFF in Middle Cingulate Cortex (MCC) which was positively correlated with postoperative pain intensity and negatively correlated with pain threshold
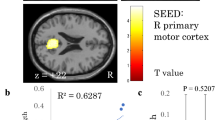
Relative to HC, DCM patients exhibited increased ALFF within left Middle Cingulate Cortex (lMCC) and left Superior Frontal Gyrus (lSFG) Fig. 3a. while decreased ALFF within right primary motor cortex (i.e., precentral gyrus, M1) and left primary visual cortex (i.e., calcarine, V1) Fig. 3b, Table 2, Additional file 1: Figure S1. We also observed a significant positive correlation between postoperative pain intensity and ALFF within MCC (R = 0.62, p < 0.001); a significant negative correlation between pain threshold and ALFF within MCC (R = −0.43, p = 0.005) Fig. 3c, d. No significant association was observed between clinical metrics and brain alteration within other brain regions.
Analysis of Amplitude of Low Frequency Fluctuation (ALFF) alterations and its relationship to clinical metrics in Degenerative Cervical Myelopathy (DCM) patients. a The increased ALFF in DCM patients. lMCC: left Middle Cingulate Cortex; lSFG: left Superior Frontal Gyrus; b The decreased ALFF in DCM patients. rM1: right precentral gyrus; lV1: left calcarine gyrus. c The heat map for illustrating the correlation coefficients between brain alterations and clinical metrics in DCM patients. PT: Pain Threshold; PVAQ: Pain Vigilance and Awareness Questionnaire; JOA: Japanese Orthopedic Association (JOA). d The scatter plot for association between postoperative pain intensity and ALFF, between PT and ALFF within MCC in DCM patients
Analysis 3 and 4: MCC ALFF provide additional value for predicting the prevalence of postoperative axial pain in DCM patients
In analysis 3, univariate paired-t test was performed to compare the ALFF between PAP and nPAP patients while controlling the effect of JOA, we observed that relative to nPAP, PAP group exhibited significant higher ALFF within MCC (T = 4.21, p = 0.0003, Fig. 4a, Table 3). Indicated that higher level of MCC ALFF was associated with a more intense postoperative axial pain in DCM patients. Subsequently, results for analysis 4 showed that the feature set including JOA, preoperative pain intensity, PVAQ, pain threshold along with age, gender, education years could successfully identify DCM patients with postoperative axial pain form patients without postoperative axial pain (Correct rate = 70.4, p = 0.002, Fig. 4b). After including the MCC ALFF to the feature set, the correct rate for PAP vs. nPAP classification increased to 88.9% (p < 0.001, Fig. 4c) and the difference between two model was significant (Difference = 18.8%, p = 0.026, Fig. 4d).
a The paired t test for revealing the Amplitude of Low Frequency Fluctuation (ALFF) differences between postoperative axial pain (PAP) group and no postoperative axial pain (nPAP) group in DCM patients. MCC: Middle Cingulate Gyrus. b The null distribution obtained from permutation test and performance of Support Vector Machine (SVM) model using only clinical metrics for PAP vs. nPAP classification. CR: Correct Rate. c The null distribution obtained from permutation test and performance of SVM model using clinical metrics combined with ALFF (i.e., within MCC) for PAP vs. nPAP classification. d Null distribution obtained from the permutation test to determine whether there is a significant difference between two models
Analysis 5: Validation analyses
No significant difference for FD, which was measured by Jenkinson method, Power method, and VanDijk method, was observed (Additional file 1: Figures S2 and S3). We also found that after controlling the effect of preoperative pain intensity, PAP group still exhibited significant higher level of ALFF along with lower pain threshold (i.e., more sensitive to pain) relative to nPAP group (Fig. 5). These results were in line with our results in analysis 1 and 3, suggesting that the observed differences from analysis 1 and 3 were not affected by the preoperative pain intensity to a large extent.
Discussion
In our current study, three major findings were observed: (1) Relative to healthy participants, Degenerative Cervical Myelopathy (DCM) patients exhibited lower threshold for pain; (2) and altered Middle Cingulate Cortex (MCC) function was associated with pain threshold which is also tightly correlated with the postoperative neck pain intensity; (3) Further, the ALFF of MCC provided additional value for predicting the occurrence of postoperative axial pain via machine learning analysis in DCM patients.
In comparison to healthy participants, DCM patients exhibited lower pain threshold; and patients with postoperative axial pain showed lower pain threshold preoperatively than those without.
In analysis 1, we found that the pain thresholds of DCM patients were significantly lower than healthy participants, and the pain thresholds were also lower in DCM patients with PAP than those without. This finding is in line with previous study conducted by Zhang et.al. in which they conducted quantitative sensory testing and revealed that patients with PAP have a lower pressure pain threshold and temporal summation (i.e., higher sensitivity to pain perception) than patients without PAP. Their findings indicated that preoperative endogenous pain modulation deficiency might be associated with axial pain after posterior cervical decompression [9]. It is not surprising that DCM patients developed abnormal pain modulation system, considering most of the patients experienced chronic pain that is associated with modifications of the central nervous system, such as central sensitization, which is responsible for alterations in pain sensitivity in acute and chronic pain situations [26,27,28]. We also found that preoperative pain threshold was negatively correlated with pre/post-operative pain intensity and preoperative pain intensity was positively correlated with postoperative pain intensity in DCM patients. These findings also supported the idea that DCM patients developed central sensitization following long-term axial pain which further aggravates or induce the postoperative axial pain.
Altered MCC function was associated with preoperative pain threshold and PAP intensity in DCM patients
In analysis 2, we found that relative to healthy participants, DCM patients exhibited significantly higher ALFF within Middle Cingulate Cortex (MCC) and Superior Frontal Gyrus (SFG), and the MCC ALFF were correlated with both preoperative pain threshold and PAP intensity. MCC, which is frequently activated during acute pain, has been shown to be responded specifically to nociceptive input from subcortical brain regions. Additionally, chronic pain also causes grey matter changes in MCC, and such changes overlaps in various chronic pain condition indicating the structural alterations of MCC could well be the biological marker for chronic pain per se [29]. Further, Davis et.al. found that greater heat pain sensitivity (i.e., lower heat pain threshold) correlated with thickening in the mid-cingulate cortex, which indicated MCC is responsible for detecting and processing nociceptive input [30]. From the functional aspect, in addition, the MCC is an important component of the cingulate-insular pathway which gates and maintains nociceptive hypersensitivity in the absence of conditioned noxious stimuli and affects the impact of pain [31]. Taken together, our observed association between pain sensitivity and MCC ALFF support the hypothesis that continuous nociceptive input causes MCC cortical reorganisation which further induces hypersensitivity in chronic pain patients.
Furthermore, we also observed significant altered ALFF within SFG, M1 and V1. These results were in line with previous reports. Kaito et.al. conducted rs-fMRI and found that the ALFF within SFG and V1 were altered in DCM patients [32]. They concluded that these brain alterations were considered as the functional reorganisation following long-term chronic spinal cord injury. We also found that relative to healthy participants, DCM patients exhibited significantly lower ALFF within primary motor cortex (M1). M1, a key region in the sensorimotor network, is involved in a various of motor functions, such as motor planning, inhibition, coordination, movement, and so on [33, 34]. It has been shown that the ALFF within M1 was significantly higher in DCM patients than healthy controls, and was also tightly correlated with the fractional anisotropy value of C2 segment which reflects the severity of myelopathy [33]. Our previous study also illustrated the potential utility of M1 ALFF for predicting the prognosis of DCM patients following decompression surgery [35]. Our current finding was in line with the previous reports, indicating potential cortical reorganisation occurs in DCM [36,37,38,39].
MCC ALFF provides additional value for PAP vs. nPAP classification
Posterior cervical decompression surgery is one of the most widely used surgical approaches, and increasing frequency of PAP after posterior decompression approach seriously affects the daily life of patients. Till now, there is still controversy about the causes of PAP and its related factors. Atsushi et al. showed that anterolithesis, current smoking, preoperative neck pain, etc. are influencing factors of axial pain after laminoplasty [7]. A systematic review summarises possible factors influencing axial pain after posterior surgery, including age, preoperative axial pain, different surgical techniques, and postoperative management [8]. It has been shown that about 40% of patients experienced axial pain after laminoplasty, but it occurred mostly in those who had preoperative axial pain [7, 8, 40]. Although multiple factors have been identified as causal factors in PAP, preoperative neck pain severity is the most commonly reported PAP marker in DCM patients, and our current results also confirmed this. Further, our univariate and multivariate analysis also identified the neural correlates of the PAP, which is associated with the pain sensitivity in DCM patients. It is also worth mentioning that despite we optimally matched the preoperative pain intensity between PAP and nPAP patients, PAP still exhibited significantly higher MCC ALFF than nPNP patients. This result indicated the independent contribution of MCC function in altered pain modulation pathway which related to hypersensitivity in PAP patients. As to the clinical implications of our findings, identifying patients with PAP could aid the clinicians to develop novel perioperative management to reduce or avoid such complication based on hypersensitivity in these patients. Preoperative analgetic use has been proved to be effective in reducing postoperative pain intensity for many other orthopedic surgeries [41,42,43]. Such perioperative preparation could reduce the central sensitization and thus relieve the pain following large trauma.
Limitation
First, the main limitation is that our patients have all received medication treatment including non-steroid-anti-inflammatory drug, etc. This may affect our results to some extent. Therefore, future studies with DCM patients who are not on medication or who have a washout period from medication are needed to confirm our findings. Postoperative fMRI data was not collected due to the possibility of artifact and heating due to surgical implants. Even though it appears to be safe and other studies have collected data on postoperative fMRI data, the majority of our patients declined to cooperate after we informed them of potential harm (e.g., loss of surgical implants) associated with postoperative fMRI. Our current study only analysed ALFF alterations between patients and healthy controls, other resting-state fMRI metrics such as functional connectivity (FC), regional homogeneity (ReHo), functional connectivity strength (FCS), need further study. Socioeconomic status is a crucial factor affecting pain process between individuals, but was not collected and thus its possible association with pain perception could not be investigated in the present study.
Conclusion
In conclusion, our findings suggest that the altered middle cingulate cortex function might be associated with preoperative pain hypersensitivity which aggravates postoperative axial pain in degenerative cervical myelopathy patients. This finding may inspire new therapeutic ideas for patients with preoperative axial pain.
Availability of data and materials
The data and codes used in this study can be availed upon reasonable request.
Abbreviations
- ALFF:
-
Amplitude of Low Frequency Fluctuation
- DCM:
-
Degenerative Cervical Myelopathy
- fMRI:
-
Functional Magnetic Resonance Imaging
- JOA:
-
Japanese Orthopedic Association
- LOOCV:
-
Leave-one-out cross-validation procedure
- PVAQ:
-
Pain Vigilance and Awareness Questionnaire
- PAP:
-
Postoperative Axial Pain
- SVM:
-
Support Vector Machine
References
Iyer A, Azad TD, Tharin S (2016) Cervical spondylotic myelopathy. Clin Spine Surg 29:408–414
Kalsi-Ryan S, Karadimas SK, Fehlings MG (2013) Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19:409–421
Lebl DR, Bono CM (2015) Update on the diagnosis and management of cervical spondylotic myelopathy. J Am Acad Orthop Surg 23:648–660
Li XY, Lu SB, Sun XY et al (2018) Clinical and magnetic resonance imaging predictors of the surgical outcomes of patients with cervical spondylotic myelopathy. Clin Neurol Neurosurg 174:137–143
Badhiwala JH, Ahuja CS, Akbar MA et al (2020) Degenerative cervical myelopathy: update and future directions. Nat Rev Neurol 16:108–124
Milligan J, Ryan K, Fehlings M, Bauman C (2019) Degenerative cervical myelopathy: diagnosis and management in primary care. Can Fam Phys 65:619–624
Kimura A, Shiraishi Y, Inoue H, Endo T, Takeshita K (2018) Predictors of persistent axial neck pain after cervical laminoplasty. Spine (Phila Pa 1976) 43: 10–15.
Wang SJ, Jiang SD, Jiang LS, Dai LY (2011) Axial pain after posterior cervical spine surgery: a systematic review. Eur Spine J 20:185–194
Chen K, Yu J, Nie C et al (2022) Preoperative dynamic quantitative sensory testing in remote pain-free areas is associated with axial pain after posterior cervical spinal surgeries. BMC Musculoskelet Disord 23:409
Fernandez Rojas R, Huang X, Ou KL (2019) A machine learning approach for the identification of a biomarker of human pain using fNIRS. Sci Rep 9:5645
Huang X, Chen J (2021) Impaired frontal-parietal control network in chronic prostatitis/chronic pelvic pain syndrome revealed by graph theoretical analysis: a DTI study. 53:1060–1071
Su Q, Qin W, Yang QQ et al (2019) Brain regions preferentially responding to transient and iso-intense painful or tactile stimuli. Neuroimage 192:52–65
Mouraux A, Iannetti GD (2018) The search for pain biomarkers in the human brain. Brain 141:3290–3307
Seminowicz DA, Moayedi M (2017) The Dorsolateral prefrontal cortex in acute and chronic pain. J Pain 18:1027–1035
Tu Y, Cao J, Bi Y, Hu L (2021) Magnetic resonance imaging for chronic pain: diagnosis, manipulation, and biomarkers. Sci China Life Sci 64:879–896
Zhou W, Jin Y, Meng Q et al (2019) A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci 22:1649–1658
Bai L, Zhang L, Chen Y et al (2022) Middle cingulate cortex function contributes to response to non-steroidal anti-inflammatory drug in cervical spondylosis patients: a preliminary resting-state fMRI study. Neuroradiology
Gu L, Hong S, Jiang J et al (2019) Bidirectional alterations in ALFF across slow-5 and slow-4 frequencies in the brains of postherpetic neuralgia patients. J Pain Res 12:39–47
Zhang B, Jung M, Tu Y et al (2019) Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth 123:e303–e311
Li W, Chen Z, Wu M et al (2017) Characterization of brain blood flow and the amplitude of low-frequency fluctuations in major depressive disorder: a multimodal meta-analysis. J Affect Disord 210:303–311
Wang J, Sun H, Cui B et al (2021) The relationship among glucose metabolism, cerebral blood flow, and functional activity: a hybrid PET/fMRI study. Mol Neurobiol 58:2862–2873
Li Z, Zeng F, Yin T et al (2017) Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. Neuroimage Clin 15:367–375
Tomasi D, Volkow ND (2019) Association between brain activation and functional connectivity. Cereb Cortex 29:1984–1996
Fukui M, Chiba K, Kawakami M et al (2007) Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire: part 3. Determination of reliability. J Orthop Sci 12:321–326
Wong WS, McCracken LM, Fielding R (2011) Factorial validity and reliability of the Chinese version of the Pain Vigilance and Awareness Questionnaire (ChPVAQ) in a sample of Chinese patients with chronic pain. Pain Med 12:1018–1025
Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M (2014) Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 44:68–75
Coppieters I, Cagnie B, De Pauw R, Meeus M, Timmers I (2021) Enhanced amygdala-frontal operculum functional connectivity during rest in women with chronic neck pain: associations with impaired conditioned pain modulation. Neuroimage Clin 30:102638
Yu S, Li W, Shen W et al (2020) Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. Neuroimage 218:116969
Teutsch S, Herken W, Bingel U, Schoell E, May A (2008) Changes in brain gray matter due to repetitive painful stimulation. Neuroimage 42:845–849
Erpelding N, Moayedi M, Davis KD (2012) Cortical thickness correlates of pain and temperature sensitivity. Pain 153:1602–1609
Wei HL, Li J, Guo X et al (2021) Functional connectivity of the visual cortex differentiates anxiety comorbidity from episodic migraineurs without aura. J Headache Pain 22:40
Takenaka S, Kan S, Seymour B et al (2020) Resting-state amplitude of low-frequency fluctuation is a potentially useful prognostic functional biomarker in cervical myelopathy. Clin Orthop Relat Res 478:1667–1680
Aleksanderek I, McGregor SM, Stevens TK, Goncalves S, Bartha R, Duggal N (2017) Cervical spondylotic myelopathy: metabolite changes in the primary motor cortex after surgery. Radiology 282:817–825
Gohmann RF, Blume C, Zvyagintsev M et al (2019) Cervical spondylotic myelopathy: changes of fractional anisotropy in the spinal cord and magnetic resonance spectroscopy of the primary motor cortex in relation to clinical symptoms and their duration. Eur J Radiol 116:55–60
Zhao R, Guo X, Wang Y et al (2022) Functional MRI evidence for primary motor cortex plasticity contributes to the disease’s severity and prognosis of cervical spondylotic myelopathy patients. Eur Radiol 32:3693–3704
Chen Z, Wang Q, Liang M et al (2018) Visual cortex neural activity alteration in cervical spondylotic myelopathy patients: a resting-state fMRI study. Neuroradiology 60:921–932
Chen Z, Zhao R, Wang Q et al (2020) Functional connectivity changes of the visual cortex in the cervical spondylotic myelopathy patients: a resting-state fMRI study. Spine (Phila Pa 1976) 45: E272–E279
Zhao R, Song Y, Guo X et al (2021) Enhanced information flow from cerebellum to secondary visual cortices leads to better surgery outcome in degenerative cervical myelopathy patients: a stochastic dynamic causal modeling study with functional magnetic resonance imaging. Front Hum Neurosci 15:632829
Zhao R, Su Q, Chen Z, Sun H, Liang M, Xue Y (2020) Neural correlates of cognitive dysfunctions in cervical spondylotic myelopathy patients: a resting-state fMRI study. Front Neurol 11:596795
Weinberg DS, Rhee JM (2020) Cervical laminoplasty: indication, technique, complications. J Spine Surg 6:290–301
Carley ME, Chaparro LE, Choinière M et al (2021) Pharmacotherapy for the prevention of chronic pain after surgery in adults: an updated systematic review and meta-analysis. Anesthesiology 135:304–325
Esparza‐Villalpando V, Pozos‐Guillén A, Masuoka‐Ito D, Gaitán‐Fonseca C, Chavarría‐Bolaños D (2018) Analgesic efficacy of preoperative dexketoprofen trometamol: a systematic review and meta-analysis. Drug Dev Res 79:47–57
Straube S, Derry S, McQuay HJ, Moore RA (2005) Effect of preoperative Cox-II-selective NSAIDs (coxibs) on postoperative outcomes: a systematic review of randomized studies. Acta Anaesthesiol Scand 49:601–613
Funding
This study has received Project of integrated traditional Chinese and Western Medicine of Tianjin Health Commission (2021076) and the National Natural Science Foundation of China (82102133).
Author information
Authors and Affiliations
Contributions
RZ designed the study and analysed the data. QS revised the manuscript, analysed the data for revision. JL wrote the manuscript and visualised the results. XC review, edited and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all subjects (patients) in this study before any procedure. Institutional Review Board of local medical centre approved our study.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, Q., Li, J., Chu, X. et al. Preoperative pain hypersensitivity is associated with axial pain after posterior cervical spinal surgeries in degenerative cervical myelopathy patients: a preliminary resting-state fMRI study. Insights Imaging 14, 16 (2023). https://doi.org/10.1186/s13244-022-01332-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-022-01332-2