Abstract
Objectives
Treatment methods of local residual or recurrent hepatocellular carcinoma (HCC) after thermal ablation are limited. Therefore, our study aimed to explore the efficacy and prognostic factors of 125I brachytherapy for local residual or recurrent lesion after thermal ablation.
Methods
A total of 114 patients with 212 local residual or recurrent HCC tumors after thermal ablation underwent 125I brachytherapy. Local progression-free survival (LPFS) and prognostic factors were analyzed by Kaplan–Meier curves and the Cox model.
Results
After a 6-month follow-up, the percentage of patients who achieved complete response (CR), partial response (PR), and stable disease (SD) was 57%, 13.2%, and 5.2%, respectively. The 1-, 2-, and 3-year LPFS rates were 58.7%, 50.0%, and 41.2%, respectively. Portal vein tumor thrombus (PVTT) (p = 0.03), the number of intrahepatic tumors (p = 0.01), and AFP level (p = 0.02) were independent risk factors for local tumor progression (LTP). The median LPFS in patients without PVTT (22 months) was much longer compared to those with PVTT (10 months). The median LPFS in patients with less than three intrahepatic lesions improved from 17 to 24 months. The median LPFS was only 5 months in the high AFP group, but was prolonged with a decrease in AFP level (24 months). No severe complications were recorded. All complications were controllable and treatable.
Conclusions
CT-guided 125I brachytherapy was a safe and effective treatment for patients with local residual or recurrent HCC after thermal ablation to improve local control rate.
Key points
-
125I brachytherapy is a treatment for locally recurrent HCC after ablation.
-
125I brachytherapy combined with ablation may improve tumor control.
-
Vascular invasion, multiple lesions, and AFP are prognostic risk factors.
-
125I brachytherapy is a safe method with a curative effect.
Similar content being viewed by others
Introduction
According to the International Agency for Research on Cancer [1], the incidence of liver cancer accounts for 4.7% of the global tumor incidence and is ranked sixth. However, the mortality rate is twice the incidence rate and ranked third in 2020, indicating that the treatment is challenging and the treatment model needs to be improved to reduce the mortality rate further. Hepatocellular carcinoma (HCC) is the main tissue type of primary liver cancer, accounting for more than 90%, and curative therapies such as surgical resection, liver transplant, and ablative techniques offer the chance of long-term response and improved survival. However, about 40% of HCC patients are locally advanced at the time of diagnosis (stage IIb/IIIa according to the Chinese liver cancer staging standard) [2]. Therefore, according to the European and American Liver Cancer Association standards [3], these patients have lost the opportunity for surgical resection. However, in China, patients with locally advanced HCC can undergo surgical resection if certain conditions are met. Unfortunately, the 5-year recurrence rate is over 70% after surgical resection. Furthermore, 55% of HCC patients who underwent surgical resection plus postoperative adjuvant therapy had tumor recurrence at a median of 22 months postoperatively [4, 5].
Local thermal ablation therapy, including radiofrequency ablation (RFA) or microwave ablation (MWA), has been widely used in recent years and provides curative results for patients with early-stage liver cancer [6,7,8]. However, for tumors near large blood vessels (e.g., the hepatic vein or inferior vena cava), the diaphragm, abdominal organs (gallbladder and gastrointestinal tissues), or the pericardium, thermal ablation may result in thermal damage to these tissues and a higher recurrence rate [9,10,11]. In addition, the size > 3 cm and a location immediately adjacent to the regions mentioned above lead to a significantly lower rate of tumor ablation, which is prone to local residual or recurrent tumors [12]. For tumor recurrent or residual tumors with the above characteristics, local treatments, such as repeated ablation or stereotactic body radiation therapy (SBRT), are used clinically [11, 13].
125I brachytherapy could inhibit tumor progression through continuously emitting low doses of X-rays and γ-rays, with the half-life dose reaching 140–160 Gy. 125I brachytherapy has been widely used in the treatment of various solid malignant tumors such as prostate cancer, lung cancer, liver cancer, pancreatic cancer, and metastatic tumors [14,15,16,17]. The advantages of 125I brachytherapy include local high dose within tumor, less radiation damage to normal tissue around the tumor, and no respiratory movement effect [18]. CT-guided 125I brachytherapy is safe to HCC adjacent to the subcapsular, large blood vessels, gallbladder, or subdiaphragm. Previous studies showed that 125I brachytherapy yielded good clinical efficacy and safety in patients with HCC [19]. Thus, the CT-guided 125I brachytherapy was applied to the local recurrent or residual tumor after thermal ablation in our center. To evaluate the efficacy and safety of CT-guided 125I brachytherapy, we retrospectively studied 114 patients with local residual or recurrent HCC after thermal ablation.
Materials and methods
Patient selection
The Institutional Review Board approved this study. HCC was diagnosed according to the practice guidelines of the American Association for the Study of Liver Diseases (AASLD) [3], and the stage was according to the Barcelona Liver Clinic (BCLC) stage [20].
The inclusion criteria were as follows: 1. The patient was diagnosed with hepatocellular carcinoma; 2. the patient had a Child–Pugh classification of grade A or grade B and an ECOG score ≤ 2; and 3. the patient had an estimated survival time of more than 3 months. The exclusion criteria were as follows: 1. patients with no indication for surgery such as coagulation dysfunction, organ failure, intolerable surgery, or complicated by severe infection and 2. patients lacking procedural information. Between April 2010 and May 2021, 126 consecutive patients were included in this retrospective study. Five patients refused or were considered unsuitable for the 125I brachytherapy procedure, and seven patients lacking procedural information were excluded. A total of 114 patients (93 men and 21 women; mean age 58 years, range 26–84) with 212 tumors who underwent CT-guided 125I brachytherapy procedure for local residual or recurrent HCC after thermal ablation and fulfilled the inclusion criteria were enrolled in this study.
CT-guided 125I brachytherapy procedure
125I seed is a synthetic anti-tumor seed with a length of 4.5 mm and a diameter of 0.8 mm. The shell of the seed is made of titanium and contains silver column with liquid 125I isotopes adsorbed; 125I seed inhibits tumor progression by emitting low-energy X- and γ-rays. The seed activity chosen was 0.8 mCi. The ray energy is 27–32 keV, the half-life is 59.6 days, the tissue semivalent layer is 2 cm, and the lead semivalent layer is 0.025 mm.
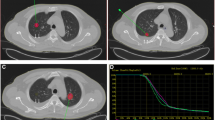
The CT-guided 125I brachytherapy procedure was described in our previously published literature [21]. Based on the preoperative enhanced computerized tomography (CT) or magnetic resonance imaging (MRI), clinical target volume (CTV), planned target volume (PTV), and puncture path were delineated by the physicist (Fig. 1). In addition, the mean prescribed radiation dose (120, 110–140 Gy), the required number of 125I seeds, seed distribution, and dose-volume histogram (DVH) were generated by the treatment planning system (TPS) (RT-RSI, Beijing Atom and High Technique Industries Inc., Beijing, China). According to PTV, the dose could achieve at least 95% of the prescribed dose (Vl00 > 95%).
a Preoperative TPS. The green straight line and the red area represent the puncture path and the gross tumor outline, respectively. b Preoperative dose-volume histograms (DVH). The prescribed dose (PD) was 120 Gy. 90% of the tumor volume received 123 Gy (D90 = 123 Gy), and 91.7% of the tumor target received 100% of the prescribed dose (V100 = 92.5%). c Postoperative practical radiation dose distribution. d Postoperative DVH, D90 = 127.5 Gy, V100 = 92.8%. The postoperative practical radiation dose almost matches the preoperative radiation dose
For the purpose of convenient operation and avoidance of vessel injuries, the patient’s body position was chosen prone, lateral or supine position. CT scan (PHILIPS 16-slice spiral CT, the Netherlands) was performed to locate the liver tumors. If necessary, contrast-enhanced CT was used to detect the boundary of tumor adjacent to vessels. According to the preoperative TPS, the puncture path was delineated on the CT scan images. Routine disinfection and local anesthesia were performed around the selected puncture points. The number and angle of the inserted 18G seed spinal needles were consistent with those of the preoperative TPS plan. The needle core was pulled out, and then, a 125I seed was released every 0.5 cm within the target lesion using a 125I seed implantation gun (Yunke Pharmaceuticals Limited Liability Company, Chengdu, China). Chest and abdominal CT scans were performed immediately after the procedure to assess the seed distribution and any complications and the CT scan images were imported into the TPS for postoperative dose verification. Patient follow-up.
The follow-up time was defined as the period from the start of 125I brachytherapy to the patient’s death or the last follow-up. Patients lost to follow-up were censored at the date of the last observation. Follow-up data included patient survival, laboratory data, and all follow-up treatment. According to the diagnostic criteria of AFP level for HCC, the AFP level of 400 ng/ml was defined as the cutoff value for the high and low AFP groups. In addition, contrast-enhanced CT and MR imaging of the abdomen was carried out to evaluate the local residual or recurrent tumor. The first period of follow-up assessment was performed one month after the 125I brachytherapy, and subsequent follow-up assessments were conducted every three months. Follow-up images were independently reviewed by one radiologist (> 10 years of experience) and one interventional physician (> 10 years of experience) in our center.
Evaluation of tumor response and control
Efficacy assessment of tumor therapy was performed according to the Modified Response Evaluation Criteria (m-RECIST) in Solid Tumors [22]. Complete response (CR) was defined as the disappearance of arterial phase enhancement in the target lesion. Partial response (PR) was the size of the lesion decreased by more than 30% in arterial phase enhancement. Progressive disease (PD) was defined as ≥ 20% increase in the sum of diameters of target lesions (enhanced arterial phase). Stable disease (SD) was between PR and PD. The LPFS was defined as the period from the start of 125I brachytherapy to the date of the first imaging assessment of PD.
Evaluation of complications
The perioperative complications were classified as major and minor, such as pain, fever, pneumothorax, or bleeding, and recorded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.3 [23] and the Society of Interventional Radiology [24].
Statistical analysis
All statistical analyses were performed using SPSS 26.0 (IBM, Chicago, Illinois, USA). Continuous variables were presented as the median and interquartile range (IQR), and categorical variables were described by frequency and percentage. The Cox model analyzed baseline tumor parameters, and the median time of LPFS was calculated using Kaplan–Meier curves and the log-rank tests. For all analyses, p values < 0.05 were considered statistically significant.
Results
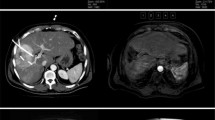
The clinical characteristics of the patients are summarized in Table 1. A total of 114 HCC patients with residual or recurrent lesions after thermal ablation were included. The preoperative average prescribed dose D90 was 124.4 (90–131) Gy, and the mean V100 was 96.7% (84.8–99.9%). The average postoperative prescribed dose D90 was 122.7 (42–235) Gy, and the average V100 was 91.8% (72–100%). The median number of seeds was 20 (5–119). Representative CT images of HCC adjacent to inferior vena cava and portal vein before TACE, subsequent microwave ablation (MWA), and two months after the 125I brachytherapy are displayed (Fig. 2).
Contrast-enhanced computed tomography (CT) of a 69-year-old man with primary liver cancer (5.6 cm) adjacent to inferior vena cava and portal vein. Contrast-enhanced CT showed abnormal enhancement of liver S1 segment at 1 month after TACE (a). CT-guided MWA was performed but contrast-enhanced CT still showed residual tumor lesion (b) after 1 month. Three months after the CT-guided.125I seed implantation, the residual tumor lesions showed no enhancement (c, d)
The CR, PR, and SD rates 6 months after the 125I brachytherapy procedure were 62.2%, 16.7%, and 7.9%, respectively. The form of tumor progression was mainly a local uncontrolled tumor or new lesions occur near the primary site. The median LPFS was 19.0 months. The 1-, 2-, and 3-year LPFS rates were 58.7%, 50.0%, and 41.2%, respectively (Fig. 3). Univariate analyses showed (Table 2) that PVTT, number of intrahepatic tumors, and AFP level were significantly associated with worse LPFS (p < 0.05). Multivariate analysis demonstrated (Table 3) that PVTT (hazard ratio [HR] = 2.04 [95% confidence interval (CI) 1.08–3.85]; p = 0.03), the number of intrahepatic tumors (HR = 2.50 [95% (CI) 1.43–4.35]; p = 0.01) and AFP level (HR = 2.36 [95% CI: 1.14–4.85]; p = 0.02) were independent risk factors for poor prognosis (Fig. 4). The median LPFS in patients without PVTT (25.2 months) was much longer compared to those with PVTT (9.9 months). The median LPFS in patients with less than three intrahepatic lesions was improved from 12.7 months to 28.3 months. The median LPFS was only 7.5 months in the high AFP group, but was prolonged with the decrease in AFP level (23.6 months).
Complications
No severe complications were detected during the perioperative period. Fourteen patients developed postoperative pain controlled with opioids. Five patients developed self-limiting pneumothorax. No radiation-related hepatitis was found in laboratory tests, and no 125I seeds migrated to other tissues or organs.
Discussion
Since most patients with HCC are diagnosed in advanced stages, only about 20% of patients have the opportunity for surgery [25]. Local ablation therapy has been widely used in recent years and has provided curative outcomes for patients with early-stage liver cancer. However, previous studies showed that in patients with unresectable or special location of hepatocellular carcinoma, tumors have a risk of incomplete ablation or major complications after thermal ablation [26,27,28]. Subsequent 125I brachytherapy can improve the local tumor control rate for patients with local recurrent or residual lesions after thermal ablation. In our study, after the postoperative half-year the percentage of patients who achieved CR, PR, and SD was 57%, 13.2%, and 5.2%, respectively. The median LPFS was 19.0 months. The 1-, 2-, and 3-year PFS rates were 58.7%, 50.0%, and 41.2%, respectively.
Thermal ablation is comparable to radical resection for HCC patients with a diameter of ≤ 3 cm [6,7,8]. However, in previous studies, a diameter > 3 cm and a location adjacent to the major vessels, the diaphragm, or the subcapsular were considered major risk factors for local residual or recurrent HCC after thermal ablation [29, 30]. Previous studies have shown that a tumor size > 3 cm and a perivascular location result in a substantial reduction in the rate of tumor ablation [31]. A multipolar RFA system instead of a monopolar system, overlapping ablation, temporary reduction of blood flow by transient vascular occlusion or increased power output, and prolonged ablation time were applied to patients with these risk factors [32,33,34]. In the previous study of Peng et al. and Morimoto et al. [35], local ablation combined with TACE was applied in patients with isolated or multiple tumors 3–7 cm in diameter and improved overall survival (OS) and recurrence-free survival compared to the only RFA group. For local residual or recurrent lesions after ablation, TACE or repeat ablation was applied. In our study, 125I brachytherapy was performed in HCC patients, especially in those with tumors larger than 3 cm or that were adjacent to high-risk locations. A total of 40 patients with a tumor diameter > 3 cm and 42 with the tumor near high-risk locations achieved local CR. Univariate and multivariate analysis indicated that PVTT, the number of intrahepatic tumors, and the level of AFP were poor prognostic factors, whereas tumor size was not. However, previous studies have shown that the maximum diameter was a poor prognostic affecting LPFS [36]. The key difference between these studies and ours may be that we only enrolled patients with local residual or recurrent HCC after thermal ablation.
In certain locations (near major bile duct trees, abdominal organs, or heart), RFA or MWA is contraindicated due to the risk of serious complications and the heat sink effect, which results in a loss of efficacy. For subcapsular tumors, especially those protruding from the liver capsule, percutaneous puncture ablation may cause the risk of liver rupture and hemorrhage, or for liver cancer that is difficult to image-guided, laparoscopic or open surgical ablation can be considered [37]. When the tumor was adjacent to the important organs in abdominal organs, such as the diaphragm, stomach, bowel loops, and gallbladder, hydrodissection was performed before thermal ablation to reduce the thermal damage to the surrounding tissue and prevent major complications such as perforation. However, hydrodissection may lead to incomplete ablation and increase the risk of peritoneal seeding [38]. In our study, LPFS had no significant difference in the adjacent organ group compared to the control group after 125I seed implantation and suggesting that 125I brachytherapy is feasible and effective for local residual or recurrent lesions after thermal ablation [16].
Internal radiotherapy was divided into temporal high-dose-rate interstitial brachytherapy (HDR-BT) and permanent low-dose-rate interstitial brachytherapy (LDR-BT) according to the dose rate. Mohnike’s study suggested that CT-guided HDR-BT yielded a low rate of major complications and high one-year local recurrence-free surviving proportion [39]. HDR-BT combined with transarterial radioembolization (TARE) may have a positive effect on survival [40]. 125I brachytherapy is also used in HCC patients and improves OS and PFS. A systematic review and meta-analysis showed that TACE plus 125I brachytherapy had significantly improved the 6-month OS compared to TACE alone [41]. In our study, CR achieved 57% and the median LPFS prolonged up to 19.0 months. At present, there is no study comparing HDR with LDR in HCC. Thus, randomized clinical trials (RCT) comparing the two methods are needed.
125I brachytherapy is associated with only minor complications. Our previous study reported no deaths or severe complications in patients who underwent 1251 brachytherapy [21]. Although 17 cases appeared with minor complications (such as fever and pain) and five patients developed self-limiting pneumothorax in our study, all recovered after symptomatic treatment. Thus, CT-guided 125I brachytherapy was a safe method and another choice for treating local residual or recurrent tumors after thermal ablation with its curative effect, minimal surgical trauma, and few complications.
The results of our study suggest that 125I brachytherapy combined thermal ablation may be an effective and safe treatment mode for locally advanced HCC patients, especially with larger than 3 cm or adjacent to abdominal organs, and could inhibit tumor growth through different mechanisms and increase the local control rate. For locally advanced HCC, the treatment mode is mostly systemic drugs combined with local therapy which may be the future development direction, for example, sorafenib combined with TACE [42]. However, our study has several limitations that warrant discussion. First, the study was retrospective in nature, and the data were collected from a single institution. Second, during the follow-up period, some patients who experienced local progressive disease received subsequent treatments such as targeted therapy and immunotherapy but were not further analyzed. Third, this study mainly analyzed the effect of 1251 seed implantation combined with thermal ablation on LTPFS but not analyzed on OS or PFS. Therefore, randomized controlled trials are needed to support our findings of 125I brachytherapy for local residual or recurrent HCC after thermal ablation.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFP:
-
A-fetoprotein
- CR:
-
Complete response
- CT:
-
Computerized tomography
- CTV:
-
Clinical target volume
- DVH:
-
Dose-volume histogram
- HCC:
-
Hepatocellular carcinoma
- LPFS:
-
Local progression-free survival
- LTP:
-
Local tumor progression
- MRI:
-
Magnetic resonance imaging
- MWA:
-
Microwave ablation
- PD:
-
Progressive disease
- PR:
-
Partial response
- PTV:
-
Planned target volume
- PVTT:
-
Portal vein tumor thrombus
- RFA:
-
Radiofrequency ablation
- TACE:
-
Transarterial chemoembolization
- SD:
-
Stable disease
- TPS:
-
Treatment planning system
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Zhou J, Sun H-C, Wang Z et al (2018) Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer 7(3):235–260
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology 68(2):723–750
Poon RT, Fan ST, Lo CM et al (2002) Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 235(3):373–382
Tabrizian P, Jibara G, Shrager B et al (2015) Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 261(5):947–955
Hasegawa K, Aoki T, Ishizawa T et al (2014) Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma. Ann Surg Oncol 21(Suppl 3):S348–S355
Xu XL, Liu X-D, Liang M et al (2018) Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology 287(2):461–472
Huang J, Yan l, Cheng Z et al (2010) A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 252(6):903–912
Sala M, Llovet JM, Vilana R et al (2004) Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 40(6):1352–1360
N’Kontchou G, Mahamoudi A, Aout M et al (2009) Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 50(5):1475–1483
Xie X, Jiang C, Peng Z et al (2015) Local recurrence after radiofrequency ablation of hepatocellular carcinoma: treatment choice and outcome. J Gastrointest Surg 19(8):1466–1475
Lencioni R, Crocetti L (2012) Local-regional treatment of hepatocellular carcinoma. Radiology 262(1):43–58
Kim N, Cheng J, Jung I et al (2020) Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol 73(1):121–129
Henry AM, Al-Qaisieh B, Gould K et al (2010) Outcomes following iodine-125 monotherapy for localized prostate cancer: the results of leeds 10-year single-center brachytherapy experience. Int J Radiat Oncol Biol Phys 76(1):50–56
Zhang T, Lu M, Peng S et al (2014) CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol 140(8):1383–1390
Zhang FJ, Li C-X, Zhang L et al (2009) Short- to mid-term evaluation of CT-guided 125I brachytherapy on intra-hepatic recurrent tumors and/or extra-hepatic metastases after liver transplantation for hepatocellular carcinoma. Cancer Biol Ther 8(7):585–590
Gai B, Zhang F (2018) Chinese expert consensus on radioactive (125)I seeds interstitial implantation brachytherapy for pancreatic cancer. J Cancer Res Ther 14(7):1455–1462
Al-Haj AN, Lobriguito AM, Lagarde CS (2004) Radiation dose profile in 125I brachytherapy: an 8-year review. Radiat Prot Dosimetry 111(1):115–119
Meng J, Wang X, Zhuang QW et al (2014) Clinical effectiveness of 125I-seed implantation in combination with nimotuzumab therapy for the advanced oral carcinoma: preliminary results. Eur Rev Med Pharmacol Sci 18(21):3304–3310
European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Yan H, Luo M, Wang L et al (2020) Clinical efficacy and prognostic factors of CT-guided (125)I brachytherapy for the palliative treatment of retroperitoneal metastatic lymph nodes. Cancer Imaging 20(1):25
Prajapati HJ,Spivey JR, Hanish SI et al (2013) mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol 24(4):965–973
Dueck AC, Mendoza TR, Mitchell SA et al (2015) Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 1(8):1051–1059
Patel IJ, Rahim S, Davidson JC et al (2019) Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol 30(8):1168-1184.e1
Orcutt ST, Anaya DA (2018) Liver resection and surgical strategies for management of primary liver cancer. Cancer Control 25(1):1073274817744621
Teratani T, Yoshida H, Shiina S et al (2006) Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 43(5):1101–1108
Komorizono Y, Oketani M, Sako K et al (2003) Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer 97(5):1253–1262
Llovet JM, Vilana R, Brú C et al (2001) Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 33(5):1124–1129
Di Vece F, Tombesi P, Ermili F et al (2014) Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: a prospective pilot study. Cardiovasc Intervent Radiol 37(3):723–729
Lee DH, Lee JM, Lee JY et al (2014) Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270(3):900–909
Brace CL, Laeseke PF, Sampson LA et al (2007) Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology 244(1):151–156
de Baere T, Deschamps F, Briggs P et al (2008) Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology 248(3):1056–1066
van Duijnhoven FH, Jansen MC, Junggeburt JMC et al (2006) Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol 13(5):651–658
Kurokohchi K, Watanabe S, Masaki T et al (2005) Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol 11(10):1426–1432
Morimoto M, Numata K, Kondou M et al (2010) Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 116(23):5452–5460
Peng ZW, Zhang Y-J, Chen M-S et al (2013) Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 31(4):426–432
Groeschl RT, Pilgrim CHC, Hanna EM et al (2014) Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 259(6):1195–1200
Kang TW, Lim HK, Lee MW et al (2013) First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol 68(12):e641–e651
Mohnike K, Wolf S, Damm R et al (2016) Radioablation of liver malignancies with interstitial high-dose-rate brachytherapy: complications and risk factors. Strahlenther Onkol 192(5):288–296
Fleckenstein FN, Roesel MJ, Krajewska M et al (2021) Combining transarterial radioembolization (TARE) and CT-guided high-dose-rate interstitial brachytherapy (CT-HDRBT): a retrospective analysis of advanced primary and secondary liver tumor treatment. Cancers (Basel) 14(1):72
Liu Q, Dai X, Zhou X et al (2019) Comparison of TACE combined with and without iodine-125 seeds implantation therapy for advanced stage hepatocellular carcinoma: a systematic review and meta-analysis. J BUON 24(2):642–649
Kudo M, Han G, Finn RS et al (2014) Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 60(5):1697–1707
Acknowledgements
We thank the patients enrolled in this study.
Funding
This study has received funding by National Natural Science Foundation of China (fund No: 81871467 to Fujun Zhang and No: 81571780 to Fei Gao).
Author information
Authors and Affiliations
Contributions
FZ and FG were guarantors of integrity of the entire study and contributed to study concepts and design. HY and HG were involved in experimental studies/data analysis. MX and XH were involved in statistical analysis. WZ and ZZ contributed to manuscript preparation and manuscript editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, W., Zhong, Z., Yan, H. et al. Clinical efficacy of CT-guided 125I brachytherapy in patients with local residual or recurrent hepatocellular carcinoma after thermal ablation. Insights Imaging 13, 185 (2022). https://doi.org/10.1186/s13244-022-01327-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-022-01327-z