Abstract
Intracerebral hemorrhage (ICH) accounts for 10–20% of all strokes worldwide and is associated with serious outcomes, including a 30-day mortality rate of up to 40%. Neuroimaging is pivotal in diagnosing ICH as early detection and determination of underlying cause, and risk for expansion/rebleeding is essential in providing the correct treatment. Non-contrast computed tomography (NCCT) is the most used modality for detection of ICH, identification of prognostic markers and measurements of hematoma volume, all of which are of major importance to predict outcome. The strongest predictors of 30-day mortality and functional outcome for ICH patients are baseline hematoma volume and hematoma expansion. Even so, exact hematoma measurement is rare in clinical routine practice, primarily due to a lack of tools available for fast, effective, and reliable volumetric tools. In this educational review, we discuss neuroimaging findings for ICH from NCCT images, and their prognostic value, as well as the use of semi-automatic and fully automated hematoma volumetric methods and assessment of hematoma expansion in prognostic studies.
Similar content being viewed by others
Key points
-
Neuroimaging is pivotal in diagnosing intracerebral hemorrhage (ICH) and predicting its cause.
-
Baseline hematoma volume and hematoma expansion at repeated imaging are the strongest prognosticators in ICH.
-
Specific imaging markers on computed tomography (CT) can aid in predicting ICH prognosis including risk of hematoma expansion and/or rebleeding.
-
ICH volume can be measured by manual, semi-automatic and automatic methods
Introduction
Spontaneous intracerebral hemorrhage (ICH) represents non-traumatic bleeding within the brain parenchyma. ICH accounts for 10–20% of all acute strokes worldwide and is the most fatal type of stroke, with a 30-day mortality rate of up to 40%, and a 1 year mortality rate reaching 60% [1]. Between 1990 and 2016, the absolute number of stroke events and mortality rate increased worldwide. However, the age-standardized incidence and death rate for ICH have decreased in all regions worldwide [2]. There were 4.1 million new cases of ICH worldwide in 2016, accounting for 51% of all stroke-related deaths and 64.5 million years of healthy life lost each year [2]. A study performed on 657 patients who survived up to 3 months after an ICH to assess the neurological impairment and functional disability based on the HRQoL (Health-Related Quality of Life) concept, showed that the majority of survivors (87%) after ICH had poor HRQoL (< 1). Advanced age, higher National Institutes of Health Stroke (NIHSS) score, higher systolic blood pressure, higher baseline ICH volume, deep hematoma location, and increase in neurologic deficit during the first 72 h after ICH onset were shown to be independent predictors of poor HRQoL [3].
Rapid diagnosis of intracerebral hemorrhage and identification of its etiology is key for early treatment decisions such as blood pressure management [4], reversal of anticoagulation [5] or neurosurgery [6]. The most common neuroimaging modalities available in acute settings are non-contrast computed tomography (NCCT) and CT angiography (CTA) [7, 8]. In addition to diagnosing ICH, NCCT gives detailed information about the hematoma location, shape and the presence of intraventricular extension. NCCT and can further be used to measure the hematoma volume which is considered the strongest predictor of 30-day mortality and functional outcome for ICH patients [9]. Accurate and efficient tools for measuring hematoma volume are therefore a major medical need.
Hematoma expansion is an important risk factor for poor clinical and functional outcome and is considered modifiable if identified early. It has been shown that 30% of ICH patients develop hematoma expansion [10], which occurs mostly during the first 6 h following stroke onset [11]. There are several imaging markers shown to be able to predict early hematoma expansion and active bleeding with good accuracy on NCCT (blend sign, black hole sign, swirl sign and island sign) and CTA (spot sign) [12].
In this educational review aimed for general radiologists and neuroradiologists at different stages of training, following a short introduction to clinical characteristics and risk factors of ICH, we focus on neuroimaging findings and signs on NCCT useful for determining ICH etiology and predicting hematoma expansion and clinical outcome. We also describe the most widely used manual, semi-automatic and fully automated methods for measuring hematoma volume.
Risk factors and clinical characteristics of ICH
Intracerebral hemorrhage can be spontaneous (also known as non-traumatic ICH), related to trauma (traumatic ICH) [13, 14]. Spontaneous ICH arises from chronic, progressive small vessel disease occurring primarily due to chronic hypertension and cerebral amyloid angiopathy [15, 16]. Other less common etiologies of non-traumatic ICH include arteriovenous or other vascular malformations, cerebral venous thrombosis, hemorrhagic transformation of ischemic stroke, mycotic aneurysms, Moya Moya disease, cerebral vasculitis, and primary or metastatic tumor. [17] (Table 1 and Fig. 3).
Hypertension, cerebral amyloid angiopathy (CAA), oral anticoagulant treatment and older age are considered the most important risk factors of ICH [18]. Additional risk factors include cigarette smoking, excessive alcohol consumption, low-density lipoprotein cholesterol levels, genetics (Apolipoprotein E gene), and ethnicity (Asian ethnicity) [1, 18, 19].
Symptoms of ICH are identical to those of ischemic stroke, as patients present with sudden neurologic deficits. Presenting with decreased level of consciousness, vomiting and headache may be indicative of ICH, but only neuroimaging can determine if the stroke is ischemic or hemorrhagic [20].
In ICH, a lower Glasgow coma scale (GCS) score and higher NIHSS score are predictors of larger baseline hematoma volume, mortality, severe disability, and hematoma expansion [10, 21]. However, the CT findings may not always correlate with the clinical symptoms of patients with ICH especially in cases of lobar bleedings where large hematomas may be seen in patients with relatively mild symptoms [22]. Wagner et al. [23] found that both neurosurgeons and neurologists had limitations in clinical estimation of ICH patients, other than of GCS and NIHSS scores, concluding that a collaborative approach by neurologists and neurosurgeons in addition to careful evaluation of the imaging findings is important to make appropriate acute treatment decisions.
High systolic blood pressure (above 160 mmHg) is associated with larger initial hematoma volume, an increased risk of hematoma expansion and poor functional outcome. The mechanism of which is likely through rupture and continuous bleeding from small cerebral blood vessels [24,25,26].
The use of antithrombotic drugs increases the risk of hematoma expansion [27]. Oral anticoagulation treatment increases initial hematoma volume and increases the risk of hematoma expansion by 6.2 times [28]. Patients taking non-vitamin K oral anticoagulant medication prior to their ICH had smaller baseline hematomas, lower risk of expansion, and better functional outcome compared to ICH patients on vitamin K oral anticoagulant medications [29,30,31,32].
Hyperglycemia at admission is present in more than half of ICH patients [33] and associated with poor functional outcome, increased mortality regardless of prior diabetes history [34, 35] and a 2.5-fold increased risk of hematoma expansion [36].
Interestingly, hematoma expansion is more common in ICH with onset at daytime than at night, however the underlying mechanisms are not well known [37].
The use of bundles of care for ICH including rapid anticoagulant reversal, intensive blood pressure lowering, access to intensive care and neurosurgery has shown to reduce 90-day case-fatality [38].
Predictive scores in ICH
The ICH score [39] (Table 2) is used as a prognostic tool and calculated from the patient’s presenting GCS score, age, the presence or absence of infratentorial hemorrhage, ICH volume and the presence of intraventricular hemorrhage. There are several available prognostic scores for ICH, most of them not fully validated and not widely used in routine clinical practice. Future scores need to be developed with standardized timing, preferably using more than one time point, incorporating standardized outcome assessments based on the modified Rankin Scale, and including patient-reported outcome measures [40].
Neuroimaging of ICH
NCCT is the gold standard imaging modality for the diagnosis of ICH [7] due to its availability, few contraindications and excellent sensitivity for bleeding. Although the typical finding in acute ICH is a hyperintense hematoma, the hyperacute ICH may be iso-attenuating to gray matter, rapidly increasing within minutes to the hyperintense state seen for hours to days after which the attenuation gradually decreases to be iso-attenuating to white matter in the subacute stage (days–weeks) and hypoattenuating in the chronic state over (weeks–months), as illustrated in Fig. 1.
Hematoma attenuation. The left panel illustrates the changes in hematoma attenuation over time. In the hyperacute phase, within the first minutes of onset, the hematoma is iso-attenuating with gray matter. Within an hour, the attenuation in the hematoma increases to the hyperattenuating typical appearance of the acute phase, lasting for several hours. The attenuation then gradually decreases over the next days to iso-attenuating with the gray or white matter in the subacute phase, and then even further to become hypoattenuating in the chronic phase, typically within weeks of onset. Finally, the hematoma is absorbed and the lesion is healed with substance loss and gliosis in the surrounding brain parenchyma
NCCT can be used to diagnose intraventricular hematoma extension, hydrocephalus, presence and degree of edema, midline shift and brainstem compression secondary to the mass effect from the hematoma. Several NCCT imaging markers (blend sign, black hole sign, swirl sign and island sign, illustrated in Fig. 2) that have been shown to predict early hematoma expansion and/or active bleeding are specifically discussed below [12].
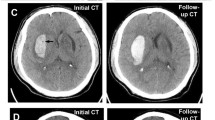
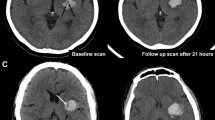
Imaging Signs. The Spot sign is seen on CTA, the other signs on NCCT. Spot sign—Spot sign is defined as one or more focal enhancements (white arrows) within an acute parenchymal hematoma on CTA, suggestive of active contrast extravasation/active bleeding into the hematoma. Swirl sign—The swirl sign is defined as regions of hypo-attenuation or iso-attenuation within the hyper-attenuated ICH, suggestive of hyperacute bleeding/active bleeding within the acute hematoma. Black hole sign—The black hole sign is defined as well-defined round or oval areas (black arrow) of hypoattenuation enclosed within the hyperattenuating hematoma, without any connection with the adjacent brain parenchyma. Blend sign—The blend sign is defined as regions of mixed hyper-attenuated and hypo-attenuated areas with identifiable boundaries located within the hemorrhage. Island sign—The island sign is defined as: (1) three or more scattered small oval or round hematomas separated from the main hematoma (white arrowheads), or (2) four or more small bubble-like or sprout-like hematomas, some or all of which may be connected with the main hematoma. Satellite sign—The satellite sign is defined as any small hemorrhage (white arrow) with an isolating distance of 1–20 mm from the main hematoma
CTA is an effective modality for diagnosing the underlying causes of ICH in the acute setting [8], specifically when the cause is suspected to be secondary to vascular abnormalities such as arteriovenous malformation or other intracranial pathologies [8, 20]. The spot sign on CTA images is caused by contrast extravasation due to active bleeding within the hematoma and is therefore a predictor of hematoma growth and poor clinical outcome [41]. However, due to the additional radiation exposure, the need for of iodine contrast medium and the risk of contrast-induced nephropathy, CTA is often not routinely used in the workup of ICH [8] but the 2022 guidelines for the management of patients with spontaneous ICH from the American Heart Association/American Stroke Association suggest it is reasonable to perform CTA within the first hours of onset to identify patients at risk for subsequent hematoma expansion [42].
Magnetic resonance imaging (MRI) has similar accuracy for diagnosing acute ICH as NCCT and is superior to CT in distinguishing between ICH and hemorrhagic transformation of an acute ischemic lesion [43]. MRI gradient echo sequences can identify microbleeds within the brain parenchyma as well as blood products that represent chronic lesions and cavernomas—findings that typically cannot be diagnosed by NCCT [43]. Contrast-enhanced MRI can be performed after the hematoma resolves to identify an underlying tumor. Magnetic resonance angiography can be useful in imaging brain vessels and can be performed without contrast medium. Nonetheless, MRI is rarely used in the acute setting for ICH diagnosis due to its cost and lower availability [20].
The role of neuroimaging for determining the ICH etiology
There are several different underlying etiologies causing ICH, the most common are illustrated in Fig. 3.
Etiology. The figure illustrates the most common etiologies of ICH. Top row: Aneurysm—Bleeding from cerebral aneurysms may rarely present predominantly as ICH (top panel), such as this case of a bi-lobulated anterior communicating artery aneurysm (white arrows in CBCT 3D reconstruction in the bottom panel). Cavernoma—Bleeding in a cavernoma in the left hemisphere (top panel) with an associated DVA (black arrowheads and white arrows for the draining vein) and another cavernoma (black arrow) in the contralateral hemisphere on MRI (SWI). CAA (Cerebral Amyloid Angiopathy)—with a large parietal lobar ICH with the typical “finger-like projections” (top panel) and multiple amyloid deposits throughout the brain on a preceding MRI (SWAN, bottom panel). Ischemia—Illustrating an ICH an association with an acute ischemic stroke (top panel) caused by an embolic occlusion of the terminal internal carotid artery (T-occlusion—indicated by the white arrows in the CTA in the lower panel). Trauma—A common cause of ICH, illustrated by a temporal ICH in the left hemisphere, associated with an extracranial hematoma (white arrowheads in the top panel) and a skull fracture (black arrows in the 3D reconstruction in the bottom panel). Tumor—A large ICH in the right hemisphere with an underlying glioma, visualized on a subsequent MRI (T1-TSE with Gadolinium in the bottom panel). Vasculitis—A large frontal ICH associated with CNS vasculitis with several stenosed intracranial arteries (examples indicated by white arrowheads in the sagittal DSA in the bottom panel). Bottom row: AVM (ArterioVenous Malformation)—A temporal ICH where the AVM nidus (asterisk in all 3 panels) is protruding into the hematoma. The main feeder is indicated by white arrowheads (middle panel CTA and bottom panel DSA). DAVF (Dural ArterioVenous Fistula) – ICH with intraventricular extension adjacent to a DAVF (hematoma indicated by † in the CTA; middle panel) with an ectasia (asterisks in the middle and bottom panels) on a large draining vein (white arrowheads in the middle and bottom panels). Hypertensive microangiopathy—Typical location in the basal ganglia as this example with an ICH in the left thalamus, shown on CT (top panel) and MRI (T1 TSE in the middle panel and T2 FLARI in the bottom panel). Moya Moya—ICH caused by increased demand from thalamostriatal collateral vessels due to stenosis/occlusion of the distal internal carotid arteries (white arrows in the CTA in the middle panel and DSA in the lower panel). It is the collateral network of numerous small vessels that give rise to the typical “puff of smoke” appearance in advanced stages. Mycotic aneurysm—illustrated by a small ICH indicated by white arrowheads on CT (top panel) and MRI (SWI in the bottom panel) from a mycotic aneurysm on a distal MCA branch (white arrowhead in the CBCT angiography in middle panel) caused by septal emboli from staphylococcal endocarditis. Additional septic emboli seen on MRI are indicated by white arrows (SWI in the bottom panel). Sinus thrombosis—Illustrated by bilateral ICH in a case with extensive thrombosis of the superior sagittal sinus. The hyperdense appearance on NCCT (black arrowheads) is illustrated in the middle panel and the absence of superior sagittal sinus (white arrows on MR venography, bottom panel) and the torcula (white arrowhead in the bottom panel). Telangiectasia—Illustrated by an Ossler patient with a small frontal ICH where MR shows gadolinium enhancement in the lesion (white arrow in the middle panel; MR T1 TSE with Gadolinium) corresponding to a small telangiectasia without shunting on DSA (black arrow in bottom panel)
Intracerebral hemorrhage location can predict its etiology. Deep/non-lobar (basal ganglia, thalamus, brainstem, deep cerebellum) ICHs usually occur due to long-standing hypertension in addition to other risk factors such as diabetes mellitus, male sex, alcohol overuse, and underweight [44]. Pooled results from several studies including a total of about 4000 patients showed that hypertension was twice as common in patients with deep ICH as in those with lobar ICH [45].
Chronic hypertension induces degenerative changes of small perforating arteries leading to rupture, and deep hemorrhages that can extend into the ventricles [46]. Hypertensive microangiopathy (also known as chronic hypertensive encephalopathy) accounts for up to 35% of all spontaneous ICHs [47], and it is caused by the effects of long-standing hypertension on the brain leading to lipohyalinosis and Charcot-Brouchard aneurysms that can rupture and cause hypertensive hemorrhages. Hypertensive microangiopathy causes brain microbleeds, best visualized on T2-weighted MRI sequences (specifically susceptibility-weighted imaging as small foci of susceptibility blooming in the basal ganglia, pons and cerebellar hemispheres. Presence of brain microbleeds is significantly correlated with history of hemorrhagic stroke and has been shown to be a predictor of future ICH [48, 49]. A study done on 2164 patients who had undergone 2416 consecutive brain MRI studies showed that presence of microhemorrhages had the highest significant correlation (p < 0.001) with history of hemorrhagic stroke. Out of 139 patients with microhemorrhages followed clinically for more than 1 month, four patients suffered new hemorrhagic strokes [48].
Cerebral amyloid angiopathy (CAA) is caused by progressive deposition of beta-amyloid on the wall of leptomeningeal and cortical vessels that results in them becoming fragile [50]. CAA is typically associated with lobar ICH and accounts for up to 20% of all spontaneous ICHs [51]. A typical imaging feature seen when using NCCT is “finger-like projections” which is included in the Edinburgh criteria (Table 3) [52] proposed by Rodrigues et al. in 2018 to identify lobar ICH associated with CAA. The presence of subarachnoid hemorrhage, in combination with either the APOE ε4 allele, or “finger-like projections” has a nearly 100% sensitivity in determining CAA. Conversely, the absence thereof can be used to rule out CAA for lobar ICH [52]. However, the Edinburgh criteria can only indicate a high probability of CAA and a major limitation of clinical use of the criteria is that the APOE-status is typically not known by the radiologist at image examination. Further studies on the use of the Edinburgh criteria based solely on the CT imaging features may be warranted.
MRI is superior to NCCT in identifying small vessel disease, which helps distinguishing CAA from hypertensive arteriopathy. Multiple lobar cerebral microbleeds and/or cortical superficial siderosis are strong imaging predictors of CAA as described in the Boston criteria (Table 4) [51, 53, 54]. The Boston criteria was first proposed by Greenberg SM et al. in 1995 for diagnosing CAA, and is composed of clinical, imaging and neuropathological parameters [55]. The Boston criteria were later modified in 2010 by Linn et al. by incorporating cortical superficial siderosis into the radiological diagnosis of probable CAA. The modified Boston criteria have an increased sensitivity with a slight decrease in specificity compared to the original Boston criteria [54].
White matter hyperintensities and enlarged perivascular spaces are predictive of CAA when located in the lobar regions, and of hypertensive arteriopathy when located in the deep and infratentorial regions [56, 57]. Susceptibility-weighted MRI can show small foci of susceptibility blooming in the cerebral white matter which aids in the clinical diagnosis of CAA [50, 58]. CT and MRI imaging features that predict CAA are shown in Fig. 4.
Cerebral amyloid angiopathy imaging features. Examples of imaging features of cerebral amyloid angiopathy (CAA) on CT and MRI. CT imaging predictors of CAA (panel A and B) showing ICH with multifocal components (black asterisks), finger-like projections (black arrowheads), subarachnoid hemorrhage component (black arrow) and subdural hemorrhage component (gray arrow). MRI of cerebral amyloid angiopathy (panel C) showing multiple lobar cerebral microbleeds (white arrows) and cortical superficial siderosis (white arrowheads)
The role of neuroimaging in predicting prognosis following ICH
Neuroimaging findings are of paramount importance to assess the prognosis following ICH. Examples of the most common neuroimaging predictors of ICH prognosis are shown in Table 5.
Hematoma volume
Hematoma volume is considered the strongest predictor of 30-day mortality and functional outcome for ICH patients [9]. The 30-day mortality rate is less than 20% for patients with hematoma volume < 30 ml, whereas volumes larger than 60 ml are associated with > 90% mortality rate. Patients with a hematoma volume of more than 30 ml are more likely to be functionally dependent at 30 days [9]. Several studies have shown that a baseline hematoma volume of 30 ml or more is a predictor of hematoma expansion and poor outcome, whereas a hematoma volume of 10 ml or less is associated with a lower probability of hematoma expansion and predictive of a favorable functional outcome [59,60,61].
Hematoma location
Lobar hemorrhages are commonly associated with CAA and feature subarachnoid extension and finger-like projections on NCCT images [52].
The INTERACT2 study showed that ICHs in the posterior limb of the internal capsule, thalamus, and infratentorial sites are associated with poor clinical outcomes. ICHs engaging specifically the thalamus and the posterior limb of internal capsule showed the highest association with death or major disability [62].
The association between hematoma volume and location was established in a study by Roh et al. [63] that showed significantly larger baseline hematoma volume for lobar ICH compared to deep ICH. In addition, hematoma expansion was found to be less common in lobar ICH compared to deep [63]. The association between larger hematoma volumes and lobar location has been confirmed in a recent study [64].
Hematoma shape and density
Hematoma shape was classified by Fujii et al. as either round, irregular, or separated [65]. Irregularly shaped hematomas have been associated with increased risk of expansion during the first 24 h of onset [66]. The association between irregular hematoma shape and early expansion has been confirmed in several studies and is also linked to larger perihematomal edema volume compared to regular-shaped hematomas [67,68,69].
In 2009, Barras et al. proposed a 5-point categorical scales based on hematoma shape and density. ICHs were categorized into either homogenous/regular (Category 1–2) or heterogenous/irregular (Category 3–5). It was found that median hematoma growth (10–25 ml) was significantly higher in the large, heterogenous and irregular shaped hematomas compared to the small, regular and homogenous hematomas [70].
Perihematomal edema
Perihematomal edema (PHE) progresses aggressively within the first 24 h and continues to increase rapidly 3 days post-ictus. The edema peaks initially at day 4–5, remains gradually elevated until day 14, then decreases [71]. Elevated oncotic pressure of the perihematomal space due to infiltration of blood components from the hematoma, along with inflammation, thrombin cascade and erythrocyte lysis products cause blood–brain barrier disruption. These factors lead to the formation of a vasogenic edema. Oxidative stress induced by vasogenic edema, and the release of cytotoxic substances can induce a secondary cytotoxic edema [72]. PHE is an independent prognostic factor for death or dependency. The attenuation of the PHE at 72 h after onset has also been shown to predict outcome, with lower attenuation values (suggestive of a more intense PHE) being predictive of a poor outcome [73]. Baseline hematoma volume is also associated with PHE growth [69]. The mean distance from the hematoma border and the outer border of the edema has been defined as the edema extension distance [74]. The edema extension distance at 72 h is associated with larger baseline hematoma, older age, lobar location and intraventricular hemorrhage extension [75].
Hematoma expansion
Hematoma growth occurs mostly within the first few hours (0–6) after onset, which makes early follow-up NCCT important for prognosis and for implementing any early interventions [11]. Early hematoma expansion has been shown to occur in 30% of ICH patients and is considered a modifiable predictor of poor outcome [10]. Sembolini et al. found that hematoma expansion was associated with a larger baseline hematoma volume, higher NIHSS score, higher cumulative mortality, and long-term functional dependence rate [76]. In the INTERACT-1 study, it was shown that hematoma growth of 1 ml was associated with a 5% increase of the risk of death or dependency [77]. The definition of hematoma expansion varies substantially between studies [65, 78, 79]. Hematoma growth has been defined in studies as either a > 33% or a ≥ 6 ml increase in volume compared to the baseline CT hematoma volume [80,81,82], or by an increase in volume of ≥ 12.5 ml compared to the baseline CT—the latter definition has been shown to be superior for outcome prediction [83]. The 2022 for the management of patients with spontaneous ICH from the American Heart Association/American Stroke Association suggest that repeated head CT during the first 24 h after onset can be useful to evaluate hematoma expansion [42].
Intraventricular hemorrhage extension and hydrocephalus
Expansion of hematomas located near the ventricular system may cause a break in the ventricular wall and a subsequent intraventricular hemorrhage (IVH). IVH is present in 45% of ICH cases, with the highest frequency for thalamic and caudate bleedings [84]. Blood within the ventricular system can cause hydrocephalus, either by obstructing the cerebrospinal fluid flow or by impairing the cerebrospinal fluid resorption at the level of the arachnoid granulations [85].
IVH is predictive of early (within 24 h) and late (up to 7 days) neurological deterioration [86] and poor outcome [87, 88]. IVH is also strongly associated with pre-hospital loss of consciousness [89]. It has been shown that the prognostic value of hematoma expansion is even higher if the IVH component is also included [90].
Specific NCCT and CTA imaging markers
There are several specific imaging signs associated with the risk of hematoma expansion and prognosis. Examples of such imaging signs on NCCT and CTA are shown in Fig. 2.
Spot sign
Spot sign is defined as one or more foci of contrast enhancement within an acute parenchymal hematoma on a CTA image. A positive spot sign indicates active bleeding and is a strong predictor for hematoma expansion and predicts poor clinical outcome [11, 91, 92]. The occurrence of a spot sign is most common within 3 h of symptom onset, but its accuracy in predicting hematoma expansion remains high regardless of time from symptom onset [93]. Spot sign on the initial CTA image has been correlated with larger hematoma volume and increased risk of hematoma expansion on 24 h follow-up imaging [94]. A meta-analysis has shown that a positive spot sign can predict hematoma expansion with 53% sensitivity and 88% specificity [95].
Swirl sign
The swirl sign is defined as regions of hypo-attenuation or iso-attenuation within the hyper-attenuated ICH on NCCT images [96] and represents active bleeding within the hematoma. The swirl sign has been associated with larger hematoma size, higher frequency of midline shift, IVH extension, and hematoma expansion where the median absolute hematoma growth was 35.6% higher in patients with positive swirl sign [96, 97]. A meta-analysis has shown that the swirl sign predicts hematoma expansion with 45% sensitivity and 79% specificity [98].
Black hole sign
The black hole sign is defined as a round or oval-like shaped area of hypoattenuation enclosed within the hyperattenuating hematoma, without any connection with the adjacent brain parenchyma on NCCT images. It represents mixed density—and thereby bleeding of different ages—within the same hematoma, suggestive of bleeding at several timepoints and therefore predictive of hematoma expansion [99]. The hypo-attenuated area is described as having a density difference of at least 28 Hounsfield units compared to the hyper-attenuated hematoma [100]. A meta-analysis has shown that the black hole sign predicts hematoma expansion with a 30% sensitivity and 91% specificity [101].
Blend sign
The blend sign on NCCT images was defined by Li et al. [102] in 2015 as a region of mixed hyper-attenuated and hypo-attenuated areas with identifiable boundaries located within the hemorrhage. The blend sign is associated with hematoma expansion and poor outcome [103] as well as increased risk of postoperative rebleeding in patients undergoing minimally invasive neurosurgery [104]. In a meta-analysis, the blend sign has been shown to predict hematoma expansion with a sensitivity of 28% and specificity of 92% [105].
Island sign
The island sign was proposed by Li et al. in 2017 as a predictor of hematoma expansion. It is defined as [1] three or more scattered small oval or round hematomas all separate from the main hematoma [2]; or four or more small bubble-like or sprout-like but not lobulated hematomas, some or all of which could be connected with the main hematoma. Island signs have been proposed to be associated with multiple bleeding sources caused by injury of adjacent arterioles, possibly by shear force from the initial hematoma. The island sign has been shown to be present in as many as 44.7% of expanding hematomas and is a highly specific (98.2% specificity) with a 44.7% sensitivity for predicting hematoma expansion [106], and it’s prognostic values has been confirmed by several other studies [107,108,109,110].
Satellite sign
The satellite sign is defined as any small hemorrhage with an isolating distance of 1–20 mm from the main hematoma and is predictive of expansion with 54% in sensitivity and 94% specificity [111].
Serrano E et al. compared two combined indicators—Combined Barras Total Score and Hematoma Maturity Score—to individual radiological NCCT signs (Black hole, blend, island, swirl, Barras classification, any hypodensity, any irregularity) (Fig. 5) to predict functional outcome (according to the modified Rankin Scale) of 114 ICH patients [112]. The Hematoma Maturity Score categorized ICH into Mature: completely homogenous without any irregularity or hypodensity, and Immature: any irregularity in density and shape. Categories of the original Barras classification were grouped into a one Combined Barras Total Score, where ICH was classified as homogenous and regular if the original Barras score was < 4, and ICH was considered heterogenous and irregular if the original Barras score of the variables was ≥ 4. Results showed that among all evaluated radiological signs, The Hematoma Maturity Score (Immature) had the highest accuracy (AUC 0.779) for the prediction of poor outcome for ICH patients, followed by Combined Barras Total Score ≥ 4 (AUC of 0.727) [112].
Hematoma Maturity Score. Examples of the two categories of Hematoma Maturity Score. Categories of Hematoma Maturity Score classified ICH as mature (completely homogeneous in density and shape) and immature (ICH with any irregularity in density or shape) illustrated by typical cases. Categories of the Barras score classified ICH as homogenous and regular if the Barras score was < 4, and heterogenous and irregular if the original Barras score of the variables was ≥ 4. The left panel illustrating the score is adopted from the original publication by Serrano et al. [112]
Volumetric assessment methods
Examples of volumetric assessment methods are shown in Fig. 6.
Volumetric methodology. Panel A illustrates the principle of the ABC/2 method where A is the largest diameter of the hematoma in axial images, B is the largest perpendicular diameter, and C is the number of slices with hematoma visible multiplied with the slice distance. Panel B illustrates the measurement done by the automated hematoma segmentation tool qER-qQuant from Qure.ai. Panel C and D illustrate the semi-automated measurement done in Sectra IDS7 where an arbitrary diameter is drawn through the hematoma (panel C) and the software then identifies the boundaries and calculate the volume (panel D)
Manual volume assessment
Manual segmentation can be performed in most Picture Archiving and Communication Systems by measuring the hematoma area (typically using area measurement tools) in consecutive images and multiply the total area with the slice thickness. However, such manual segmentation is time consuming and therefore rarely used in routine health care.
The TADA formula, commonly referred to as “ABC/2”, where A is the largest diameter of the hematoma as measured on axial images; B is the largest diameter perpendicular to A on the same image slice, and C is the number of slices in which the hematoma is seen, multiplied by the slice thickness. This formula was introduced by Kwak et al. [113] and Broderick et al. [114] and was validated by Kothari et al. [115], who proposed a modified ABC/2 (mABC/2). In the modified method each image slice containing the hematoma was compared to the image slice containing the largest hematoma area in order to estimate “C”. Here, each image slice where the hematoma area was > 75% of the largest area was weighted as 1. A weighting of 0.5 was given to slices where the hematoma area ranged between 25 and 75% of the largest hematoma area. Slices with a hematoma area < 25% of the largest area were excluded. The sum of these weighted slices multiplied by slice thickness determined the value of C [115].
In general, the ABC/2 methods assume an ellipsoid-shaped hematoma, leading to both under- and overestimation of the true hematoma volume in non-ellipsoid-shaped hematomas [116,117,118,119]. The modified ABC/2 method was shown to underestimate hemorrhage volumes compared to standard ABC/2 and computer-assisted semi-automatic segmentation [116], as seen in Fig. 7. In addition, the larger and the more irregular in shape the hematoma was, the larger the difference is between the techniques [116]. Maeda et al. found that the ABC/2 method underestimated the ICH volume by 15% compared to planimetric methods [119]. The underestimation was confirmed by Hussein et al. who found that for hematomas smaller than 20 ml, there was a 10% underestimation of ICH volume when using the ABC/2 method compared to a computer-based analysis. This underestimation increased to 37% for hematomas larger than 40 ml [120]. Contrary to this, Wang et al. found a systematic 10% overestimation of the volume for hematomas smaller than 20 ml when using the ABC/2-formula compared to a computer-assisted volumetric analysis. This overestimation increased to 17% for hematomas between 20 and 40 ml, and 37% for hematomas larger than 40 ml [121].
In a study on vitamin K oral anticoagulant treated patients, Huttner et al. showed that more than half of the hematomas were irregularly shaped and for these, the ABC/2 method significantly overestimated the hematoma volume compared with computer-assisted planimetric analysis and that modifying the denominator to ABC/3 in irregularly shaped hematomas, increased the accuracy [122]. Scherer et al. compared the ABC/2 method to manual segmentation and a novel semi-automatic threshold-based region growing tool and found substantial agreement between manual and automatic segmentations and confirmed a systematic volume overestimation by the ABC/2 formula [123]. Using a different volume measurement software (Quantomo 5, Cybertrial, Inc, Calgary, Canada), Dowlatshahi et al. [124] found the volumetric measurements to be accurate for smaller hematomas, but less so for hematomas larger than 43.8 ml. The discrepancies between studies resulting in both under- and overestimation by the ABC/2-method is a major concern. With access to accurate and effective methods for volumetric measurement, the ABC/2 method appear obsolete and will likely be less used in the future.
Semi-automated volume measurements
Several studies have investigated computer-assisted semi-automated methods that use Hounsfield unit segmentation to estimate hematoma volume from images obtained by NCCT [125,126,127]. Another computer-aided measuring tool used in radiology is the Sectra Volume Measurement tool (Sectra IDS7, Sectra, Linköping, Sweden), where a line is manually drawn between two margins of the hematoma, and the software identifies the margins of the entire hematoma and calculates the volume.
Fully automated ICH identification and volume measurement
Recently, several fully automated imaging analysis tools based on machine learning algorithms such as Rapid AI (Ischemia view Inc., Menlo Park, California, United States.), CINA® ICH (Avicenna.AI, La Ciotat, France), and qER/qQuant (Qure.ai, Mumbai, India) have been shown to accurately identify ICHs from NCCT images [128,129,130]. The triage and notification tool qER has been shown to accurately identify intracranial bleeds, cranial fractures, mass effect or midline shift from NCCT images (Fig. 2) [129]. The qQuant tool can be used to automatically quantify ICH volume.
The Rapid ICH module has been shown to detect various types of ICH (excluding hemorrhagic transformations) with a sensitivity of 95% and specificity of 94% within less than 2 min from NCCT images. In a study by Heit et al. [128] in 2021, ICH volumes measured by Rapid ICH correlated well with expert manual segmentation (correlation coefficient r = 0.983). However, the Rapid ICH volumetric quantification tool is currently only available for investigational use.
Another recently developed fully automated image analysis tool based on machine learning algorithms is the CINA® ICH software included in Canon’s AUTOStroke package. The CINA® software does not measure hematoma volume, but can detect ICH with 95.6% accuracy, 91.4% sensitivity, and 97.5% specificity [130]. A study by Rava et al. showed that the CINA® software can detect intracranial hemorrhage (intraparenchymal, intraventricular, subdural or subarachnoid) with an overall sensitivity of 93 ± 3%, and an overall specificity of 93 ± 1% [131]. However, these studies identified limitations in the software’s ability to detect small hemorrhages (< 5 mm).
Conclusion
In this educational review aimed primarily for radiologists and neuroradiologists, we provide an overview of the most important neuroimaging findings for determining etiology and predicting outcome for ICH and describe the most widely used manual, semi-automated and automated methods for volumetric measurement for ICH from NCCT and CTA images. Identifying imaging markers on NCCT (blend sign, black hole sign, swirl sign and island sign) and CTA (spot sign) are important for prediction of early hematoma expansion and should be considered in the radiology report. Hematoma volume is the strongest predictor of 30-day mortality and functional outcome for patients with ICH and should as such be included in in the radiology report; hence, rapid and accurate tools for measuring ICH volume will become a major advancement in routine healthcare. The commonly used ABC/2 method has been shown to both under- and overestimate the volume of large and irregularly shaped ICHs as is therefore a poor substitute to accurate volume segmentation.
Future studies to assess and validate the ability of novel imaging tools to accurately identify and measure hematoma volume, perihematomal edema volume and hematoma expansion are warranted.
Abbreviations
- APOE:
-
Apolipoprotein E
- CAA:
-
Cerebral amyloid angiopathy
- CT:
-
Computed tomography
- CTA:
-
Computed tomography angiography
- DALYs:
-
Disability-adjusted life years
- DICOM:
-
Digital Imaging and Communications in Medicine
- GCS:
-
Glasgow coma scale
- ICH:
-
Intracerebral hemorrhage
- IVH:
-
Intraventricular hemorrhage
- MRI:
-
Magnetic resonance imaging
- NCCT:
-
Non-contrast computed tomography
- NIHSS:
-
National Institutes of Health Stroke Scale
- PHE:
-
Perihematomal edema
References
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9(2):167–176
Johnson CO, Nguyen M, Roth GA et al (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):439–458
Christensen MC, Mayer S, Ferran JM (2009) Quality of life after intracerebral hemorrhage: results of the factor seven for acute hemorrhagic stroke (FAST) trial. Stroke 40(5):1677–1682
Tran QK, Najafali D, Tiffany L et al (2021) Effect of blood pressure variability on outcomes in emergency patients with intracranial hemorrhage. West J Emerg Med 22(2):177–185
Kuramatsu JB, Gerner ST, Schellinger PD et al (2015) Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 313(8):824–836
Luzzi S, Elia A, Del Maestro M et al (2019) Indication, timing, and surgical treatment of spontaneous intracerebral hemorrhage: systematic review and proposal of a management algorithm. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.01.016
Salmela MB, Mortazavi S, Jagadeesan BD et al (2017) ACR Appropriateness Criteria(®) Cerebrovascular Disease. J Am Coll Radiol 14(5s):S34-s61
Khosravani H, Mayer SA, Demchuk A et al (2013) Emergency noninvasive angiography for acute intracerebral hemorrhage. AJNR Am J Neuroradiol 34(8):1481–1487
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (1993) Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 24(7):987–993
Brouwers HB, Chang Y, Falcone GJ et al (2014) Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol 71(2):158–164
Romero JM, Brouwers HB, Lu J et al (2013) Prospective validation of the computed tomographic angiography spot sign score for intracerebral hemorrhage. Stroke 44(11):3097–3102
Cai JX, Zhu HC, Yang D et al (2020) Accuracy of imaging markers on noncontrast computed tomography in predicting intracerebral hemorrhage expansion. Neurol Res 42(11):973–979
Kim JY, Bae HJ (2017) Spontaneous Intracerebral Hemorrhage: management. J Stroke 19(1):28–39
O’Brien T, Mitra B, Le Sage N et al (2020) Clinically significant traumatic intracranial hemorrhage following minor head trauma in older adults: a retrospective cohort study. Brain Inj 34(6):834–839
Al-Shahi Salman R, Labovitz DL, Stapf C (2009) Spontaneous intracerebral haemorrhage. BMJ 339:b2586
Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9(7):689–701
Gross BA, Jankowitz BT, Friedlander RM (2019) Cerebral Intraparenchymal Hemorrhage: A Review. JAMA 321(13):1295–1303
Ikram MA, Wieberdink RG, Koudstaal PJ (2012) International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep 14(4):300–306
O’Donnell MJ, Xavier D, Liu L et al (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376(9735):112–123
Hemphill JC 3rd, Greenberg SM, Anderson CS et al (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46(7):2032–2060
Wang X, Arima H, Al-Shahi Salman R et al (2015) Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke 46(2):376–381
Wagner A, Schebesch KM, Zeman F et al (2018) Primary cCT imaging based clinico-neurological assessment-calling for addition of telestroke video consultation in patients with intracerebral hemorrhage. Front Neurol 9:607
Wagner A, Schebesch KM, Isenmann S et al (2019) Interdisciplinary decision making in hemorrhagic stroke based on CT imaging-differences between neurologists and neurosurgeons regarding estimation of patients’ symptoms, glasgow coma scale, and National Institutes of Health Stroke Scale. Front Neurol 10:997
Maruishi M, Shima T, Okada Y, Nishida M, Yamane K (2001) Involvement of fluctuating high blood pressure in the enlargement of spontaneous intracerebral hematoma. Neurol Med Chir 41(6):300–305
Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A (2004) Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 35(6):1364–1367
Rodriguez-Luna D, Piñeiro S, Rubiera M et al (2013) Impact of blood pressure changes and course on hematoma growth in acute intracerebral hemorrhage. Eur J Neurol 20(9):1277–1283
Lovelock CE, Molyneux AJ, Rothwell PM (2007) Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol 6(6):487–493
Flaherty ML, Tao H, Haverbusch M et al (2008) Warfarin use leads to larger intracerebral hematomas. Neurology 71(14):1084–1089
Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ (2013) New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 70(12):1486–1490
Hagii J, Tomita H, Metoki N et al (2014) Characteristics of intracerebral hemorrhage during rivaroxaban treatment: comparison with those during warfarin. Stroke 45(9):2805–2807
Takahashi H, Jimbo Y, Takano H et al (2016) Intracerebral hematoma occurring during warfarin versus non-vitamin K antagonist oral anticoagulant therapy. Am J Cardiol 118(2):222–225
Inohara T, Xian Y, Liang L et al (2018) Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA 319(5):463–473
Scott JF, Robinson GM, French JM, O’Connell JE, Alberti KG, Gray CS (1999) Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet 353(9150):376–377
Fogelholm R, Murros K, Rissanen A, Avikainen S (2005) Admission blood glucose and short term survival in primary intracerebral haemorrhage: a population based study. J Neurol Neurosurg Psychiatry 76(3):349–353
Koga M, Yamagami H, Okuda S et al (2015) Blood glucose levels during the initial 72 h and 3-month functional outcomes in acute intracerebral hemorrhage: the SAMURAI-ICH study. J Neurol Sci 350(1–2):75–78
Qureshi AI, Palesch YY, Martin R et al (2011) Association of serum glucose concentrations during acute hospitalization with hematoma expansion, perihematomal edema, and three month outcome among patients with intracerebral hemorrhage. Neurocrit Care 15(3):428–435
Yao X, Wu B, Xu Y, Siwila-Sackman E, Selim M (2015) Day-night variability of hematoma expansion in patients with spontaneous intracerebral hemorrhage. J Biol Rhythms 30(3):242–250
Parry-Jones AR, Sammut-Powell C, Paroutoglou K et al (2019) An intracerebral hemorrhage care bundle is associated with lower case fatality. Ann Neurol 86(4):495–503
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32(4):891–897
Witsch J, Siegerink B, Nolte CH et al (2021) Prognostication after intracerebral hemorrhage: a review. Neurol Res Pract 3(1):22
Tseng WC, Wang YF, Wang TG, Hsiao MY (2021) Early spot sign is associated with functional outcomes in primary intracerebral hemorrhage survivors. BMC Neurol. https://doi.org/10.1186/s12883-021-02146-3
Greenberg SM, Ziai WC, Cordonnier C et al (2022) 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 53(7):e282–e361
Kidwell CS, Chalela JA, Saver JL et al (2004) Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 292(15):1823–1830
Jolink WMT, Wiegertjes K, Rinkel GJE, Algra A, De Leeuw FE, Klijn CJM (2020) Location-specific risk factors for intracerebral hemorrhage Systematic review and meta-analysis. Neurology 95(13):E1807–E1818
Jackson CA, Sudlow CL (2006) Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage? J Neurol Neurosurg Psychiatry 77(11):1244–1252
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344(19):1450–1460
Meretoja A, Strbian D, Putaala J et al (2012) SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 43(10):2592–2597
Tsushima Y, Aoki J, Endo K (2003) Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol 24(1):88–96
Dobrynina LA, Zabitova MR, Kalashnikova LA, Gnedovskaya EV, Piradov MA (2018) Hypertension and cerebral microangiopathy (cerebral small vessel disease): genetic and epigenetic aspects of their relationship. Acta Naturae 10(2):4–15
Greenberg SM, Charidimou A (2018) Diagnosis of cerebral amyloid angiopathy: evolution of the boston criteria. Stroke 49(2):491–497
Charidimou A, Gang Q, Werring DJ (2012) Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 83(2):124–137
Rodrigues MA, Samarasekera N, Lerpiniere C et al (2018) The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol 17(3):232–240
Greenberg SM, Vernooij MW, Cordonnier C et al (2009) Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8(2):165–174
Linn J, Halpin A, Demaerel P et al (2010) Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 74(17):1346–1350
Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT (1995) Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 38(2):254–259
Boulouis G, Charidimou A, Greenberg SM (2016) Sporadic cerebral amyloid angiopathy: pathophysiology, neuroimaging features, and clinical implications. Semin Neurol 36(3):233–243
Reddy ST, Savitz SI (2020) Hypertension-related cerebral microbleeds. Case Rep Neurol 12(3):266–269
Charidimou A, Boulouis G, Gurol ME et al (2017) Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 140(7):1829–1850
Chen S, Zhao B, Wang W, Shi L, Reis C, Zhang J (2017) Predictors of hematoma expansion predictors after intracerebral hemorrhage. Oncotarget 8(51):89348–89363
Broderick JP, Diringer MN, Hill MD et al (2007) Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke 38(3):1072–1075
Dowlatshahi D, Smith EE, Flaherty ML, Ali M, Lyden P, Demchuk AM (2011) Small intracerebral haemorrhages are associated with less haematoma expansion and better outcomes. Int J Stroke 6(3):201–206
Delcourt C, Sato S, Zhang S et al (2017) Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 88(15):1408–1414
Roh D, Sun CH, Murthy S et al (2019) Hematoma expansion differences in lobar and deep primary intracerebral hemorrhage. Neurocrit Care 31(1):40–45
Ironside N, Chen CJ, Dreyer V, Christophe B, Buell TJ, Connolly ES (2020) Location-specific differences in hematoma volume predict outcomes in patients with spontaneous intracerebral hemorrhage. Int J Stroke 15(1):90–102
Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O (1994) Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg 80(1):51–57
Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R (1998) Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 29(6):1160–1166
Blacquiere D, Demchuk AM, Al-Hazzaa M et al (2015) Intracerebral hematoma morphologic appearance on noncontrast computed tomography predicts significant hematoma expansion. Stroke 46(11):3111–3116
Boulouis G, Morotti A, Brouwers HB et al (2016) Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurol 73(8):961–968
Yang J, Arima H, Wu G et al (2015) Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke 46(4):1009–1013
Barras CD, Tress BM, Christensen S et al (2009) Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke 40(4):1325–1331
Venkatasubramanian C, Mlynash M, Finley-Caulfield A et al (2011) Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 42(1):73–80
Zheng H, Chen C, Zhang J, Hu Z (2016) Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovasc Dis 42(3–4):155–169
Huan RZ, Li Y, Tan JH, Tang J, Huang N, Cheng Y (2021) The hounsfield unit of perihematomal edema is associated with poor clinical outcomes in intracerebral hemorrhage. World Neurosurg 146:E829–E836
Parry-Jones AR, Wang X, Sato S et al (2015) Edema extension distance: outcome measure for phase ii clinical trials targeting edema after intracerebral hemorrhage. Stroke 46(6):e137–e140
Hurford R, Vail A, Heal C et al (2019) Oedema extension distance in intracerebral haemorrhage: association with baseline characteristics and long-term outcome. Eur Stroke J 4(3):263–270
Sembolini A, Romoli M, Pannacci U et al (2020) Acute hematoma expansion after spontaneous intracerebral hemorrhage: risk factors and impact on long-term prognosis. Neurol Sci 41(9):2503–2509
Delcourt C, Huang Y, Arima H et al (2012) Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology 79(4):314–319
Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T (1996) Enlargement of spontaneous intracerebral hemorrhage incidence and time course. Stroke 27(10):1783–1787
Brott T, Broderick J, Kothari R et al (1997) Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28(1):1–5
He GN, Guo HZ, Han X, Wang EF, Zhang YQ (2018) Comparison of CT black hole sign and other CT features in predicting hematoma expansion in patients with ICH. J Neurol 265(8):1883–1890
Demchuk AM, Dowlatshahi D, Rodriguez-Luna D et al (2012) Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol 11(4):307–314
Sprigg N, Flaherty K, Appleton JP et al (2018) Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet 391(10135):2107–2115
Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE (2011) Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology 76(14):1238–1244
Hallevi H, Albright KC, Aronowski J et al (2008) Intraventricular hemorrhage: anatomic relationships and clinical implications. Neurology 70(11):848–852
Bu Y, Chen M, Gao T, Wang X, Li X, Gao F (2016) Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc Neurol 1(1):23–27
You S, Zheng D, Delcourt C et al (2019) Determinants of early versus delayed neurological deterioration in intracerebral hemorrhage. Stroke 50(6):1409–1414
Li Q, Li R, Zhao LB et al (2020) Intraventricular hemorrhage growth: definition, prevalence and association with hematoma expansion and prognosis. Neurocrit Care 33(3):732–739
Yogendrakumar V, Ramsay T, Fergusson D et al (2019) New and expanding ventricular hemorrhage predicts poor outcome in acute intracerebral hemorrhage. Neurology 93(9):e879–e888
Witsch J, Falcone GJ, Leasure AC et al (2021) Intracerebral hemorrhage with intraventricular extension associated with loss of consciousness at symptom onset. Neurocrit Care. https://doi.org/10.1007/s12028-020-01180-2
Yogendrakumar V, Ramsay T, Fergusson DA et al (2020) Redefining hematoma expansion with the inclusion of intraventricular hemorrhage growth. Stroke 51(4):1120–1127
Wada R, Aviv RI, Fox AJ et al (2007) CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 38(4):1257–1262
Goldstein JN, Fazen LE, Snider R et al (2007) Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 68(12):889–894
Brouwers HB, Falcone GJ, McNamara KA et al (2012) CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care 17(3):421–428
Rizos T, Dorner N, Jenetzky E et al (2013) Spot signs in intracerebral hemorrhage: useful for identifying patients at risk for hematoma enlargement? Cerebrovasc Dis 35:234
Du FZ, Jiang R, Gu M, He C, Guan J (2014) The accuracy of spot sign in predicting hematoma expansion after intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One 9(12):e115777
Selariu E, Zia E, Brizzi M, Abul-Kasim K (2012) Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol 12:109
Ng D, Churilov L, Mitchell P, Dowling R, Yan B (2018) The CT swirl sign is associated with hematoma expansion in intracerebral hemorrhage. AJNR Am J Neuroradiol 39(2):232–237
Yu Z, Zheng J, He M et al (2019) Accuracy of swirl sign for predicting hematoma enlargement in intracerebral hemorrhage: a meta-analysis. J Neurol Sci 399:155–160
Li Q, Zhang G, Xiong X et al (2016) Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke 47(7):1777–1781
Li Q, Yang WS, Chen SL et al (2018) Black hole sign predicts poor outcome in patients with intracerebral hemorrhage. Cerebrovasc Dis 45(1–2):48–53
Zheng J, Yu Z, Guo R, Li H, You C, Ma L (2018) Meta-analysis of predictive significance of the black hole sign for hematoma expansion in intracerebral hemorrhage. World Neurosurg 115:e711–e716
Li Q, Zhang G, Huang YJ et al (2015) Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke 46(8):2119–2123
Sporns PB, Schwake M, Schmidt R et al (2017) Computed tomographic blend sign is associated with computed tomographic angiography spot sign and predicts secondary neurological deterioration after intracerebral hemorrhage. Stroke 48(1):131–135
Wu G, Shen Z, Wang L, Sun S, Luo J, Mao Y (2017) Post-operative re-bleeding in patients with hypertensive ICH is closely associated with the CT blend sign. BMC Neurol 17(1):131
Yu Z, Zheng J, Guo R et al (2017) Performance of blend sign in predicting hematoma expansion in intracerebral hemorrhage: a meta-analysis. Clin Neurol Neurosurg 163:84–89
Li Q, Liu QJ, Yang WS et al (2017) Island sign: an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke 48(11):3019–3025
Zhang F, Li H, Qian J et al (2018) Island sign predicts long-term poor outcome and mortality in patients with intracerebral hemorrhage. World Neurosurg 120:e304–e312
Huang YW, Zhang Q, Yang MF (2018) A reliable grading system for prediction of hematoma expansion in intracerebral hemorrhage in the basal ganglia. Biosci Trends 12(2):193–200
Zheng J, Yu Z, Wang C et al (2018) Evaluating the predictive value of island sign and spot sign for hematoma expansion in spontaneous intracerebral hemorrhage. World Neurosurg 117:e167–e171
Wei Y, Zhu G, Gao Y et al (2020) Island sign predicts hematoma expansion and poor outcome after intracerebral hemorrhage: a systematic review and meta-analysis. Front Neurol 11:429
Shimoda Y, Ohtomo S, Arai H, Okada K, Tominaga T (2017) Satellite sign: a poor outcome predictor in intracerebral hemorrhage. Cerebrovasc Dis 44(3–4):105–112
Serrano E, López-Rueda A, Moreno J et al (2022) The new hematoma maturity score is highly associated with poor clinical outcome in spontaneous intracerebral hemorrhage. Eur Radiol 32(1):290–299
Kwak R, Kadoya S, Suzuki T (1983) Factors affecting the prognosis in thalamic hemorrhage. Stroke 14(4):493–500
Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J (1990) Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg 72(2):195–199
Kothari RU, Brott T, Broderick JP et al (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27(8):1304–1305
Krishnan K, Mukhtar SF, Lingard J et al (2015) Performance characteristics of methods for quantifying spontaneous intracerebral haemorrhage: data from the efficacy of nitric oxide in stroke (ENOS) trial. J Neurol Neurosurg Psychiatry 86(11):1258–1266
Delcourt C, Carcel C, Zheng D et al (2019) Comparison of ABC methods with computerized estimates of intracerebral hemorrhage volume: The INTERACT2 study. Cerebrovasc Dis Extra 9(3):148–154
Khan M, Baird GL, Elias R et al (2017) Comparison of intracerebral hemorrhage volume calculation methods and their impact on scoring tools. J Neuroimaging 27(1):144–148
Maeda AK, Aguiar LR, Martins C, Bichinho GL, Gariba MA (2013) Hematoma volumes of spontaneous intracerebral hemorrhage: the ellipse (ABC/2) method yielded volumes smaller than those measured using the planimetric method. Arq Neuropsiquiatr 71(8):540–544
Hussein HM, Tariq NA, Palesch YY, Qureshi AI (2013) Reliability of hematoma volume measurement at local sites in a multicenter acute intracerebral hemorrhage clinical trial. Stroke 44(1):237–239
Wang CW, Juan CJ, Liu YJ et al (2009) Volume-dependent overestimation of spontaneous intracerebral hematoma volume by the ABC/2 formula. Acta Radiol 50(3):306–311
Huttner HB, Steiner T, Hartmann M et al (2006) Comparison of ABC/2 estimation technique to computer-assisted planimetric analysis in warfarin-related intracerebral parenchymal hemorrhage. Stroke 37(2):404–408
Scherer M, Cordes J, Younsi A et al (2016) Development and validation of an automatic segmentation algorithm for quantification of intracerebral hemorrhage. Stroke 47(11):2776–2782
Dowlatshahi D, Kosior JC, Idris S et al (2012) Planimetric hematoma measurement in patients with intraventricular hemorrhage: is total volume a preferred target for reliable analysis? Stroke 43(7):1961–1963
Wu TY, Sobowale O, Hurford R et al (2016) Software output from semi-automated planimetry can underestimate intracerebral haemorrhage and peri-haematomal oedema volumes by up to 41 %. Neuroradiology 58(9):867–876
Kosior JC, Idris S, Dowlatshahi D et al (2011) Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral haemorrhage. Int J Stroke 6(4):302–305
Chen MH, Li Z, Ding JP, Lu XQ, Cheng YA, Lin JY (2020) Comparison of common methods for precision volume measurement of hematoma. Comput Math Method Med. https://doi.org/10.1155/2020/6930836
Heit JJ, Coelho H, Lima FO et al (2021) Automated cerebral hemorrhage detection using RAPID. AJNR Am J Neuroradiol 42(2):273–278
Chilamkurthy S, Ghosh R, Tanamala S et al (2018) Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet 392(10162):2388–2396
McLouth J, Elstrott S, Chaibi Y et al (2021) Validation of a deep learning tool in the detection of intracranial hemorrhage and large vessel occlusion. Front Neurol 12:656112
Rava RA, Seymour SE, LaQue ME et al (2021) Assessment of an artificial intelligence algorithm for detection of intracranial hemorrhage. World Neurosurg 150:e209–e217
Acknowledgements
Dr Lee Nolan-Lönn is acknowledged for professional language editing. The authors wish to thank all colleagues and patients at the department of medical imaging and physiology at Skåne University Hospital/Lund.
Funding
Open access funding provided by Lund University. Author JW has obtained funding by the Crafoord Foundation (20200548, #2020-04841), VINNOVA (Grant No. 2020-04841) and AIDA (#2024).
Author information
Authors and Affiliations
Contributions
AH, TU, and JW researched literature and conceived the manuscript. TU and JW are guarantors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hillal, A., Ullberg, T., Ramgren, B. et al. Computed tomography in acute intracerebral hemorrhage: neuroimaging predictors of hematoma expansion and outcome. Insights Imaging 13, 180 (2022). https://doi.org/10.1186/s13244-022-01309-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-022-01309-1