Abstract
Many benign breast entities have a clinical and imaging presentation that can mimic breast cancer. The purpose of this review is to illustrate the wide spectrum of imaging features that can be associated with benign breast diseases with an emphasis on the suspicious imaging findings associated with these benign conditions that can mimic cancer. As radiologic-pathologic correlation can be particularly challenging in these cases, the radiologist’s familiarity with these benign entities and their imaging features is essential to ensure that a benign pathology result is accepted as concordant when appropriate and that a suitable management plan is formulated.
Similar content being viewed by others
Keypoints
-
A heterogeneous group of benign breast conditions can mimic breast cancer.
-
Understanding the imaging spectrum of benign breast diseases ensures appropriate radiologic–pathologic correlation.
-
Appropriate radiologic–pathologic correlation is essential to avoid delay in proper management.
Background
The clinical presentation of several benign breast conditions, common and rare, can mimic breast cancer. Suspicious imaging features may be part of the imaging spectrum of many benign breast conditions, making them indistinguishable from breast cancer. Although biopsy is often required to confirm the diagnosis, understanding the range of clinical and imaging findings is important to ensure appropriate radiologic-pathologic correlation and clinical management.
We have classified mimickers of breast cancer into three groups: inflammatory breast conditions, proliferative breast conditions, and benign breast tumors (Fig. 1). Benign inflammatory breast conditions that mimic malignancy include infectious mastitis and breast abscess, granulomatous mastitis, and lymphocytic mastopathy. Proliferative breast conditions that mimic malignancy include fat necrosis, stromal fibrosis, and sclerosing adenosis. Benign tumors that mimic malignancy include hamartoma, pseudoangiomatous hyperplasia, tubular adenoma, desmoid fibromatosis, and granular cell tumor. The purpose of this review is to illustrate the wide range of suspicious mammographic, sonographic, and magnetic resonance imaging (MRI) features associated with benign breast diseases. Recognition of these conditions is essential to ensuring careful and accurate radiologic-pathologic correlation, and to formulating a clinical management plan.
Inflammatory breast conditions
Benign inflammatory breast conditions constitute a heterogeneous group of breast conditions characterized by a marked inflammatory process. These conditions are clinically important because they closely mimic and are often clinically and radiologically indistinguishable from inflammatory breast cancer [1]. Thorough imaging assessment of these cases is important. Biopsy is usually indicated to establish the correct diagnosis and to rule out breast cancer. Less-common inflammatory conditions that will not be described here include those associated with connective tissue disorders such as Churg–Strauss syndrome, amyloidosis, granulomatosis with polyangiitis (formerly known as Wegener’s granulomatosis), and sarcoidosis.
Infectious mastitis and breast abscess
Breast abscess is a complication of infectious mastitis. Abscesses can be associated with lactation, in the case of puerperal abscesses, or independent of pregnancy, in the case of nonpuerperal abscesses [2]. Puerperal abscesses tend to be peripheral in location and are often easily recognized clinically. Nonpuerperal abscesses can pose a diagnostic challenge and are more commonly seen in younger women. They are usually periareolar and typically have worse outcomes and a higher rate of recurrence than puerperal abscesses. The risk factors for nonpuerperal breast abscesses are thought to include smoking and diabetes [3, 4].
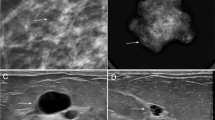
Mammographically, mastitis and breast abscess present with skin thickening, asymmetry, a mass, or architectural distortion (Fig. 2a) [2]. Sonographic features of breast abscesses include one or more hypoechoic collections of variable shapes and sizes that are often continuous and multiloculated (Fig. 2b, c). Breast abscesses typically demonstrate a thick echogenic rim and increased vascularity, suggesting malignancy [2]. Associated mastitis presents as an area of increased parenchymal echogenicity, representing inflamed glandular parenchyma. Skin thickening, distended lymphatic vessels, and inflammatory axillary adenopathy can also be seen. On MRI, breast abscesses will typically be T2-hyperintense, have progressive enhancement kinetics, and sometimes have the characteristic thin rim of peripheral enhancement (Fig. 2d–f) [2].
Breast abscess. A 45-year-old woman presented with a palpable area in the right breast. Mediolateral oblique (a) mammogram shows a focal asymmetry in the upper outer breast (arrow) and associated trinagular palpable marker. Power Doppler ultrasound images (b, c) reveal two hypoechoic, oval masses with peripheral vascularity in the same region (asterisks). Axial post-contrast T1-weighted MRI (d), sagittal T2-weighted MRI (e), and postcontrast subtraction T1-weighted MRI (f) show two T2-hyperintense, rim-enhancing masses (arrows). Needle biopsy showed acute inflammatory cells consistent with abscess without evidence of malignancy
As inflammatory breast cancer is the most important differential consideration with this clinical presentation, caution must be exercised to exclude an underlying malignancy. Patients with a clinical presentation typical of a breast abscess require a short-term, 7- to 14-day follow-up after treatment with antibiotics and drainage [2]. Inflammatory breast cancer should be strongly considered in patients with breast erythema and swelling in the absence of an abscess on ultrasound evaluation, especially in older non-lactating patients and in patients who are at increased risk of breast cancer. Mammography is indicated in these patients and should not be delayed. In lactating patients, although mammography is initially delayed until the acute symptoms of mastitis resolve following a course of antibiotics, mammography is indicated when there is a clinical suspicion for malignancy and in those with a prolonged clinical course [5, 6].
Although mastitis and breast abscess can be difficult to distinguish from inflammatory breast cancer, a number of imaging features tend to differ between them. While the skin thickening associated with inflammatory breast cancer is likely to be diffuse, the thickening associated with breast abscess and mastitis tends to be localized to the area involved with mastitis. A study by Chow found that suspicious microcalcifications are the most specific finding for breast cancer in patients with inflammatory breast symptoms of unclear etiology [7]. Nguyen et al. suggested that a mass with a hypoechoic wall and associated interstitial fluid is more suggestive of a breast abscess and not usually seen in the setting of breast cancer [8]. In addition, ultrasound evaluation of the axillary lymph nodes, in cases where malignancy is the primary consideration, is more likely to show markedly abnormal lymph node enlargement with the characteristic cortical thickening and hilar displacement of metastatic lymph nodes. In contrast, breast abscesses are more likely to be associated with reactive lymphadenopathy characterized by mild diffuse cortical thickening [2]. MRI can sometimes be used to differentiate the two entities: inflammatory breast cancer is more likely to show heterogenous enhancement with washout kinetics, while breast abscess is more likely to have an increased T2 signal and benign enhancement kinetics [2]. In patients with a prolonged course and patients whose condition does not respond to antibiotics, breast biopsy is indicated and should not be delayed.
Granulomatous mastitis
Granulomatous mastitis is an inflammatory breast condition of unknown etiology. Pathologically, it is characterized by a non-caseating granulomatous inflammatory process of the breast lobules without an identifiable infectious or inflammatory etiology [9, 10]. Fat necrosis, abscess formation, and fibrosis are commonly associated end-stage features of this disease process [1]. Granulomatous mastitis is a rare diagnosis of exclusion. An inflammatory or infectious etiology such as plasma cell mastitis, granulomatosis with polyangiitis, sarcoidosis, or tuberculous mastitis must be excluded [11]. These conditions are usually distinguishable histologically. Granulomatous mastitis typically affects parous women of childbearing age, often within 6 months of pregnancy, although the timing of onset after pregnancy can vary widely and has been reported to be as long as 9 years post-partum in some cases [10]. Granulomatous mastitis typically presents as a firm palpable mass that is sometimes associated with skin erythema or pain [11]. Other symptoms include draining sinus tracts and nipple discharge.
Mammographically, granulomatous mastitis can have a variety of presenting features such as masses, asymmetries, or trabecular and skin thickening (Fig. 3a). In some cases, the findings are mammographically occult. Sonographically, one or more commonly multiple irregular hypoechoic masses have been described (Fig. 3b) [11,12,13,14]. These masses can sometimes be continuous and can appear tubular. In other cases, ultrasound shows only parenchymal distortion with increased shadowing, without a discrete mass (Fig. 3c) [11]. Associated skin thickening and edema have also been described [11]. On MRI, the two most common findings are masses with circumscribed margins and rim enhancement and heterogeneous non-mass enhancement in a segmental or regional distribution (Fig. 3d, e) [15]. Reactive lymphadenopathy may also be present (Fig. 3e). Most cases of granulomatous mastitis have benign persistent enhancement kinetics, although washout kinetics can also be seen, making MRI unreliable in distinguishing between inflammatory breast cancer and granulomatous mastitis [16,17,18]. Positron emission tomography (PET)/CT can show fluorodeoxyglucose avidity (Fig. 3f).
Granulomatous mastitis. A 34-year-old woman who is 2 years post-partum presented with a palpable left breast mass, diffuse breast swelling, tenderness, and erythema for several weeks. Bilateral mediolateral oblique mammogram (a) show diffuse skin thickening (solid arrow), global asymmetry, and trabecular thickening (dashed arrows) in the left breast, asymmetric from the right breast. Extended field of view grayscale ultrasound (b) shows an ill-defined hypoechoic, irregular mass (arrows). Color Doppler ultrasound (c) shows increased vascularity, edema (arrow head), and skin thickening (dashed arrow). T1weighted axial delayed post-contrast (d) and sagittal subtraction (e) post-contrast MRI shows diffuse skin thickening (solid arrow) and trabecular thickening with heterogenous enhancement involving the left superior breast (dashed arrows). There is associated axillary adenopathy (solid arrow in e). Axial PET/CT (f) shows diffuse fluorodeoxyglucose avidity involving the left breast (arrow). Findings and clinical presentation were suspicious for inflammatory breast cancer. The patient underwent three core needle breast biopsies of the mass in the left breast over the span of a month, and a skin punch biopsy. All biopsies showed dense stromal fibrosis, chronic inflammation, and features suggestive of granulomatous mastitis without atypia or malignancy
Because the clinical and radiological presentation of granulomatous mastitis is indistinguishable from breast cancer, core needle biopsy is indicated to establish the diagnosis. Once concordance of the biopsy results is established, granulomatous mastitis is traditionally treated with corticosteroids or surgical intervention, such as wide local excision of localized disease or rarely mastectomy when the disease is extensive and resistant to corticosteroid therapy [19]. In cases resistant to corticosteroids, methotrexate can be used. In recent years, expectant conservative management is becoming the treatment of choice, especially in mild cases. Spontaneous resolution has been reported in about half of patients [20]. The prognosis of granulomatous mastitis is favorable, although some cases can be refractory to therapy and can develop significant sinus tracts and scarring [11].
Lymphocytic mastopathy
Lymphocytic mastopathy is a perivascular and perilobular inflammatory process of the breast parenchyma incited by the infiltration of lymphocytes [21,22,23,24]. Clinically, it presents as one or more painless, mobile, discrete breast masses, closely mimicking malignancy. Multicentric and bilateral involvement is frequent. In most cases, lymphocytic mastopathy is associated with diabetes; however, other autoimmune disorders such as Hashimoto’s thyroiditis, Sjögren’s syndrome, and systemic lupus erythematosus have also been associated with lymphocytic mastopathy [1, 21, 25, 26]. Lymphocytic mastopathy has also been described in the absence of an underlying systemic condition. When associated with diabetes, this entity is often referred to as diabetic mastopathy and is most often seen in patients with a long-standing history of insulin-dependent diabetes. It has also been described in men in association with gynecomastia [26, 27].
Mammographically, lymphocytic mastopathy presents as an ill-defined mass or asymmetry. Many times, it is mammographically occult. On sonography, it frequently presents as a hypoechoic irregular mass with posterior acoustic shadowing or an area of shadowing without a discrete mass (Fig. 4). Increased vascularity can also be seen (Fig. 5). MRI findings include heterogeneously enhancing masses and non-mass enhancement [28,29,30]. To date, there is no evidence that lymphocytic mastopathy is associated with an increased risk of developing lymphoma [22, 25]. The process is usually self-limited. Recurrence has been reported in patients who undergo surgical excision [21]. Therefore, close clinical and imaging follow-up is indicated to ensure that there is no interval development of new breast masses [22].
Diabetic mastopathy. A 56-year-old patient presented with bilateral palpable breast masses and an 8-year history of type 2 diabetes. Bilateral craniocaudal mammogram (a) shows bilateral non-calcified, obscured masses correlating with the palpable triangular markers (arrows). Grayscale right (b) and left (c) breast ultrasound shows irregular, hypoechoic masses with posterior acoustic shadowing (arrows). Power Doppler (d) ultrasound demonstrated internal vascularity (arrow) involving these masses. Core needle biopsy of both masses showed perilobular lymphocytic infiltration. Repeat core needle biopsy 8 months after the initial biopsy showed chronic lymphocytic lobulitis. No evidence of malignancy. Findings are consistent with diabetic mastopathy
Lymphocytic mastopathy. A 37-year-old woman presented with right breast palpable retroareolar mass, swelling, erythema, and tenderness. Craniocaudal and lateral mammography (a, b) shows a focal asymmetry (solid arrows) and skin thickening (dashed arrows) associated with the triangular palpable marker in the lateral breast. Grayscale (c) and color Doppler breast ultrasound (d) reveals multiple retroareolar, vascular masses (arrows). Core needle biopsy showed acute and chronic inflammation and granulation tissue. The patient’s symptoms resolved within a month. The patient presented 2 years later with a contralateral palpable breast mass and nipple inversion. Craniocaudal and lateral mammographic views (e, f) reveal a focal asymmetry in the central breast associated with the palpable marker (arrows). Grayscale (c) and color Doppler (d) breast ultrasound reveals a vascular heterogeneous mass (arrows). Core needle biopsy revealed benign breast tissue with lymphoplasmacytic infiltrate. The patient’s symptoms improved over the following 6 months. She did not have a history of diabetes or other known immunologic condition
Proliferative breast conditions
Fat necrosis
Fat necrosis is a proliferative condition commonly seen as a result of breast surgery, infection, trauma, or radiation. Fat necrosis comprises 2.75% of breast lesions and is incited by the destruction of adipocytes, which causes an inflammatory process [31]. Clinical features of fat necrosis include palpable masses, pain, and skin changes such as skin tethering, skin thickening, and dimpling [32]. Fat necrosis has a wide spectrum of imaging features, some of which show the typical, classically benign findings, such as dystrophic calcifications or oil cysts. However, there are other imaging findings that can closely mimic malignancy: suspicious mammographic features of fat necrosis include irregular and spiculated masses, architectural distortion, asymmetries, coarse heterogeneous and even branching or pleomorphic calcifications. Ultrasound features of fat necrosis that mimic malignancy include irregular hypoechoic masses with posterior acoustic shadowing (Fig. 6). Characteristically benign features of fat necrosis on MRI include one or more fat-containing masses, which demonstrate T1 hyperintensity on non-fat supresed T1 sequences with corresponding drop in signal on fat-suppressed T1 and T2 sequences. Conversely, MRI features of fat necrosis that resemble malignancy are irregular enhancing masses, with or without a thick, irregular rim. Non-mass enhancement has also been reported [31]. If a PET/CT is performed, these lesions can be hypermetabolic [31].
Fat necrosis. A 52-year-old woman with remote history of mastopexy presented with a palpable breast mass. Mediolateral oblique mammogram (a) shows an irregular mass with spiculated margins, associated coarse rim calcifications (solid arrow), and focal skin thickening (dashed arrow). Longitudinal grayscale ultrasound (b) shows a non-parallel irregular hypoechoic mass (arrow). Fat-suppressed axial post-contrast T1-weighted breast MRI (c) shows an irregular mass with spiculated margins (arrow) with associated singal void artifact related to post-biopsy clip marker. Ultrasound-guided biopsy showed fat necrosis. Repeat biopsy under MRI guidance confirmed the diagnosis of fat necrosis
The management of fat necrosis is based on the imaging features. Typical benign imaging features do not require further workup. Suspicious features including irregular masses, suspicious calcifications, and architectural distortions require image-guided biopsy. The radiologic-pathologic correlation of the suspicious imaging features of fat necrosis require the radiologist’s familiarity with the wide spectrum of suspicious imaging features of fat necrosis as well as careful review of the biopsy technique and confirmation of adequate sampling. Determining concordance can be challenging, especially on MRI-guided biopsies where the target may not remain visible and adequacy of sampling may be difficult to judge. If needed, a short-term follow-up MRI after biopsy may be recommended.
Stromal fibrosis
Stromal fibrosis in the breast is a benign pathologic entity characterized by proliferation of stroma with obliteration of the mammary acini and ducts on pathologic analysis. This process results in a localized area of fibrous tissue associated with hypoplastic mammary ducts and lobules. Stromal fibrosis is not an uncommon pathologic diagnosis, representing 2%-9% of biopsied breast lesions. This condition can manifest as a palpable mass or be clinically occult. The most common mammographic findings are calcifications, followed by masses and asymmetries (Fig. 7). With the advent of tomosynthesis, stromal fibrosis can present as architectural distortion. Sonographic findings of stromal fibrosis include masses and non-mass lesions. In most cases, the masses are oval or round, but stromal fibrosis can also present as irregular masses in 13% of cases (Fig. 8) [33]. MRI features include masses, non-mass enhancement and foci of enhancement [34]. The masses can demonstrate variable shapes and kinetics. It is important to note that stromal fibrosis can be seen in the setting of malignancy and has a reported upgrade rate to malignancy of 7% [35]. Therefore, evaluation of sampling adequacy and establishing radiologic-pathologic correlation in these cases is of utmost importance. Establishing a close relationship with the pathologist is essential to assess adequacy of the sample and whether repeat biopsy is indicated. Short-term follow-up is recommended to ensure stability [34,35,36].
Stromal fibrosis. A 55-year-old woman presented for a focal asymmetry detected on screening mammography. Craniocaudal (a) and lateral (b) spot compression mammographic views show an oval non-calcified mass with angular margins (arrows). Grayscale transverse (c) and longitudinal (d) ultrasound show a mixed-echogenicity oval mass correlating with the mammographic finding (arrows). Ultrasound-guided biopsy yielded stromal fibrosis. Six-month follow-up mammography and ultrasound were recommended and demonstrated stability
Stromal fibrosis. A 48-year-old female presented with a palpable breast mass. Spot compression tangential (a), mediolateral (b), and craniocaudal (c) mammographic views did not show a corresponding abnormality. Grayscale (d) and power Doppler (f) ultrasound images reveal an irregular mass with posterior acoustic shadowing corresponding to the palpable area. Core needle biopsy of the mass showed stromal fibrosis without evidence of malignancy
Sclerosing adenosis
Sclerosing adenosis is a proliferative breast condition of the terminal lobular unit characterized by distortion of the lobules with an increased number of acini and desmoplasia. Sclerosing adenosis is often seen in perimenopausal women and can coexist with other benign proliferative lesions such as sclerosing papilloma, and complex sclerosing lesions [37]. Like stromal fibrosis, sclerosing adenosis can have features that resemble malignancy both clinically and radiologically. Additionally, this entity can be seen in the setting of malignancy [37]. Suspicious mammographic imaging features of sclerosing adenosis include amorphous, pleomorphic, and punctate calcifications. If a mass is seen as the presenting imaging feature, it can have irregular margins, although it more commonly has circumscribed margins. Architectural distortion can also be an imaging feature of sclerosing adenosis. Sonograpically, sclerosing adenosis can present as a circumscribed mass, with variable echogenicity. It can, however, mimic malignancy when presenting with suspicious features such as an irregular mass or focal areas of shadowing without a mass (Fig. 9).
Sclerosing adenosis. A 35-year-old woman presented with a palpable breast mass. Mediolateral, craniocaudal, and spot tangential mammographic views do not reveal an abnormality (a–c, respectively). Grayscale and color Doppler ultrasound images (d, e) reveal a vascular, mixed cystic, and solid mass correlating with the area of palpable abnormality. Core needle biopsy showed sclerosing adenosis in a background of dense fibrosis without atypia or carcinoma. A 6-month follow-up ultrasound was rcommended and demonstrated stability
The management of sclerosing adenosis ultimately relies once again on the imaging features. If the radiographic presentation is of a circumscribed mass or amorphous or punctate calcifications in a grouped distribution with adequate sampling, a pathologic diagnosis of sclerosing adenosis can be considered concordant. If the imaging findings are more suspicious such as an irregular mass or pleomorphic calcifications in a segmental or linear distribution, repeat biopsy is recommended to exclude a coexisting malignancy. If the sclerosing adenosis is associated with a radial scar, surgical excision is recommended. In cases of well-sampled architectural distortion, a short-term follow-up is recommended. A short-term follow-up is also recommended for circumscribed masses to ensure stability.
Benign breast tumors
Hamartoma
Hamartomas are uncommon, slow-growing tumors that represent 4.8% of benign breast tumors [38,39,40]. Often termed a “breast within a breast”, breast hamartomas are circumscribed benign tumors that are composed of variable amounts of fat, fibrous tissue, and glandular tissue. Mammographically, a hamartoma has a characteristic benign appearance of a fat-containing circumscribed mass. On ultrasound, a hamartoma presents as a parallel mass with circumscribed margins and heterogonous echotexture due to variable amounts of fat and glandular tissue. On MRI, hamartomas present as a heterogeneous circumscribed mass with a thick capsule, usually with heterogenous progressive enhancement kinetics [39]. Although most hamartomas have a typical benign appearance, they can mimic circumscribed malignant tumors, such as phyllodes tumor or breast sarcoma, if large or when presenting with marked heterogenous enhancement (Fig. 10) [41].
Hamartoma. A 14-year-old presented with unilateral breast enlargement. A mass was not palpable on examination. Color Doppler (a) and longitudinal panoramic ultrasound grayscale images (b) show a hypervascular, 17-cm isoechoic breast mass replacing the entire breast. T1-weighted non-fat-suppressed (c) and T1-weighted fat-suppressed (d) post-contrast MRI images show a well-circumscribed, heterogeneously enhancing breast mass suspicious for a sarcoma (arrow). T2-weighted sagittal images (e) show moderate T2 signal with marked heterogeneity (arrow). Pathology at the time of surgical excision showed hamartoma with areas of pseudoangiomatous stromal hyperplasia and fibroadenomatoid change
Pseudoangiomatous stromal hyperplasia
Pseudoangiomatous stromal hyperplasia (PASH) is a benign breast entity that presents with a wide spectrum of imaging features. It is characterized by proliferation of the fibrous stroma lined by a complex network of slit-like spaces and slender spindle cells. PASH is associated with hormone exposure, including oral contraceptive use, and is primarily seen in premenopausal and perimenopausal women on hormone replacement therapy [42, 43]. It has been postulated that PASH may result from an abnormal reactivity of myofibroblasts to hormonal exposure, although the true etiology is still unclear [44, 45]. Histologically, the differential diagnosis includes a low-grade angiosarcoma and phyllodes tumor [46]. This pathologic entity can frequently coexist with both benign and malignant breast lesions. In one study, PASH was seen in up to 23% of biopsied cases which reflects its wide spectrum of imaging findings [47].
Mammographically, PASH usually presents as an oval or round, noncalcified mass with circumscribed margins ranging from 0.3 to 11 cm (Fig. 11a, c) [43]. In some cases, it can present as a focal asymmetry (Fig. 12a–c). On sonography, it has a more variable appearance, from the more common and benign-appearing oval, circumscribed hypoechoic mass (Fig. 11b, d) to the irregular, mixed echogenicity mass (Fig. 12d) [43]. On MRI, PASH usually presents as a circumscribed mass resembling a fibroadenoma, but it can also present as non-mass enhancement in a focal or segmental distribution (Fig. 12e, f) [42, 43, 48]. The enhancement usually follows a progressive kinetics pattern. Presence of T2-hyperintense slit-like spaces with cystic components favors the PASH diagnosis favors the diagnosis of PASH (Fig. 12g).
Pseudoangiomatous stromal hyperplasia. A 41-year-old woman presented with a palpable breast mass. Mediolateral oblique mammogram (a) shows a high-density well-circumscribed mass (arrow). Longitudinal grayscale ultrasound (b) shows a large, oval circumscribed mass. Core needle biopsy showed pseudoangiomatous stromal hyperplasia (PASH). A different patient, a 40-year-old woman, was found to have a new breast mass (arrow) on mammography, craniocaudal spot view (c). Transverse grayscale (d) ultrasound reveals a corresponding oval isoechoic circumscribed mass. Core needle biopsy showed PASH
Suspicious presentation of pseudoangiomatous stromal hyperplasia. A 56-year-old woman with a new focal asymmetry in the outer breast that was identified on her (a) screening mammogram and persisted on (b) additional diagnostic spot compression views (arrows). The area was palpable by the patient and the clinician. The focal asymmetry had developed since her prior available mammogram performed 4 years earlier (c). Breast ultrasound (d) shows a corresponding irregular hypoechoic mass with associated posterior acoustic shadowing (arrow). Breast MRI sagittal post-contrast T1-weighted delayed images (e) and axial post-contrast T1-weighted images (f) show irregular enhancing masses (arrows). Corresponding T2 hyperintense cystic components with slit-like spaces (arrow) are identified (g), which favors PASH when present. Ultrasound-guided and MRI-guided biopsies were performed in this case and showed PASH. The area remained mammographically stable for over 4 years
Although it can coexist with malignant lesions [49, 50], PASH itself is a benign entity without an increased risk for malignancy [51]. To date, only a single case of PASH with malignant transformation has been reported [52]. In cases where PASH is identified as an incidental finding on pathology, no additional intervention is necessary, and clinical and imaging follow-up is recommended [53]. When PASH is identified as a mass on imaging or is the targeted lesion on biopsy, then surgical excision can be considered for larger lesions (> 2 cm) and for women with an increased risk of developing breast cancer or strong family history [43, 54, 55]. In the presence of an enlarging mass, surgical excision is recommended [45, 53]. Recurrence after excision of PASH has been reported at rates from 5 to 22% [43, 48].
Tubular adenoma
Tubular adenoma is a rare benign tumor of the breast representing 0.13%-2.9% of all benign breast neoplasms [56,57,58]. It is more frequently seen in younger women of childbearing age. Although tubular adenoma and fibroadenoma are both epithelial tumors, a tubular adenoma is histologically distinguished from a fibroadenoma by its tightly packed tubular or acinar epithelium and sparse connective tissue. Conversely, a fibroadenoma has an extensive connective tissue component. Despite this, the cytologic features of tubular adenoma may still resemble those of a fibroadenoma. Therefore, histopathology remains key for confirmation of this diagnosis. Like fibroadenomas, tubular adenomas are not known to increase the risk of cancer, and in contrast to fibroadenomas, are not associated with pregnancy or oral contraceptive use [59, 60].
Both fibroadenomas and tubular adenomas present as circumscribed masses and are occasionally palpable. Mammographically, tubular adenomas have been associated with tightly grouped microcalcifications, which can be suspicious in morphology and may warrant biopsy [61]. Sonographically, tubular adenomas usually present as oval parallel masses with circumscribed margins, similar to fibroadenomas; however, tubular adenomas can also present as irregular hypoechoic masses (Fig. 13) [61]. Management should be determined by the clinical examination, imaging studies, and core biopsy results [62]. In many cases, as with a growing breast mass, surgical excision should be considered to obtain a definitive diagnosis [56].
Tubular adenoma. A 36-year-old woman presented with a palpable breast mass. Spot cradiocaudal and lateral mammogram (a, b) shows a high-density irregular mass (solid arrow), adjacent to an incidental oil cyst (dotted arrow). Grayscale (c) and power Doppler ultrasound (d) images show an irregular hypoechoic mass with increased vascularity (arrows). Core needle biopsy showed tubular adenoma. The mass remained stable sonographically for 30 months and mammographically for 9 years
Desmoid fibromatosis
Desmoid fibromatosis is a rare benign mesenchymal tumor characterized by the proliferation of fibroblasts and myofibroblasts, accounting for 0.2% of all breast tumors [63]. Development of desmoid fibromatosis is associated with trauma, surgery, and Gardner syndrome [63, 64]. Despite its benign nature, it has a tendency for local recurrence [65]. Less than 10% of desmoid tumors are found in the breast; more common locations include the abdominal wall, retroperitoneum, and the mesentery. The clinical course of desmoid fibromatosis varies between patients.
The imaging features of desmoid fibromatosis are often indistinguishable from those of malignancy. Mammographically, desmoid fibromatosis presents as an irregular, high-density mass with spiculated margins (Fig. 14) [66]. On sonography, a hypoechoic irregular mass with posterior acoustic shadowing is usually seen [67]. MRI usually shows a heterogeneously enhancing mass, often with a progressive enhancement pattern. MRI is the modality of choice to assess extent of disease and to evaluate for chest wall involvement [68, 69].
Desmoid fibromatosis. A 47-year-old woman presented with a palpable mass. Lateral spot compression mammogram (a) shows an irregular, high-density mass with spiculated margins adjacent to the chest wall (arrow). Grayscale ultrasound (b) shows a corresponding irregular, hypoechoic anti-parallel mass with indistinct margins. T1-weighted post-contrast fat-saturated MRI of the chest (c) shows invasion of the pectoralis major muscle by this irregular enahancing mass (dotted arrow). Core needle biopsy showed desmoid fibromatosis. The patient underwent surgical excision
The standard treatment for desmoid fibromatosis is surgical excision with wide margins, particularly in cases of tumor growth or progressive disease [70,71,72,73,74,75]. Additional treatment options include non-steroidal anti-inflammatory drugs, chemotherapeutic agents, hormonal therapy, and radiation treatment [71, 72]. Recently published studies have shown no significant difference in event-free survival between asymptomatic patients who underwent surgery and cases managed non-surgically, thereby suggesting that conservative management should precede surgical management [71, 72]. The Desmoid Working Group and European Society for Medical Oncology have provided additional guidance, adding the possibility of active surveillance to the above-stated treatment choices with the supervision of a multidisciplinary team experienced in desmoid fibromatosis tumors. In the presence of tumor growth or progressive disease, surgery remains the gold standard [69, 72,73,74].
Granular cell tumor
Granular cell tumor is an extremely rare tumor of neural origin. It is more commonly found in the head and neck and chest wall regions, with only 4%-6% of cases located in the breast [76, 77]. Granular cell tumor has a predilection for premenopausal and African American women and is preferentially found in the upper inner quadrants of the breast. The clinical and imaging features of granular cell tumor are indistinguishable from those of breast cancer. On physical examination, it can present as a fixed, painless, palpable mass with associated skin dimpling. Mammographically, granular cell tumors can present as a mass with indistinct or spiculated margins (Fig. 15) [42]. On sonography, a hypoechoic mass with irregular or circumscribed margins is usually seen. On MRI, a mass with persistent or washout kinetics is usually seen [78]. Malignant granular cell tumors occur in 1% of these cases [77]. The most common sites of metastasis are the lymph nodes and lungs. PET can differentiate between benign and malignant granular cell tumors, with the benign tumors showing a lower standardized uptake value than the cutoff value of 2.5 [79]. Biopsy is indicated for diagnosis. Although granular cell tumors are benign, wide local surgical excision is indicated owing to their locally infiltrative nature [75, 80].
Granular cell tumor. A 65-year-old woman presented for further evaluation of a focal asymmetry detected on screening mammopgrahy. Craniocaudal and lateral spot compression views (a, b) show a small irregular mass with spiculated margins (arrows). Color Doppler (c) and grayscale (d) ultrasound shows a corresponding irregular mass with internal vascularity (arrows). Core needle biopsy yielded granular cell tumor which was treated with surgical excision
Conclusion
Awareness of multiple malignancy mimickers, rare and common, is vital to clinical practice and plays a key role in radiologic-pathologic concordance, ensuring appropriate clinical management.
Availability of data and materials
Not applicable.
Abbreviations
- PASH:
-
Pseudoangiomatous stromal hyperplasia
References
Sabaté JM, Clotet M, Gómez A, De Las HP, Torrubia S, Salinas T (2005) Radiologic evaluation of uncommon inflammatory and reactive breast disorders. Radiographics 25(2):411–424
Trop I, Dugas A, David J et al (2011) Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics 31(6):1683–1699
Schäfer P, Fürrer C, Mermillod B (1988) An association of cigarette smoking with recurrent subareolar breast abscess. Int J Epidemiol 17(4):810–813
Rizzo M, Gabram S, Staley C et al (2010) Management of breast abscesses in nonlactating women. Am Surg 76(3):292–295
Berna-Serna JD, Madrigal M (2004) Percutaneous management of breast abscesses. An experience of 39 cases. Ultrasound Med Biol 30(1):1–6
Eryilmaz R, Sahin M, Hakan Tekelioglu M, Daldal E (2004) Management of lactational breast abscesses. Breast 14(5):375–379
Chow CK (2005) Imaging in inflammatory breast carcinoma. Breast Dis 22:45–54
Nguyen SL, Doyle AJ, Symmans PJ (2000) Interstitial fluid and hypoechoic wall: two sonographic signs of breast abscess. J Clin Ultrasound 28(7):319–324
Fletcher A, Magrath IM, Riddell RH, Talbot IC (1982) Granulomatous mastitis: a report of seven cases. J Clin Pathol 35(9):941–945
Going JJ, Anderson TJ, Wilkinson S, Chetty U (1987) Granulomatous lobular mastitis. J Clin Pathol 40(5):535–540
Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G (2009) Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol 193(2):574–581
Han BK, Choe YH, Park JM et al (1999) Granulomatous mastitis: mammographic and sonographic appearances. AJR Am J Roentgenol 173(2):317–320
Yilmaz E, Lebe B, Usal C, Balci P (2001) Mammographic and sonographic findings in the diagnosis of idiopathic granulomatous mastitis. Eur Radiol 11(11):2236–2240
Memis A, Bilgen I, Ustun EE, Ozdemir N, Erhan Y, Kapkac M (2002) Granulomatous mastitis: imaging findings with histopathologic correlation. Clin Radiol 57(11):1001–1006
Poyraz N, Emlik GD, Batur A, Gundes E, Keskin S (2016) Magnetic resonance imaging features of idiopathic granulomatous mastitis: a retrospective analysis. Iran J Radiol 13(3):e20873
Rieber A, Tomczak RJ, Mergo PJ, Wenzel V, Zeitler H, Brambs HJ (1997) MRI of the breast in the differential diagnosis of mastitis versus inflammatory carcinoma and follow-up. J Comput Assist Tomogr 21:128–132
de Bazelaire C, Groheux D, Chapellier M et al (2012) Breast inflammation: indications for MRI and PET-CT. Diagn Interv Imaging 93:104–115
Sripathi S, Ayachit A, Bala A, Kadavigere R, Kumar S (2016) Idiopathic granulomatous mastitis: a diagnostic dilemma for the breast radiologist. Insights Imaging 7(4):523–529
Patel RA, Strickland P, Sankara IR, Pinkston G, Many W, Rodriguez M (2010) Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med 25(3):270–273
Lai EC, Chan WC, Ma TK, Tang AP, Poon CS, Leong HT (2005) The role of conservative treatment in idiopathic granulomatous mastitis. Breast J 11(6):454–456
Ely KA, Tse G, Simpson JF, Clarfeld R, Page DL (2000) Diabetic mastopathy. A clinicopathologic review. Am J Clin Pathol 113(4):541–545
Thorncroft K, Forsyth L, Desmond S, Audisio RA (2007) The diagnosis and management of diabetic mastopathy. Breast J 13(6):607–613
Pereira MA, de Magalhães AV, da Motta LD et al (2010) Fibrous mastopathy: clinical, imaging, and histopathologic findings of 31 cases. J Obstet Gynaecol Res 36(2):326–335
Chan CL, Ho RS, Shek TW, Kwong A (2013) Diabetic mastopathy. Breast J 19(5):533–538
Valdez R, Thorson J, Finn WG, Schnitzer B, Kleer CG (2003) Lymphocytic mastitis and diabetic mastopathy: a molecular, immunophenotypic, and clinicopathologic evaluation of 11 cases. Mod Pathol 16(3):223–228
Goulabchand R, Hafidi A, Van de Perre P et al (2020) Mastitis in autoimmune diseases: review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med 9(4):958
Weinstein SP, Conant EF, Orel SG, Lawton TJ, Acs G (2001) Diabetic mastopathy in men: imaging findings in two patients. Radiology 219(3):797–799
Wong KT, Tse GM (2002) Yang WT (2002) Ultrasound and MR imaging of diabetic mastopathy. Clin Radiol 57(8):730–735
Sakuhara Y, Shinozaki T, Hozumi Y, Ogura S, Omoto K, Furuse M (2002) MR imaging of diabetic mastopathy. AJR Am J Roentgenol 179(5):1201–1203
Tuncbilek N, Karakas HM, Okten O (2004) Diabetic fibrous mastopathy: dynamic contrast-enhanced magnetic resonance imaging findings. Breast J 10(4):359–362
Tayyab SJ, Adrada BE, Rauch GM, Yang WT (2018) A pictorial review: multimodality imaging of benign and suspicious features of fat necrosis in the breast. Br J Radiol 91(1092):20180213
Nakada H, Inoue M, Furuya K et al (2019) Fat necrosis after breast-conserving oncoplastic surgery. Breast Cancer 26(1):125–130
Shin S, Ko ES, Han BK, Ko EY (2014) Stromal fibrosis of the breast: outcome analysis. Acta Radiol 55(4):409–415
Lee SJ, Mahoney MC, Khan S (2011) MRI features of stromal fibrosis of the breast with histopathologic correlation. AJR Am J Roentgenol 197(3):755–762
Malik N, Lad S, Seely JM, Schweitzer ME (2014) Underestimation of malignancy in biopsy-proven cases of stromal fibrosis. Br J Radiol 87(1039):20140182
Yilmaz R, Bayramoglu Z, Kartal MG et al (2018) Stromal fibrosis: imaging features with diagnostic contribution of diffusion-weighted MRI. Br J Radiol 91(1085):20170706
Sklair-Levy M, Samuels TH, Catzavelos C, Hamilton P, Shumak R (2001) AJR Am J Roentgenol 177(3):573–577
Cho SH, Park SH (2013) Mimickers of breast malignancy on breast sonography. J Ultrasound Med 32(11):2029–2036
Farrokh D, Hashemi J, Ansaripour E (2011) Breast hamartoma: mammographic findings. Iran J Radiol 8(4):258–260. https://doi.org/10.5812/iranjradiol.4492
Presazzi A, Di Giulio G, Calliada F (2015) Breast hamartoma: ultrasound, elastosonographic, and mammographic features. Mini pictorial essay J Ultrasound 18(4):373–377
Charpin C, Mathoulin M, Andrac L et al (1994) Reappraisal of breast hamartomas: a morphological study of 41 cases. Pathol Res Pract 190(4):362–371
Adrada BE, Krishnamurthy S, Carkaci S, Posleman-Monetto FE, Ewere A, Whitman GJ (2015) Unusual benign tumors of the breast. J Clin Imaging Sci 5:27
Raj SD, Sahani VG, Adrada BE et al (2017) Pseudoangiomatous stromal hyperplasia of the breast: multimodality review with pathologic correlation. Curr Probl Diagn Radiol 46(2):130–135
Ferreira M, Albarracin CT, Resetkova E (2008) Pseudoangiomatous stromal hyperplasia tumor: a clinical, radiologic and pathologic study of 26 cases. Mod Pathol 21(2):201–207
Powell CM, Cranor ML (1995) Rosen PP (1995) Pseudoangiomatous stromal hyperplasia (PASH): a mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol 19(3):270–277
Drinka EK, Bargaje A, Erşahin ÇH et al (2012) Pseudoangiomatous stromal hyperplasia (PASH) of the breast: a clinicopathological study of 79 cases. Int J Surg Pathol 20(1):54–58
Ibrahim RE, Sciotto CG, Weidner N (1989) Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer 63(6):1154–1160
Jones KN, Glazebrook KN, Reynolds C (2010) Pseudoangiomatous stromal hyperplasia: imaging findings with pathologic and clinical correlation. AJR Am J Roentgenol 195(4):1036–1042
Cohen MA, Morris EA, Rosen PP, Dershaw DD, Liberman L, Abramson AF (1996) Pseudoangiomatous stromal hyperplasia: mammographic, sonographic, and clinical patterns. Radiology 198(1):117–120
Solomou E, Kraniotis P, Patriarcheas G (2012) A case of a giant pseudoangiomatous stromal hyperplasia of the breast: magnetic resonance imaging findings. Rare Tumors 4(2):73–77
Hargaden GC, Yeh ED, Georgian-Smith D et al (2008) Analysis of the mammographic and sonographic features of pseudoangiomatous stromal hyperplasia. AJR Am J Roentgenol 191(2):359–363
Nassar H, Elieff MP, Kronz JD, Argani P (2010) Pseudoangiomatous stromal hyperplasia (PASH) of the breast with foci of morphologic malignancy: a case of PASH with malignant transformation? Int J Surg Pathol 18(6):564–569
Smilg P (2018) Pseudoangiomatous stromal hyperplasia: presentation and management—a clinical perspective. SA J Radiol 22(2):1366
Bowman E, Oprea G, Okoli J et al (2012) Pseudoangiomatous stromal hyperplasia (PASH) of the breast: a series of 24 patients. Breast J 18(3):242–247
Yoon KH, Koo B, Lee KB et al (2020) Optimal treatment of pseudoangiomatous stromal hyperplasia of the breast. Asian J Surg 43(7):735–741
Sengupta S, Pal S, Biswas BK, Phukan JP, Sinha A, Sinha R (2014) Preoperative diagnosis of tubular adenoma of breast–10 years of experience. N Am J Med Sci 6(5):219
Salemis NS, Gemenetzis G, Karagkiouzis G et al (2012) Tubular adenoma of the breast: a rare presentation and review of the literature. J Clin Med Res 4(1):64
Tavasoli F, Deville P (2003) WHO classification of tumours, tumours of the breast and female genital organs. IARC Press, pp 153–158
Maiorano E, Albrizio M (1995) Tubular adenoma of the breast: an immunohistochemical study of ten cases. Pathol Res Pract 191(12):1222–1230
O’Hara MF, Page DL (1985) Adenomas of the breast and ectopic breast under lactational influences. Hum Pathol 16(7):707–712
Soo MS, Dash N, Bentley R, Lee LH, Nathan G (2000) Tubular adenomas of the breast: imaging findings with histologic correlation. AJR Am J Roentgenol 174(3):757–761
Tavassoli FA (1999) Pathology of the breast. McGraw Hill Professional
Eastley N, McCulloch T, Esler C et al (2016) Extra-abdominal desmoid fibromatosis: a review of management, current guidance and unanswered questions. Eur J Surg Oncol 42(7):1071–1083
Neuman HB, Brogi E, Ebrahim A, Brennan MF, Van Zee KJ (2008) Desmoid tumors (fibromatoses) of the breast: a 25-year experience. Ann Surg Oncol 15(1):274–280
Glazebrook KN, Reynolds CA (2009) Mammary fibromatosis. AJR Am J Roentgenol 193(3):856–860
Cederlund C-G, Gustavsson S, Linell F, Moquist-Olsson I, Andersson I (1984) Fibromatosis of the breast mimicking carcinoma at mammography. Br J Radiol 57(673):98–101
Leibman A, Kossoff M (1991) Sonographic features of fibromatosis of the breast. J Ultrasound Med 10(1):43–45
Linda A, Londero V, Bazzocchi M, Zuiani C (2008) Desmoid tumor of the breast: radiologic appearance with a focus on its magnetic resonance features. Breast J 14(1):106
Nakazono T, Satoh T, Hamamoto T, Kudo S (2003) Dynamic MRI of fibromatosis of the breast. AJR Am J Roentgenol 181(6):1718–1719
Alman B, Attia S, Baumgarten C et al (2020) The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer 127:96–107
Fiore M, Rimareix F, Mariani L et al (2009) Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 16(9):2587–2593
Bonvalot S, Ternès N, Fiore M et al (2013) Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 20(13):4096–4102
Kasper B, Baumgarten C, Garcia J, et al (2017) An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol 28(10):2399–2408
Duazo-Cassin L, Le Guellec S, Lusque A et al (2019) Breast desmoid tumor management in France: toward a new strategy. Breast Cancer Res Treat 176(2):329–335
Lorenzen J, Cramer M, Buck N et al (2019) Desmoid type fibromatosis of the breast: ten-year institutional results of imaging, histopathology, and surgery. Breast Care 16:77–84
Gavriilidis P, Michalopoulou I, Baliaka A, Nikolaidou A (2013) Granular cell breast tumour mimicking infiltrating carcinoma. Case Rep. https://doi.org/10.1136/bcr-2012-008178
Jagannathan DM (2015) Benign granular-cell tumor of the breast: case report and literature review. Radiol Case Rep 10(2):1116
Scaranelo A, Bukhanov K, Crystal P, Mulligan AM, O’malley FP, (2007) Granular cell tumour of the breast: MRI findings and review of the literature. Br J Radiol 80(960):970–974
Hoess C, Freitag K, Kolben M et al (1998) FDG PET evaluation of granular cell tumor of the breast. J Nucl Med 39(8):1398–1401
Brown AC, Audisio RA, Regitnig P (2011) Granular cell tumour of the breast. Surg Oncol 20(2):97–105
Acknowledgements
The manuscript was edited by Sarah Bronson, ELS, of the Research Medical Library at The University of Texas MD Anderson Cancer Center.
Funding
None.
Author information
Authors and Affiliations
Contributions
MG and BA have contributed to the conception of this work. MG, BA, and EA have contributed significantly to the writing of this manuscript. All authors have contributed to the editing of this manuscript and to providing case examples. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guirguis, M.S., Adrada, B., Santiago, L. et al. Mimickers of breast malignancy: imaging findings, pathologic concordance and clinical management. Insights Imaging 12, 53 (2021). https://doi.org/10.1186/s13244-021-00991-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-021-00991-x