Abstract
Adenomyosis is a challenging clinical condition that is commonly being diagnosed in women of reproductive age. To date, many aspects of the disease have not been fully understood, making management increasingly difficult. Over time, minimally invasive diagnostic and treatment methods have developed as more women desire uterine preservation for future fertility or to avoid major surgery. Several uterine-sparing treatment options are now available, including medication, hysteroscopic resection or ablation, conservative surgical methods, and high-intensity focused ultrasound each with its own risks and benefits. Uterine artery embolization is an established treatment option for uterine fibroids and has recently gained ground as a safe and cost-effective method for treatment of uterine adenomyosis with promising results. In this review, we discuss current trends in the management of uterine adenomyosis with a special focus on uterine artery embolization as an alternative to hysterectomy.
Similar content being viewed by others
Key points
-
Uterine artery embolization (UAE) seems to be the most promising uterine-sparing, minimally invasive treatment option for adenomyosis.
-
Results of ongoing randomized controlled trial (QUESTA) will soon show whether UAE can be validated as a treatment option for adenomyosis.
-
Ability to preserve fertility will be one of the main factors determining whether UAE can replace hysterectomy in treatment of adenomyosis, but further randomized controlled trials are needed.
Introduction
Adenomyosis is defined by the abnormal location of endometrial tissue within the myometrium associated with hypertrophy or hyperplasia of the myometrial stroma [1, 2]. Although pathogenesis and etiology of adenomyosis remain unknown, two main theories have been proposed: invagination of the endometrial basal layer and metaplasia of embryonic stem cells [3]. Prevalence of adenomyosis varies widely from 5 to 70% [4,5,6,7] with recent studies showing about 20% prevalence [8,9,10] among which the majority were premenopausal. Despite the absence of specific (pathognomonic) diagnostic features for uterine adenomyosis, typical symptoms include menorrhagia, chronic pelvic pain, and dysmenorrhea [11]. These symptoms are commonly encountered in other gynecological disorders including leiomyomas and endometriosis, often confounding the clinical diagnosis [12].
For more than a century, diagnosis was dependent on histopathologic examination of post-hysterectomy specimens till the introduction of noninvasive ultrasound and MR techniques [13]. Since then, several studies have illustrated high sensitivities and specificities for both two-dimensional transvaginal sonography (TVS) and magnetic resonance imaging (MRI) [13,14,15,16,17]. Current treatment options for symptomatic adenomyosis include hysterectomy, medication, conservative surgery, or minimally invasive techniques including uterine artery embolization [18]. To date, hysterectomy remains the definitive treatment. This is mainly due to difficult diagnosis, the diffuse nature of the disease, and little evidence-based literature needed to standardize treatments [19]. This consequently results in a management dilemma, particularly in symptomatic patients who wish to preserve their uterus [18].
Uterine artery embolization (UAE) was first described in 1995 by Ravina et al. [20] then later established as an effective treatment option for patients with symptomatic uterine fibroids [21, 22]. Since then, UAE has been investigated as a noninvasive treatment option for adenomyosis with initial promising results [23, 24]. What remains to be known is whether UAE can be validated as a safer, noninvasive, uterine-sparing alternative to hysterectomy. This article summarizes current trends in management of uterine adenomyosis with special focus on the emerging role of UAE.
Etiology
The precise etiology and pathophysiology leading to the development of adenomyosis remains undetermined. Several theories have been introduced, including traumatic, immunological, hormonal, metaplastic, and stem cell [25]. Traumatic and immunological theories suggest disruption of endometrial-myometrial interface with invagination of eutopic (normally located) endometrial cells [26, 27], while hormonal, metaplastic, and stem cell theories rely on the altered behavior of atopic (displaced) cells [28,29,30]. These mechanisms, in addition to various risk factors, such as age, parity, previous uterine surgery, smoking, ectopic pregnancy, antidepressant, and tamoxifen therapies, are believed to contribute to the development of adenomyosis [19]. Regardless of etiology, histopathologic features remain the same, and definitive diagnosis is established by the presence of “ectopic, non-neoplastic, endometrial glands and stroma surrounded by hypertrophic and hyperplastic myometrium” on hysterectomy specimens [1].
Diagnosis
Adenomyosis remains an underdiagnosed condition. This is largely due to lack of pathognomonic symptoms related to this condition [31]. Symptomatic patients varyingly present with menorrhagia, dysmenorrhea, chronic pelvic pain, dyspareunia, and subfertility [32,33,34], and up to 30% of patients are asymptomatic [34]. Furthermore, confounding coexisting pathologies (usually fibroids and endometriosis) add to the difficulty of diagnosis, as both entities present with similar clinical features [31].
Role of ultrasound and MRI in diagnosis
With the introduction and advancement of ultrasound and MR techniques, various criteria have been utilized in the noninvasive narrowing of the clinical differential [15, 35, 36], determining the depth of myometrial invasion and monitoring treatment response [37].
Transvaginal ultrasound (TVS) represents a cost-effective initial screening modality for adenomyosis. Ultrasound features of adenomyosis can be divided into direct or indirect features (Fig. 1). Direct features are due to the presence of endometrial tissue within the myometrium, and indirect features are due to a hypertrophied myometrium as described by Atri et al. [38]. Table 1 describes ultrasound features of adenomyosis as described in previous literature [14, 16, 38,39,40,41,42,43]. To report the diagnostic accuracy of TVS in adenomyosis, several meta-analyses have been published [17, 44,45,46]. Estimated pooled sensitivities of 72 to 82%, pooled specificities of 81 to 85%, and pooled positive likelihood ratios 3.7 to 4.67 have been reported [17, 44]; however, one meta-analysis suggested that variability between studies does not allow for accurate statistical pooling [45]. With the introduction of color and power Doppler ultrasound, three-dimensional TVS and elastography techniques to the work-up of adenomyosis, there is promise for further improvement in diagnostic accuracy [46].
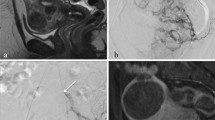
Direct and indirect imaging features of adenomyosis on ultrasound. a Small posterior wall myometrial cysts (open arrows). b Poorly defined endo-myometrial interface (solid arrow). c Diffuse myometrial heterogeneity with hyperechoic linear striations (three arrows). d Diffuse asymmetric widening of the posterior myometrial wall with hyperechoic nodules (four arrows)
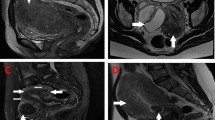
Magnetic resonance imaging (MRI) represents a second line, detailed imaging modality for the detection of adenomyosis (Fig. 2). Similar to ultrasound, various direct and indirect features can be used to describe adenomyosis, but need more knowledge of uterine anatomy and its cyclic variations [36]. Table 1 describes MRI features of adenomyosis as described in previous literature [14,15,16, 35, 47]. Few prospective studies have evaluated the diagnostic accuracy of MRI in the diagnosis of adenomyosis [15, 16, 48]. These studies have reported sensitivity between 70 and 93% and specificities between 86 and 93%. Despite being less operator dependent, MRI needs more reader experience and optimization of imaging technique to achieve higher diagnostic accuracy [36].
Classification of adenomyosis
The use of complex imaging techniques has revealed various subtypes of adenomyosis, often associated with histopathologic variation in glandular and muscular components [31]. Furthermore, initial studies have linked various imaging criteria to symptoms of adenomyosis [49,50,51]. Therefore, the need for a more holistic approach to identify various disease characteristics incorporating symptomatology, morphology, and pathologic features is rising in order to improve the diagnostic accuracy and adequately guide treatment decisions. Important factors to be included in classification systems would be the site and location of pathology, configuration, and size/volume relative to the total myometrial thickness [31]. Most recent ultrasound and MR classification and reporting systems have been developed by Van den Bosch et al. [52] and Bazot [36, 53] respectively, but the clinical relevance remains to be tested.
Treatment
As with many aspects of adenomyosis, treatment remains controversial. Important factors to be considered and discussed with patients are age, symptom severity, desire for future conception, and associated comorbidities [32, 54, 55]. Recent studies estimate a prevalence of adenomyosis among females younger than 40 years to be about 20–30%, while in the rest of the patients, diagnosis is usually established in the fourth or fifth decade [55,56,57]. Furthermore, diffuse adenomyosis, depth of invasion, and coexisting fibroids and/or endometriosis are associated with increased frequency/severity of symptoms and reproductive complications [31, 49, 58,59,60]. Currently, treatment is on a case by case basis, and hysterectomy remains the definitive treatment in patients who are willing and do not wish for future fertility. With the introduction of assisted reproductive techniques, delayed age of conception, and availability of minimally invasive treatment options, the shift from hysterectomy as the “go to” option seems inevitable.
Medical treatment
Medical treatment is the first-line treatment option for adenomyosis aiming to relieve symptoms and maintain fertility with the least possible side effect. This is achieved by disrupting pathways leading to inflammation, neuroangiogenesis, and impaired apoptosis [61]. Currently, several hormonal and non-hormonal options, namely gonadotropin-releasing hormone (GnRH) analogues, progestins, combined oral contraceptives, and non-steroidal anti-inflammatory drugs are being used in an “off label” manner for the symptomatic treatment of adenomyosis [57, 62]. Also, newer drugs, such as aromatase inhibitors, have been investigated by Badawy et al. and Tosti et al. [63, 64], while other therapies such as selective progesterone receptor modulators, GnRH antagonists, valproic acid, and anti-platelet therapies are still under investigation [55].
The main advantage of medication is symptomatic relief without the need for surgical treatment. Nevertheless, many drawbacks still need to be addressed. This includes the temporary relieve of symptoms, and the common (i.e., menopausal symptoms, irregular bleeding, amenorrhea) and occasionally severe (i.e., thromboembolic) side effects of some drugs. Lack of evidence needed to base choice of drugs also raises the need to perform research into the comparative efficacy of currently used drugs and develop a more standardized approach for patients wanting to conceive while using medication. With a better understanding of pathogenetic mechanisms of adenomyosis, advances in drug development will soon be possible [55].
Minimally invasive techniques
These are second-line treatment options aiming to cure symptoms and preserve the uterus in patients with failed medical therapy. Conservative surgical treatments aim to remove adenomyosis and preserve the remaining normal uterine muscles through laparotomy, laparoscopy, hysteroscopy, or combined approach. Excisional adenomyomectomy involves the complete removal of focal lesions (adenomyomas), while myometrectomy is the surgical debulking of diffuse adenomyosis. Non-excisional treatments aim to induce necrosis of focal or diffuse adenomyosis through selective vascular occlusion or focused ultrasound/thermal energy without direct tissue dissection. In some cases, a combination of surgical and non-excisional methods, i.e., hysteroscopic resection/ablation, are used to achieve maximum cytoreduction and reduce myometrial tissue damage.
Conservative surgical treatment
Debulking/cytoreductive surgeries aim to remove visibly diseased tissue with repair of the remaining myometrial tissue [65]. Several laparotomic techniques have been described, including wedge resection and its modifications, transverse H-shaped incision [66], wedge-shaped uterine wall removal [67], double and triple flap [68, 69], and asymmetric dissection methods [70]. Laparoscopic techniques have also been described in more focal pathology, where longitudinal or transverse incisions [71, 72] are used to access adenomyotic lesions followed by resection using monopolar needle or laser knife [73, 74], bag removal, and repair in layers or using double flaps [72, 75]. To date, there is no consensus on the best surgical method, but initial results are promising. In a systematic review by Grigoris et al., dysmenorrhea reduction, menorrhagia control, and pregnancy success rates ranged from 81 to 82%, 50 to 69%, and 47 to 61% among partial versus complete adenomyosis excisions respectively [76], and a recent review by Younes et al. showed 75% symptom relief on short-term follow-ups [77]. The main issue with conservative surgical methods is the high risk for complications, i.e., uterine rupture and complicated pregnancy [54, 65] (especially in diffuse lesions and on long-term follow-up), making this option safer in focal adenomyomas.
Hysteroscopic resection/ablation
Hysteroscopic resection/ablation is a combined treatment method involving the dissection and or coagulation of cystic adenomyotic lesions and crypts [78,79,80,81,82]. Hysteroscopic resections can be performed using yttrium aluminum garnet (YAG) laser, rollerball resection, thermal balloon ablation, cryoablation, circulated hot fluid ablation, microwave ablation, bipolar radiofrequency ablation, and electrocoagulation [19].
High-intensity focused ultrasound (HIFU)
High-intensity focused ultrasound (HIFU) is the use of intense ultrasound energy directly targeting abnormal tissues and their vascularity through heating and cavitation, sparing the normal surrounding tissues. This process can be guided and monitored through MRI or ultrasound [83]. High-intensity focused ultrasound has been used since 2008 for the treatment of adenomyosis [84]. Since then, literature has shown promising results regarding symptom relief and uterine preservation with few reported complications (namely pain, numbness, vaginal or urinary discharge, fever, skin burn, or contact dermatitis) [83]. Recent studies have also investigated the use of ultrasound contrast agents (microbubbles) and hormonal (GnRH) and non-hormonal (metformin) treatments to enhance the efficacy of HIFU. Microbubbles are believed to improve the ablative effects of HIFU by changing the acoustic characteristics, thus increasing energy deposition in target tissues, while GnRH and metformin inhibit cellular proliferation and induce apoptosis [85,86,87]. Limited literature on treatment outcomes for HIFU in adenomyosis has shown highly variable results regarding symptom and uterine volume reduction [88,89,90,91,92,93,94,95,96,97]. Rates of menorrhagia, dysmenorrhea, and uterine volume reduction varied widely from 12.4 to 44.8%, 25 to 100%, and 12.7 to 54% respectively, increasing gradually overtime (from 1 to 24 months). Nevertheless, paucity of literature comparing HIFU to other minimally invasive treatment options, limited availability, overall cost, unknown fertility outcomes, and strict indications, including lesions no more than 10 cm in diameter [88, 90], no pelvic adhesions [84, 89, 90, 93], body weight less than 100 kg [98], and abdominal wall thickness less than 5 cm [93] may limit its widespread use.
Uterine artery embolization (UAE)
Uterine artery embolization is the use of transarterial catheters aiming to induce more than 34% necrosis within adenomyotic tissues [99, 100]. The technique for UAE in adenomyosis is similar to that used in fibroids. In many parts of the world, UAE is performed under conscious sedation. Vascular access is gained through a femoral or radial artery puncture using 4–6-French (F) arterial sheath for femoral [99, 101] and 4-F sheath for radial access [102]. Under fluoroscopic guidance, aortography is followed by selective and super selective arteriography using 4–5-F catheters for the internal iliac and 2–3-F microcatheters for the uterine artery and its branches respectively. Embolization is usually performed using variable-sized permanent particulate agents [103, 104]. Special attention is paid to visualization of the cervicovaginal and ovarian artery branches (Fig. 3). Distal embolization avoids vaginal necrosis and unwanted reflux of microspheres into the ovarian artery [105].
Digital subtraction angiography (DSA) images (of the same patient in Fig. 2) with selective injections of the left (a) uterine artery demonstrate with multiple tortuous uterine artery branches and (b) lesion blush (most prominent at the anatomic site of the posterior uterine wall). Right uterine artery injection (not shown) was unremarkable for pathology. Post-embolization DSA images show occlusion of toursous feeding vessels (c) with absence of lesion blush (d)
Despite being established in fibroids as a cost-effective, short recovery alternative to surgery with minimal complications [19, 23, 100], it was believed to have lower efficacy in adenomyosis [106]. In the past 15 years, UAE has been considerably studied for the treatment of symptomatic adenomyosis [107]. Earlier studies by Popovic, Keung, and Zhou et al. demonstrate long-term improvement in patient symptoms (in over 60% of patients) and a short-term decrease in uterine volumes (in over 20% of patients), especially in vascular lesions [23, 107, 108]. Current literature by Dueholm and Bruijn et al. show up to 67% long-term (40 month) treatment success and up to and 72% patient satisfaction rates respectively [24, 100]. In the latest systematic review and meta-analysis by de Bruijn et al., patients were divided into four groups to report short- and long-term outcomes. Short-term improvement was achieved in 89.6% of patients with pure adenomyosis and 94.3% of patients with adenomyosis with fibroids, while long-term improvement was achieved in 74.0% of patients with pure adenomyosis and 84.5% of patients with adenomyosis with fibroids [109].
Overall, UAE shows favorable clinical outcomes, but randomized controlled trials are still lacking [110]. In an attempt to fill this gap in knowledge, the “Quality of Life after Embolization vs Hysterectomy in Adenomyosis” (QUESTA) trial was set up. This multicenter non-blinded randomized controlled trial is currently ongoing in the Netherlands. It has started since November 2015, and its primary outcomes are expected by May 2020 [101]. The calculated sample size for this trial was 96 patients (divided into 52 embolization and 34 hysterectomy, including a 10% expected drop-out) made on assumptions from the embolization versus hysterectomy (EMMY) trial outcomes [111].
Inclusion criteria were premenopausal women with symptomatic pure adenomyosis or dominant adenomyosis when both adenomyosis and fibroids coexist and women with an indication for hysterectomy (either failed or refused medical treatment). Exclusion criteria were patients under 18 years of age, pelvic infection, suspected or confirmed malignancy, current or future desire to conceive, any absolute contraindication to angiography, deep infiltrating endometriosis requiring surgery or obstructing the bowel, or coexisting hysteroscopically removable submucous fibroids. Following selection, TVUS and MRIs were performed to confirm the adenomyosis and eligible patients are informed of the trial. Patients with written informed consents were randomly allocated (in a 2:1 ratio) between both experimental intervention (UAE) and standard care control groups (hysterectomy), while patients refusing randomization are given the standard of care (hysterectomy) [101].
Following the procedure (UAE or hysterectomy), patients are followed up immediately, then at 6 weeks, 3 months, 6 months, 12 months, and 24 months using an online questionnaire system. Three outcome parameters were measured. Primary outcomes (quality of life) were measured at 6, 12, and 24 months using a combination of World Health Organization Quality of Life Scale and Short Form-12 Questionnaires. Secondary outcomes (clinical, symptom and quality of life, recovery related, cost utility analysis, laboratory, and pathology outcomes) were measured at 6 weeks and 3, 6, 12, and 24 months. Imaging outcomes were also determined to identify potential predictive parameters for therapy effect using specific TVUS criteria (uterine size/fibroid volume reduction in case of associated fibroids, vascular index by 3D power Doppler) at baseline, 6 weeks, and 6 months and MRI criteria (uterine size/fibroid volume reduction in case of associated fibroids, junctional zone reduction, infarction rate, and presence of endometriosis) at baseline and at 6 months postprocedure [101].
UAE as an alternative to hysterectomy
To date, UAE seems to be the most investigated and highest potential minimally invasive treatment option for adenomyosis. Results of ongoing randomized controlled (QUESTA) trial will soon show whether UAE can be validated as a treatment option for adenomyosis. Although comparative information regarding quality of life, patient satisfaction, side effects, and complications post UAE versus hysterectomy will soon be available, questions regarding fertility post UAE remain to be answered. Current American College of Obstetrics and Gynecology and Society of Interventional Radiology guidelines still consider desire for future fertility a relative contraindication to UAE, but conflicting reports regarding effects of UAE on fertility [112] still give room for debate. Nevertheless, further randomized studies are still needed to give a clear answer for physicians and patients alike.
In conclusion, lack of information is the main hurdle to overcome the complexity in management of adenomyosis. With randomized controlled trials and more evidence-based research, optimal treatment protocols can be developed according to patient needs. Whether or not UAE can replace hysterectomy will largely depend on the results of ongoing QUESTA trial and other randomized trials comparing fertility outcomes among minimally invasive therapies.
Abbreviations
- EMMY:
-
Embolization versus hysterectomy
- GnRH:
-
Gonadotropin-releasing hormone
- HIFU:
-
High-intensity focused ultrasound
- QUESTA:
-
Quality of Life after Embolization vs Hysterectomy in Adenomyosis
- UAE:
-
Uterine artery embolization
- YAG:
-
Yttrium aluminum garnet
References
Bird CC, McElin TW, Manalo-Estrella P (1972) The elusive adenomyosis of the uterus--revisited. Am J Obstet Gynecol 112:583–593
Benagiano G, Brosens I (2006) History of adenomyosis. Best Pract Res Clin Obstet Gynaecol 20:449–463 https://doi.org/10.1016/j.bpobgyn.2006.01.007
García-Solares J, Donnez J, Donnez O, Dolmans MM (2018) Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril 109:371–379 https://doi.org/10.1016/j.fertnstert.2017.12.030
Azziz R (1989) Adenomyosis: current perspectives. Obstet Gynecol Clin N Am 16:221–235
Vercellini P, Oldani S, Parazzini F, Panazza S, Bramante T, Crosignani PG (1995) Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod 10(5):1160–1162
Bergholt T, Berendt N, Eriksen L, Jacobsen M, Hertz JB (2001) Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod Update 16:2418–2421
Parazzini F, Mais V, Cipriani S, Busacca M, Venturini P, GISE (2009) Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol 143:103–106 https://doi.org/10.1016/j.ejogrb.2008.12.010
Wallwiener M, Taran F-A, Rothmund R et al (2013) Laparoscopic supracervical hysterectomy (LSH) versus total laparoscopic hysterectomy (TLH): an implementation study in 1,952 patients with an analysis of risk factors for conversion to laparotomy and complications, and of procedure-specific re-operations. Arch Gynecol Obs 288:1329–1339 https://doi.org/10.1007/s00404-013-2921-x
Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D (2012) How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod 27:3432–3439 https://doi.org/10.1093/humrep/des332
Di Donato N, Montanari G, Benfenati A et al (2014) Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol 181:289–293 https://doi.org/10.1016/j.ejogrb.2014.08.016
Tamai K, Togashi K, Ito T, Morisawa N, Fujiwara T, Koyama T (2005) MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics 25:21–40 https://doi.org/10.1148/rg.251045060
Ascher SM, Jha RC, Reinhold C (2003) Benign myometrial conditions: leiomyomas and adenomyosis. Top Magn Reson Imaging 14:281–304
Habiba M, Benagiano G (2015) Uterine adenomyosis. Springer, Cham
Reinhold C, Tafazoli F, Mehio A et al (1999) Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics 19:S147–S160 https://doi.org/10.1148/radiographics.19.suppl_1.g99oc13s147
Dueholm M, Lundorf E, Hansen ES, Sørensen JS, Ledertoug S, Olesen F (2001) Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril 76:588–594
Bazot M, Cortez A, Darai E et al (2001) Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod 16:2427–2433
Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS (2010) Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obs Gynecol Scand 89:1374–1384 https://doi.org/10.3109/00016349.2010.512061
Radzinsky VE, Khamoshina MB, Nosenko EN et al (2016) Treatment strategies for pelvic pain associated with adenomyosis. Gynecol Endocrinol 32:19–22 https://doi.org/10.1080/09513590.2016.1232673
Taran FA, Stewart EA, Brucker S (2013) Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd 73:924–931 https://doi.org/10.1055/s-0033-1350840
Ravina JH, Herbreteau D, Ciraru-Vigneron N et al (1995) Arterial embolisation to treat uterine myomata. Lancet 346:671–672
de Bruijn AM, Ankum WM, Reekers JA et al (2016) Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am J Obstet Gynecol 215:e741–e745
Edwards RD, Moss JG, Lumsden MA et al (2007) Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med 356:360–370 https://doi.org/10.1056/NEJMoa062003
Popovic M, Puchner S, Berzaczy D, Lammer J, Bucek RA (2011) Uterine artery embolization for the treatment of adenomyosis: a review. J Vasc Interv Radiol 22:901–909quiz 909 https://doi.org/10.1016/j.jvir.2011.03.013
de Bruijn AM, Smink M, Hehenkamp WJK et al (2017) Uterine artery embolization for symptomatic adenomyosis: 7-year clinical follow-up using UFS-Qol questionnaire. Cardiovasc Intervent Radiol 40:1344–1350 https://doi.org/10.1007/s00270-017-1686-1
Tinelli A, Malvasi A (2015, 2015) Uterine myoma, myomectomy and minimally invasive treatments. Springer International Publishing, pp 1–281 https://doi.org/10.1007/978-3-319-10305-1
Habiba M, Benagiano G, Brosens I (2016) The pathophysiology of adenomyosis. In: Habiba M, Benagiano G (eds) Uterine adenomyosis, vol 4. Springer, Cham, pp 45–70 https://doi.org/10.1007/978-3-319-13012-5_3
Ota H, Hatazawa J, Igarashi S, Tanaka T (1998) Is adenomyosis an immune disease? Hum Reprod 4:360–367
Bergeron C, Amant F, Ferenczy A (2006) Pathophysiology and physiology of adenomyosis. Best Pr Res Clin Obs Gynaecol 20:511–521
Matsumoto Y, Iwasaka T, Yamasaki F, Sugimori H (1999) Apoptosis and Ki-67 expression in adenomyotic lesions and in the corresponding eutopic endometrium. Obstet Gynecol 94:71–77
Du H, Taylor HS (2009) Stem cell and female reproduction. Reprod Sci 16:126–139
Gordts S, Grimbizis G, Campo R (2018) Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil Steril 109:380–388.e1 https://doi.org/10.1016/j.fertnstert.2018.01.006
Struble J, Reid S, Bedaiwy MA (2016) Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol 23:164–185 https://doi.org/10.1016/j.jmig.2015.09.018
Garcia L, Isaacson K (2011) Adenomyosis: review of the literature. J Minim Invasive Gynecol 18:428–437 https://doi.org/10.1016/j.jmig.2011.04.004
Peric H, Fraser IS (2006) The symptomatology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 20:547–555 https://doi.org/10.1016/j.bpobgyn.2006.01.006
Togashi K, Nishimura K, Itoh K, Morisawa N, Fujiwara T, Koyama T (1988) Adenomyosis: diagnosis with MR imaging. Radiology 166:111–114 https://doi.org/10.1148/radiology.166.1.3336669
Bazot M, Daraï E (2018) Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril 109:389–397 https://doi.org/10.1016/j.fertnstert.2018.01.024
Sugino N (2018) Uterine fibroids and adenomyosis. Springer Nature Singapore Pte Ltd, Singapore
Atri M, Reinhold C, Mehio AR, Chapman WB, Bret PM (2000) Adenomyosis: US features with histologic correlation in an in-vitro study. Radiology 215:783–790 https://doi.org/10.1148/radiology.215.3.r00jn06783
Bazot M, Darai E, Rouger J, Detchev R, Cortez A, Uzan S (2002) Limitations of transvaginal sonography for the diagnosis of adenomyosis, with histopathological correlation. Ultrasound Obstet Gynecol 20:605–611 https://doi.org/10.1046/j.1469-0705.2002.00852.x
Fedele L, Bianchi S, Dorta M, Arcaini L, Zanotti F, Carinelli S (1992) Transvaginal ultrasonography in the diagnosis of diffuse adenomyosis. Fertil Steril 58:94–97
Kepkep K, Tuncay YA, Goynumer G, Tutal E (2007) Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound Obstet Gynecol 30:341–345 https://doi.org/10.1002/uog.3985
Sun YL, Wang CB, Lee CY et al (2010) Transvaginal sonographic criteria for the diagnosis of adenomyosis based on histopathologic correlation. Taiwan J Obstet Gyneco 49:40–44 https://doi.org/10.1016/S1028-4559(10)60007-1
Exacoustos C, Brienza L, Di Giovanni A et al (2011) Adenomyosis: three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol 37:471–479 https://doi.org/10.1002/uog.8900
Meredith SM, Sanchez-Ramos L, Kaunitz AM (2009) Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. Am J Obstet Gynecol 201:107e1–107e6 https://doi.org/10.1016/j.ajog.2009.03.021
Dartmouth K (2014) A systematic review with meta-analysis: the common sonographic characteristics of adenomyosis. Ultrasound 22:148–157 https://doi.org/10.1177/1742271X14528837
Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM (2018) Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol 25:257–264 https://doi.org/10.1016/j.jmig.2017.08.653
Togashi K, Konishi I, Itoh H, Nishimura K, Fujisawa IOH (1989) Enlarged uterus: differentiation between adenomyosis and leiomyoma with MR imaging. Radiology 171:531–534
Reinhold C, McCarthy S, Bret PM et al (1996) Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology 199:151–158 https://doi.org/10.1148/radiology.199.1.8633139
Naftalin J, Hoo W, Pateman K, Mavrelos D, Foo X, Jurkovic D (2014) Is adenomyosis associated with menorrhagia? Hum Reprod 29:473–479 https://doi.org/10.1093/humrep/det451
Naftalin J, Hoo W, Nunes N, Holland T, Mavrelos D, Jurkovic D (2016) Association between ultrasound features of adenomyosis and severity of menstrual pain. Ultrasound Obstet Gynecol 47:779–783 https://doi.org/10.1002/uog.15798
Eisenberg VH, Arbib N, Schiff E, Goldenberg M, Seidman DS, Soriano D (2017) Sonographic signs of adenomyosis are prevalent in women undergoing surgery for endometriosis and may suggest a higher risk of infertility. Biomed Res Int 2017:9 https://doi.org/10.1155/2017/8967803
Van den Bosch T, de Bruijn AM, de Leeuw RA et al (2018) A sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol https://doi.org/10.1002/uog.19096
Bazot M (2017) Pathologie Myometriale. In: Nahum H (ed) Imagerie de la femme - Gynecologie. Lavoisier, Paris, p 668
Oliveira MAP, Crispi CP Jr, Brollo LC, De Wilde RL (2018) Surgery in adenomyosis. Arch Gynecol Obstet 297:581–589 https://doi.org/10.1007/s00404-017-4603-6
Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F (2018) Role of medical therapy in the management of uterine adenomyosis. Fertil Steril 109:398–405 https://doi.org/10.1016/j.fertnstert.2018.01.013
Tan J, Moriarty S, Taskin O et al (2018) Reproductive outcomes after fertility-sparing surgery for focal and diffuse adenomyosis: a systematic review. J Minim Invasive Gynecol 25:608–621 https://doi.org/10.1016/j.jmig.2017.12.020
Pontis A, D’Alterio MN, Pirarba S, de Angelis C, Tinelli R, Angioni S (2016) Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol 32:696–700 https://doi.org/10.1080/09513590.2016.1197200
Pinzauti S, Tosti C, Centini G et al (2015) Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: association with symptoms. Ultrasound Obstet Gynecol 46:730–736 https://doi.org/10.1002/uog.14834
Mochimaru A, Aoki S, Oba MS, Kurasawa K, Takahashi T, Hirahara F (2015) Adverse pregnancy outcomes associated with adenomyosis with uterine enlargement. J Obstet Gynaecol Res 41:529–533 https://doi.org/10.1111/jog.12604
Taran FA, Weaver AL, Coddington CC, Stewart EA (2010) Understanding adenomyosis: a case control study. Fertil Steril 94:1223–1228 https://doi.org/10.1016/j.fertnstert.2009.06.049
Vannuccini S, Tosti C, Carmona F et al (2017) Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod BioMed Online 35:592–601 https://doi.org/10.1016/j.rbmo.2017.06.016
Fedele L, Bianchi S, Frontino G (2008) Hormonal treatments for adenomyosis. Best Pr Res Clin Obs Gynaecol 22:333–339 https://doi.org/10.1016/j.bpobgyn.2007.07.006
Badawy AM, Elnashar AM, Mosbah AA (2012) Aromatase inhibitors or gonadotropin-releasing hormone agonists for the management of uterine adenomyosis: a randomized controlled trial. Acta Obstet Gynecol Scand 91:489–495 https://doi.org/10.1111/j.1600-0412.2012.01350.x
Tosti C, Vannuccini S, Lazzeri L, Luisi S, Petraglia F, Troìa L (2016) Current and future medical treatment of adenomyosis. J Endometr 8:127–135
Osada H (2018) Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril 109:406–417 https://doi.org/10.1016/j.fertnstert.2018.01.032
Fujishita A, Masuzaki H, Khan KN, Kitajima M, Ishimaru T (2004) Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Investig 57:132–138 https://doi.org/10.1159/000075830
Saremi A, Bahrami H, Salehian P, Hakak N, Pooladi A, Bahrami H (2014) Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod BioMed Online 28:753–760
Huang X, Huang Q, Chen S et al (2015) Efficacy of laparoscopic adenomyomectomy using double-flap method for diffuse uterine adenomyosis. BMC Womens Health 15:24
Kim TH, Lee HH, Chung SH, Lee W (2012) The triple-flap method for huge uterine adenomyosis with pelvic adhesions. Reprod BioMed Online 25:649author reply 650 https://doi.org/10.1016/j.rbmo.2012.09.011
Nishida M, Takano K, Arai Y, Ozone H, Ichikawa R (2010) Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril 94:715–719 https://doi.org/10.1016/j.fertnstert.2009.03.046
Nabeshima H, Murakami T, Terada Y, Noda T, Yaegashi N, Okamura K (2003) Total laparoscopic surgery of cystic adenomyoma under hydroultrasonographic monitoring. J Am Assoc Gynecol Laparosc 10:195–199
Takeuchi H, Kitade M, Kikuchi I et al (2006) Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol 13:150–154 https://doi.org/10.1016/j.jmig.2005.12.004
Suginami H, Tokushige M, Taniguchi F (2008) Surgical treatment of adenomyosis. Obstet Gynecol (Tokyo) 75:72–78
Kishi Y, Yabuta M, Taniguchi F (2014) Who will benefit from uterus-sparing surgery in adenomyosis-associated subfertility? Fertil Steril 102:802–807e1 https://doi.org/10.1016/j.fertnstert.2014.05.028
Wang PH, Chao HT, Liu WM, Cheng MH, Chao KC, Fuh JL (2009) Is the surgical approach beneficial to subfertile women with symptomatic extensive adenomyosis? J Obstet Gynaecol Res 35:495–502
Grimbizis GF, Mikos T, Tarlatzis B (2014) Uterus-sparing operative treatment for adenomyosis. Fertil Steril 101:472–487.e8 https://doi.org/10.1016/j.fertnstert.2013.10.025
Younes G, Tulandi T (2018) Conservative surgery for adenomyosis and results: a systematic review. J Minim Invasive Gynecol 25:265–276 https://doi.org/10.1016/j.jmig.2017.07.014
Giana M, Montella F, Surico D, Vigone A, Bozzola C, Ruspa G (2005) Large intramyometrial cystic adenomyosis: a hysteroscopic approach with bipolar resectoscope: case report. Eur J Gynaecol Oncol 26:462–463
Ryo E, Takeshita S, Shiba M, Ayabe T (2006) Radiofrequency ablation for cystic adenomyosis: a case report. J Reprod Med 51:427–430
Kamio M, Taguchi S, Oki T et al (2007) Isolated adenomyotic cyst associated with severe dysmenorrhea. J Obstet Gynaecol Res 33:388–391 https://doi.org/10.1111/j.1447-0756.2007.00543.x
Takeuchi H, Kitade M, Kikuchi I, Kumakiri J, Kuroda K, Jinushi M (2010) Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: a review of nine cases. Fertil Steril 94:862–868 https://doi.org/10.1016/j.fertnstert.2009.05.010
Sun W, Guo X, Zhu L, Fei X, Zhang Z, Li D (2018) Hysteroscopic treatment of a uterine cystic adenomyosis. J Minim Invasive Gynecol 25:374–375 https://doi.org/10.1016/j.jmig.2017.07.015
Cheung VYT (2017) Current status of high-intensity focused ultrasound for the management of uterine adenomyosis. Ultrasonography 36:95–102 https://doi.org/10.14366/usg.16040
Fukunishi H, Funaki K, Sawada K, Yamaguchi K, Maeda T, Kaji Y (2008) Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol 15:571–579 https://doi.org/10.1016/j.jmig.2008.06.010
Jingqi W, Lu Z, Jun Z et al (2018) Clinical usefulness of the microbubble contrast agent SonoVue in enhancing the effects of high-intensity focused ultrasound for the treatment of adenomyosis. J Ultrasound Med https://doi.org/10.1002/jum.14638
Xiao-Ying Z, Ying-Shu G, Jiu-Mei C et al (2018) Effect of pre-treatment with gonadotropin-releasing hormone analogue GnRH-α on high-intensity focussed ultrasound ablation for diffuse adenomyosis: a preliminary study. Int J Hyperth:1–9 https://doi.org/10.1080/02656736.2018.1440014
Hou Y, Qin Z, Fan K, Xu Y, Huang X (2018) Combination therapeutic effects of high intensity focused ultrasound and metformin for the treatment of adenomyosis. Exp Ther Med 15:2104–2108 https://doi.org/10.3892/etm.2017.5601
Fan TY, Zhang L, Chen W et al (2012) Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol 81:3624–3630 https://doi.org/10.1016/j.ejrad.2011.05.036
Liu X, Wang W, Wang Y, Wang Y, Li Q, Tang J (2016) Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Medicine (Baltimore) 95:e2443 https://doi.org/10.1097/MD.0000000000002443
Long L, Chen J, Xiong Y et al (2015) Efficacy of high-intensity focused ultrasound ablation for adenomyosis therapy and sexual life quality. Int J Clin Exp Med 8:11701–11707
Zhou M, Chen JY, Tang LD, Chen WZ, Wang ZB (2011) Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 95:900–905 https://doi.org/10.1016/j.fertnstert.2010.10.020
Wang W, Wang Y, Tang J (2009) Safety and efficacy of high intensity focused ultrasound ablation therapy for adenomyosis. Acad Radiol 16:1416–1423 https://doi.org/10.1016/j.acra.2009.06.005
Lee JS, Hong GY, Park BJ, Kim TE (2015) Ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroid & adenomyosis: a single center experience from the Republic of Korea. Ultrason Sonochem 27:682–687 https://doi.org/10.1016/j.ultsonch.2015.05.033
Ferrari F, Arrigoni F, Miccoli A et al (2016) Effectiveness of magnetic resonance-guided focused ultrasound surgery (MRgFUS) in the uterine adenomyosis treatment: technical approach and MRI evaluation. Radiol Med 121:153–161 https://doi.org/10.1007/s11547-015-0580-7
Polina L, Nyapathy V, Mishra A, Yellamanthili H, Vallabhaneni MP (2012) Noninvasive treatment of focal adenomyosis with MR-guided focused ultrasound in two patients. Indian J Radiol Imaging 22:93 https://doi.org/10.4103/0971-3026.101078
Kim KA, Yoon SW, Lee C et al (2011) Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: symptomatic relief and pain reduction. Fertil Steril 95:1152–1155 https://doi.org/10.1016/j.fertnstert.2010.09.024
Shui L, Mao S, Wu Q et al (2015) High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem 27:677–681 https://doi.org/10.1016/j.ultsonch.2015.05.024
Wang S, Meng X, Dong Y (2016) The evaluation of uterine artery embolization as a nonsurgical treatment option for adenomyosis. Int J Gynaecol Obstet 133:202–205 https://doi.org/10.1016/j.ijgo.2015.09.016
Kim MD, Kim YM, Kim HC et al (2011) Uterine artery embolization for symptomatic adenomyosis: a new technical development of the 1-2-3 protocol and predictive factors of MR imaging affecting outcomes. J Vasc Interv Radiol 22:497–502 https://doi.org/10.1016/j.jvir.2011.01.426
Dueholm M (2018) Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol https://doi.org/10.1016/j.bpobgyn.2018.01.016
de Bruijn AM, Lohle PN, Huirne JA et al (2018) Uterine artery embolization versus hysterectomy in the treatment of symptomatic adenomyosis: protocol for the randomized questa trial. JMIR Res Protoc 20:e47 https://doi.org/10.2196/resprot.8512
Resnick NJ, Kim E, Patel RS, Lookstein RA, Nowakowski FS, Fischman AM (2014) Uterine artery embolization using a transradial approach: initial experience and technique. J Vasc Interv Radiol 25:443–447 https://doi.org/10.1016/j.jvir.2013.11.010
Bilhim T, Pisco JM, Duarte M, Oliveira AG (2011) Polyvinyl alcohol particle size for uterine artery embolization: a prospective randomized study of initial use of 350-500μm particles versus initial use of 500-700μm particles. J Vasc Interv Radiol 22:21–27 https://doi.org/10.1016/j.jvir.2010.09.018
Firouznia K, Ghanaati H, Sanaati M, Jalali AH, Shakiba M (2009) Pregnancy after uterine artery embolization for symptomatic fibroids: a series of 15 pregnancies. AJR Am J Roentgenol 192:1588–1592 https://doi.org/10.2214/AJR.07.3904
Das C, Rathinam D, Manchanda S, Srivastava D (2017) Endovascular uterine artery interventions. Indian J Radiol Imaging 27:488 https://doi.org/10.4103/ijri.IJRI_204_16
Rabinovici J, Stewart EA (2006) New interventional techniques for adenomyosis. Best Pr Res Clin Obs Gynaecol 20:617–636 https://doi.org/10.1016/j.bpobgyn.2006.02.002
Keung JJ, Spies JB, Caridi TM (2017) Uterine artery embolization: a review of current concepts. Best Pract Res Clin Obstet Gynaecol 46:66–73 https://doi.org/10.1016/j.bpobgyn.2017.09.003
Zhou J, He L, Liu P et al (2016) Outcomes in adenomyosis treated with uterine artery embolization are associated with lesion vascularity: a long-term follow-up study of 252 cases. PLoS One 11:e0165610 https://doi.org/10.1371/journal.pone.0165610
de Bruijn AM, Smink M, Lohle PNM et al (2017) Uterine artery embolization for the treatment of adenomyosis: a systematic review and meta-analysis. J Vasc Interv Radiol 28:1629–1642.e1 https://doi.org/10.1016/j.jvir.2017.07.034
Caridi TM, Spies JB (2018) Management of adenomyosis. A review of characteristic imaging findings and treatment options, with an emphasis on the use of uterine artery embolization. Endovasc Today 17:57–61
Hehenkamp WJK, Volkers NA, Birnie E, Reekers JA, Ankum WM (2008) Symptomatic uterine fibroids: treatment with uterine artery embolization or hysterectomy—results from the randomized clinical embolisation versus hysterectomy (EMMY) trial. Radiology 246:823–832 https://doi.org/10.1148/radiol.2463070260
Mohan PP, Hamblin MH, Vogelzang RL (2013) Uterine artery embolization and its effect on fertility. J Vasc Interv Radiol 24:925–930 https://doi.org/10.1016/j.jvir.2013.03.014
Acknowledgements
The authors would like to acknowledge Prof. Adel Gamil for providing ultrasound images for this manuscript.
Funding
No funding was received for this work.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
RD contributed to the manuscript preparation and revision. SAG and MGN contributed to the manuscript editing and revision, image collection, editing, and preparation. RM and YL contributed to the preparation of the manuscript draft and editing and revision of final manuscript. All authors significantly contributed to the preparation of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dessouky, R., Gamil, S.A., Nada, M.G. et al. Management of uterine adenomyosis: current trends and uterine artery embolization as a potential alternative to hysterectomy. Insights Imaging 10, 48 (2019). https://doi.org/10.1186/s13244-019-0732-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-019-0732-8