Abstract
Background
Most children with Autism Spectrum Disorder (ASD) have co-occurring language impairments and some of these autism-specific language difficulties are also present in their non-autistic first-degree relatives. One of the possible neural mechanisms associated with variability in language functioning is alterations in cortical gamma-band oscillations, hypothesized to be related to neural excitation and inhibition balance.
Methods
We used a high-density 128-channel electroencephalography (EEG) to register brain response to speech stimuli in a large sex-balanced sample of participants: 125 youth with ASD, 121 typically developing (TD) youth, and 40 unaffected siblings (US) of youth with ASD. Language skills were assessed with Clinical Evaluation of Language Fundamentals.
Results
First, during speech processing, we identified significantly elevated gamma power in ASD participants compared to TD controls. Second, across all youth, higher gamma power was associated with lower language skills. Finally, the US group demonstrated an intermediate profile in both language and gamma power, with nonverbal IQ mediating the relationship between gamma power and language skills.
Limitations
We only focused on one of the possible neural contributors to variability in language functioning. Also, the US group consisted of a smaller number of participants in comparison to the ASD or TD groups. Finally, due to the timing issue in EEG system we have provided only non-phase-locked analysis.
Conclusions
Autistic youth showed elevated gamma power, suggesting higher excitation in the brain in response to speech stimuli and elevated gamma power was related to lower language skills. The US group showed an intermediate pattern of gamma activity, suggesting that the broader autism phenotype extends to neural profiles.
Similar content being viewed by others
Background
Autism Spectrum Disorder (ASD) is a highly heritable neurodevelopmental condition associated with difficulties in social interaction/communication, the presence of stereotyped/repetitive behavior, and restricted interest or atypical response to sensor information [1]. Although language impairment is not among the core characteristics of ASD, about 75% of children with this disorder have co-occurring language difficulties [2, 3]. Language functioning is highly heterogeneous and can vary from severe impairment (e.g., nonverbal or minimally verbal ASD) to above-average language skills [4,5,6]. Given the variability of language skills in this population, it is most likely that there are multiple neurobiological mechanisms that are related to language impairment in ASD [7,8,9]. Moreover, given the well-known broader autism phenotype, some of these autism-specific language deficits may also be presented in the first-degree relatives of children with ASD [10, 11].
One of the possible neural mechanisms related to autistic behaviors is cortical gamma-band (30–150 Hz) oscillations measured with electro- and magnetoencephalography (EEG/MEG). Animal and cellular studies with optogenetic manipulations have shown that gamma oscillations are associated with the balance between neural excitation (E) and inhibition (I) and generated mostly by gamma-aminobutyric acidergic (GABAergic) interneurons, expressing calcium-binding protein parvalbumin (PV + basket cells) [12,13,14,15,16,17]. In general, aberrant gamma activity in autistic individuals has been reported in a number of studies as a potential biomarker related to both core and co-occurring conditions of ASD [18, 19]. Additionally, animal models of autism and electrophysiological studies in combination with magnetic resonance spectroscopy have suggested that E/I imbalance and the dysfunction of the GABAergic system may be a physiological mechanism contributing to expression of autistic phenotypes [20,21,22,23,24,25,26,27]. Given the close relation of gamma oscillations to clinically relevant processes such as language processing, nonverbal IQ, and social functioning [9, 28, 29], these oscillations are of particular interest in ASD research.
A number of previous studies have demonstrated atypical gamma activity in toddlers, children, youth and adults with ASD and their first-degree relatives in response to low-level auditory as well as high-level linguistic stimuli and the relationship between these brain responses and both expressive and receptive language skills [18, 30,31,32,33,34,35,36,37,38]. For example, with respect to low-level auditory processing, reduced gamma power and/or inter-trial phase consistency was reported in both autistic children and their non-autistic siblings/parents, using 40 Hz amplitude-modulated tones and amplitude modulated sweeps; these altered brain responses were associated with lower receptive as well as overall language skills [30, 34]. Both reduced and elevated gamma activity in autistic adults and their first-degree relatives was also reported when presenting linguistic stimuli of different complexity, such as syllables, words, and sentences [31, 32]. Resting-state or baseline gamma oscillations were also related to both expressive and overall language abilities of toddlers with idiopathic ASD and their siblings and/or parents as well as individuals with single-gene disorders with elevated autism behaviors, such as Fragile X Syndrome [9, 39, 40], and could even be an early biomarker of further language functioning in ASD [9, 39]. Summarizing, atypical neural activity at the gamma frequency band was related to language abilities of both autistic individuals and their first-degree relatives and may be a non-invasive objective measure of language functioning in the endophenotype. Remarkably, according to the previous findings, gamma activity perhaps was not associated with a specific domain of language skills (e.g., expressive vs. receptive) in relation to the specific stage of child development (toddlers vs. youth), but rather reflected a general feature of the functioning of autistic brain in relation to overall language abilities.
The goal of the present study was to investigate the relationship between gamma activity in response to speech stimuli and language skills in a large sample of sex-matched autistic youth, typically developing (TD) youth, and unaffected siblings (US) of youth with ASD. We aimed to estimate EEG spectral power at a gamma frequency band (as the E/I balance marker [13, 41]) using a language learning paradigm. The study also explored which phenotypic characteristics could explain the possible relationship between gamma power in response to speech stimuli and language skills of the US group. The strengths of the study were fourfold. First, instead of passive paradigms with low-level auditory stimuli or perception of speech samples, we used a language learning paradigm that activates broader neural networks associated with language processing in a representative sample of participants. Second, our sample consisted of a roughly equal number of male and female individuals; this is essential, as the previous studies showed that male and female individuals can have different profiles with respect to language and communication abilities [42,43,44,45,46]. Third, we used a standardized formal assessment to evaluate the language skills of each child. Finally, to characterize the relationship between gamma power and language skills of the US group, we used mediation modeling that allows for probing of causal relationships between variables.
Methods
Participants
A total of 286 native English-speaking youth aged 7 to 18 years participated in the study: 125 autistic youth (58 female, 67 male), 121 TD youth (61 female, 60 male), and 40 US of youth with ASD (24 female, 16 male); all participants from the US group were siblings of autistic youth from the present study. Sex was based on parent report of sex assigned at birth. Data were collected from four sites as a part of the GENDAAR Autism Center for Excellence network, including Seattle Children’s Research Institute, Boston Children’s Hospital, the University of California in Los Angeles, and Yale University.
The study was approved by the Yale Institutional Review Board, the UCLA Office of Human Research Protection Program, Boston Children’s Hospital Institutional Review Board, USC Office for the Protection of Research Subjects, and the University of Virginia Institutional Review Board for Health Sciences Research. All performed procedures were in accordance with the Declaration of Helsinki. All minor children provided verbal assent to participate in the study and were informed that they can withdraw from the study at any time during the experiment. A written consent form was obtained from a parent of each child participating in the study.
Clinical and behavioral assessment
All youth with ASD were diagnosed with the Autism Diagnosis Observation Schedule—Second Edition (ADOS-2) [47] and DSM–IV–TR [48]. Participants were included in the study if they had either verbal or nonverbal IQ ≥ 70 based on the Differential Ability Scales—Second Edition (DAS-II) School Aged Cognitive Battery [49]. Exclusion criteria were twin status, history and/or presence of known chromosomal syndromes/single-gene conditions related to autism (e.g., Fragile X Syndrome), co-occurring neurological conditions (e.g., epilepsy), significant visual and auditory impairments, or sensory-motor difficulties that would prevent completion of study procedures. TD children had no first or second degree family members with ASD, and no elevation of autism traits according to parent report on the Social Responsiveness Scale—Second Edition (SRS-2) [50] (T-score < 60) or the Social Communication Questionnaire [51] (raw score < 11). Adaptive skills were measured with the Vineland Adaptive Behavior Scales—Second Edition (Vineland-II); standard scores in communication, socialization, and daily living skills domains were calculated for each child [52]. Language abilities were scored with the Clinical Evaluation of Language Fundamentals—Fourth Edition (CELF-4) [53], a standardized assessment tool that covers basic structural language skills at different linguistic levels (vocabulary, morphosyntax, semantics, and pragmatics) in both production and comprehension. The participants were administered only with the tests that were necessary to calculate CELF-4 Core Language Standard Score, which was used as a measure of overall language skills. All participants from the TD group as well as the US group had normal language skills based on the CELF-4 Core Language Standard Score. Demographic information is presented in Table 1.
Experimental paradigm and procedure
The implicit word segmentation paradigm from [54,55,56,57,58,59] was used. During the first (exposure) phase, participants were presented auditorily with three-syllabic pseudowords generated from the set of 12 different phonemes (e.g., pa-bi-ku); resulting in 180 exposures over approximately 2.5 min duration. The second (test) phase consisted of 96 trials; duration was 2 min 16 s. Analyses were restricted to the second (test) phase of experiment. Each trial consisted of a tree-syllabic pseudoword with the average duration of ~ 900 ms, followed by a 500–750 ms intertrial interval. A random half of these trials (48) used the same pseudowords presented in the first phase during exposure (e.g., pa-bi-ku), i.e., ‘familiar’ items. The remaining random 48 trials were constructed by combining the last syllable of each familiar pseudoword with the first two phonemes of other pseudowords (e.g., pa-bi-ku and go-la-tu became ku-go-la and tu-pa-bi), i.e., ‘unfamiliar’ items. The auditory stimuli were presented using a speaker (Logitech speaker system X320) with the same loudness across all participants and sites (65 dB). Children were instructed to look at the screen and to listen carefully to the ‘robot language’. A static robot was presented on the screen simultaneously with the auditory stimuli during the experiment. Figure 1 represents a schematic structure of the experiment.
EEG data acquisition and processing
At all four sites, EEG data were collected with EGI 128-channel Net Amps 300 system with HydroCel nets (Magstim EGI Inc., Eugene OR), using Net Station 4.4.2, 4.5.1, or 4.5.2 with a standard Net Station acquisition template.Footnote 1 Nets were available without outriders (eye electrodes 125, 126, 127, and 128) for participants with facial sensory sensitivities. The participant’s behavior was video recorded during EEG collection. Data were collected at 500 Hz sampling rate, referenced to Cz electrode (vertex), and impedances were < 50 kΩ.
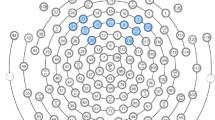
To calculate power spectral density (PSD) for the gamma-band frequency range (35–54.99 Hz) we used the Batch EEG Automated Processing Platform, BEAPP [60] in MATLAB 2021a, consisting of: (1) format the MFF file for Matlab; (2) band-pass filter 1–100 Hz; (3) down sampling from 500 to 250 Hz; (4) implementation of the Harvard Automated Preprocessing Pipeline for EEG (HAPPE) module for artifact detection and rejection [61], including removal of 60 Hz line noise, rejection of bad channels, wavelet enhanced thresholding, Independent Component Analysis (ICA) with automated component rejection, bad channel interpolation, and re-referencing to average; (5) segmentation of the continuous file into 1 s epochs (each epoch consisted of one three-syllabic pseudoword); (6) rejection of bad segments (± 40 μV); (7) calculation of the PSD using Hanning window on clean segments. We focused on the low gamma frequency band (35–54.99 Hz) to avoid potential effects of 60 Hz line noise and the notch filter used for its removal. A total of nine regions of interest (ROIs) were used for the analysis as depicted in Fig. 2. PSD was calculated for each electrode, averaged within each ROI, and normalized with natural logarithm transformation.
EEG montage with channels indicated. Channel numbers for regions are (1) frontal left (20, 23, 24, 27, 28), (2) frontal midline (5, 6, 11, 12, 16), (3) frontal right (3, 117, 118, 123, 124), (4) central left (35, 36, 41, 42, 47), (5) central midline (7, 31, 55, 80, 106), (6) central right (93, 98, 103, 104, 110), (7) posterior left (51, 52, 59, 60, 65), (8) posterior midline (62, 71, 72, 76), (9) posterior right (85, 90, 91, 92, 97)
The US group had fewer artifact-free epochs in the test ‘familiar’ condition in comparison to ASD and TD groups; ASD and TD groups did not differ in the number of artifact-free epochs in any condition: ‘familiar’ items, ASD, Mepoch = 43, range 30–47; TD, Mepoch = 43, range 34–47; US, Mepoch = 41, range 12–45; F(2, 283) = 5.61, p = 0.004. ‘Unfamiliar’ items, ASD, Mepoch = 43, range 28–47; TD, Mepoch = 43, range 33–47; US, Mepoch = 42, range 11–46; F(2, 283) = 1.19, p = 0.30.
EEG and behavioral data were available for all participants.
Statistical analysis
All linear models used in the analysis were estimated in R [62] with the lme4 package [63]; mediation models were estimated with the sem [64] and lavaan [65] packages. The data were plotted with ggplot2 [66], and semPlot [67] packages; the figures, representing neural responses, were created with python data visualization library matplotlib [68]. The structure of the models will be specified further in the Results section.
Results
Group and condition differences in gamma power
In order to assess between-group difference in gamma power in response to speech stimuli, we fitted a linear mixed-effect model for each ROI with gamma power as a dependent variable, condition (familiar vs. unfamiliar), group (ASD, TD and US; the intercept corresponded to the ASD group), condition × group interaction, and sex as main effects, and participants as a random intercept; the structure of the model was as follows: lmer(power ~ condition × group + sex + (1 | ID), data = data, control = lmerControl(optimizer = "Nelder_Mead")). A correction for multiple comparisons (false discovery rate, FDR) was applied to the models, and p-values for significant predictors were corrected with p.adjust.method in R. Additional analysis addressing ROI as a factor in the model, as well as ROI vs. composite whole-head EEG measure (gamma power averaged across all ROIs) can be found in Additional file 1.
The results showed neither between-condition difference in the gamma power nor condition × group interaction in any ROI, indicating that the pattern of neural response for familiar and unfamiliar pseudowords was similar in ASD, TD, and US groups (Table 2). At the same time, the results revealed a statistically significant between-group difference in gamma power in six out of nine ROIs after applying FDR-correction (Fig. 3): autistic youth had elevated power in comparison to TD youth (Fig. 4, see Table 2). No statistical difference was found between the ASD and US groups in any ROI. Descriptive statistics of mean values of gamma power in these six regions showed that the US group had lower power in comparison to autistic youth but higher power when comparing to TD youth (Table 3). A main effect of sex was identified in four ROIs, so as the power of gamma activity was higher in the male group.
Absolute power spectra for six regions of interest which showed statistically significant between-group differences in gamma power (35–54.99 Hz) in response to speech stimuli. The plots represent the broad frequency range (ASD = Autism Spectrum Disorder; TD = typically developing; US = unaffected siblings of children with ASD)
Between-group differences in gamma power (35–54.99 Hz) in response to speech stimuli in six regions of interest (ASD = Autism Spectrum Disorder; TD = typically developing; US = unaffected siblings of children with ASD). The significance is labeled with *p < 0.05, **p < 0.01, ns = non-significant. All p-values are FDR-corrected
The relationship between gamma power and language skills
In order to examine whether variations in gamma power in response to speech stimuli had behavioral or clinical relevance, we fitted a linear mixed-effect model for those significant six ROIs with gamma power as a dependent variable, CELF-4 Core Language Standard Score as a factor (behavioral measure of language skills), and participants as a random intercept, according to the formula: lmer(power ~ CELF-4 Core Language Standard Score + (1 | ID), data = data).
The results revealed a significant relationship between gamma power in three out of six ROIs and behavioral language abilities: higher gamma was associated with lower language skills (Fig. 5): central midline, Est = –1.826e-03, SE = 5.922e-04, t = –3.08, p = 0.002; posterior left, Est = –1.595e-03, SE = 7.268e-04, t = –2.19, p = 0.03; posterior right, Est = –1.575e-03, SE = 7.814e-04, t = –2.02, p = 0.04. After correction for multiple comparisons (p.adjust.method in R) these effects remained significant: FDR-corrected p-values are 0.006, 0.04, 0.04 for central midline, posterior left, and posterior right ROIs, respectively. Other ROIs did not show statistically significant effects: frontal midline, Est = –1.160e-03, SE = 6.214e-04, t = –1.87, p = 0.06; central right, Est = –8.117e-04, SE = 6.580e-04, t = –1.23, p = 0.22; posterior midline, Est = –1.274e-03, SE = 7.977e-04, t = –1.60, p = 0.11.
Considering age and sex
As previous studies have demonstrated that the gamma power can change during child development [69,70,71,72,73,74] and we observed a main effect of sex in four ROIs, we fitted a linear mixed-effect model for three ROIs that showed significant effects (central midline, posterior left, posterior right) to assess the relationship between gamma power and language skills while accounting for age and sex: lmer(power ~ CELF-4 Core Language Standard Score + age + sex + (1 | ID), data = data). We applied a correction for multiple comparisons to the models, so all p-values are FDR-corrected. The full models’ outcomes are presented in Table 4. After correction for multiple comparisons and accounting for age and sex, the relationship between gamma power and language skills for central midline ROI remained significant, Est = –0.00, SE = 0.00, t = –2.79, p = 0.015. Age and sex effects were not related to gamma power (see Table 4). For the posterior left and right ROIs, the association between gamma power and language skills was not significant when controlling for age and sex.
Summary
Between-group comparisons showed that autistic youth had elevated gamma power in comparison to TD youth. Higher gamma power was related to lower language skills in the central midline ROI.
The phenotype of unaffected siblings of children with ASD
This follow-up post-hoc analysis focused on the US group specifically, as this group demonstrated an ‘intermediate’ neural phenotype between the ASD and TD groups. To assess the language skills of the US group in comparison to ASD and TD groups, we fitted a linear model with main effects of group (the intercept corresponded to the US group), sex (as assigned at birth), and group × sex interaction; sex was included into the model as the previous studies showed that male and female individuals can have different profiles with respect to language and communication abilities [42,43,44,45,46]. The structure of the model was as follows: lm(CELF-4 Core Language Standard Score ~ group + sex + group × sex, data = data). The results revealed a main effect of group, indicating that the US group had significantly higher language skills in comparison to the ASD group, Est = –12.24, SE = 1.41, t = –8.68, p < 0.001; but significantly lower language skills when comparing to TD participants, Est = 6.44, SE = 1.42, t = 4.54, p < 0.001 (see Table 5, Fig. 6A). It is important to note that all participants from the US group had language skills at or above average on the CELF-4 Core Language Standard Score (M = 110, range 88–129).
Language skills of unaffected siblings of children with Autism Spectrum Disorder: A—a comparison of CELF-4 Core Language Standard Score in three groups of children (ASD = Autism Spectrum Disorder; TD = typically developing; US = unaffected siblings of children with ASD); B—a mediation model for central midline region of interest: red path represents a statistically significant indirect relationship between gamma power and language skills via nonverbal IQ
To explore which phenotypic characteristics inform the relationship between gamma power and language skills of US participants, we fitted a mediation model (for the ROI in which the association between gamma power and CELF-4 Core Language Standard Score remained significant when controlling for age, sex, and the correction for multiple comparisons) and included sex, age, nonverbal IQ, verbal IQ, Vineland Socialization Standard Score, and SRS-2 total raw score as mediators. The model assessed the direct effects of gamma power on language skills as well as indirect effects through all mediators included in the models. Also, the model calculated the overall indirect effect and the total effect. The full model outcome is presented in Table 6, and standardized estimates of path coefficients are depicted in Fig. 6B.
The results of the mediation model showed a statistically significant indirect effect of nonverbal IQ as a mediator between gamma power and language skills of US participants, Est = –6.89, SE = 2.96, z = –2.33, p = 0.02, C.I. [–12.69, –1.09]. Also, the model revealed significant overall indirect and total effects: overall indirect effect, Est = –10.92, SE = 4.42, z = –2.47, p = 0.01, C.I. [–19.59, –2.25]; total effect, Est = –25.99, SE = 6.49, z = –4.00, p < 0.001, C.I. [–38.72, –13.26]; SRMS = 0.180, AIC = 3828.398, TLI = –0.027, CFI = 0.450, power (1—β) = 0.499. The same mediation analysis was provided for the ASD and TD groups to assess whether the mediation role of nonverbal IQ in the relationship between gamma power and language skills is universal for all groups. The results did not reveal any indirect paths between gamma power and language skills in both groups, indicating hypothetically that this effect can be specific to the US group (see Additional file 1 with the full model outcomes for the ASD and TD groups).
As the models revealed an indirect or mediation effect of nonverbal IQ, we provided follow-up between-group comparison in nonverbal IQ, using the same structure of model as for CELF-4 Core Language Standard Score: linear model with main effect of group (the intercept corresponded to the US group), sex (as assigned at birth), and group × sex interaction. The results showed a main effect of group, indicating that the US group had a significantly higher nonverbal IQ in comparison to the ASD group, Est = –7.01, SE = 1.39, t = –5.04, p < 0.001; however, no difference was found between US and TD groups: Est = 1.83, SE = 1.39, t = 1.31, p = 0.19. No main effect of sex and an effect of group × sex interaction was identified: sex, Est = –0.06, SE = 1.11, t = –0.05, p = 0.96; ASD, female, Est = –0.70, SE = 1.39, t = –0.51, p = 0.61; TD, female, Est = 0.80, SE = 1.40, t = 0.57, p = 0.57.
Summary
US individuals had higher language skill in comparison to youth with ASD but lower in comparison to TD youth. In the US group, nonverbal IQ mediated the relationship between gamma power and language skills.
Discussion
The present study investigated neural activity at the gamma frequency band in response to speech stimuli in autistic youth and their first-degree relatives, as well as the relationship between this neural activity and language skills. In general, results revealed an elevation in EEG spectral power at the gamma frequency band in youth with ASD and their siblings and showed that variability in gamma activity was associated with language skills measured in formal assessment. The analysis in the US group showed that nonverbal IQ mediated the relationship between brain response and language abilities.
In accordance with the previous findings [75,76,77], we showed altered gamma activity in youth with ASD when comparing them to TD youth but extend this to neural activity during speech processing. Given the nature of the task (perception of speech stimuli presented auditorily), we proposed that the main brain areas generated gamma activity were temporal regions in the left and right hemispheres, which is consistent with the previous studies that identified alterations in gamma response in the auditory cortex of autistic individuals [30, 32, 34, 74]. In general, gamma-band abnormalities in autism were reported in multiple studies [19, 20, 35, 78,79,80] and were considered as a potential biomarker [18, 19] related to both core characteristics and co-occurring conditions in ASD, including language functioning [30]. Gamma oscillations are one of the indexes of E/I balance, and they arise from the inhibition of pyramidal cells via binding the inhibitory GABAergic neurotransmitter [12,13,14]. Thus, increased spectral power at the gamma frequency range may reflect increased E/I ratio [25]. This, in turn, may result in a selective enhancement of excitation and increased ‘noise’ in the cortex, which in turn impacts synaptic plasticity during development and results in less effective information processing [24, 25]. Elevated excitatory activity in the autistic brain may also explain a high rate of epilepsy in this population: it is known that the rate of epilepsy in autistic individuals is approximately 20% [81] whereas in general population it is ~ 1%. The hypothesis of increased E/I ratio and altered gamma activity in ASD was supported by both animal models of autism and cellular studies of neural tissues [82,83,84].
The observed elevation in EEG spectral power at the gamma frequency range during a speech task in autistic youth and the intermediate pattern of gamma activity in the US participants was related to behavioral language functioning: higher gamma power was associated with lower language skills. A number of previous studies have demonstrated that gamma oscillations play a significant role in the local networks involved in language processing. For instance, in the left temporal region, which is a crucial cortical area for speech processing, gamma oscillations are specifically associated with coding temporal fine units of speech [41, 85,86,87] and analyzing the properties of a sound in the short temporal integration windows [88, 89]. In addition, biophysical models of neural computations suggested that gamma oscillations are sufficient for phoneme identification during speech processing [41]. Thus, all these types of speech processing could be affected by alterations in gamma activity due to an E/I imbalance. While previous findings have shown a tight relationship between gamma power and age [69,70,71,72,73,74], we revealed a significant association between neural activity at the gamma band and language in our broad developmental-range sample when accounting for age. In general, given the specificity of this neural activity the changes in gamma power during child development could be associated with the maturation of GABAergic inhibitory neurotransmission and age-related changes of E/I balance [70]. This maturation starts very early in development by switching from a depolarizing to hyperpolarizing action of GABA receptors, that is, excitatory-to-inhibitory shift of GABA receptors [90,91,92]. Previous findings have shown that gamma power increases rapidly during first years of life [78] with a less rapid increase in the adolescence and early adulthood [93] and then decreases in the late brain development [94]. Therefore, age-related changes of gamma activity in the early stages of brain development may reflect neural maturation that promotes efficient cognitive development, including language development.
The analysis of the US group identified an intermediate pattern of gamma activity as well as language skills based on CELF-4 Core Language Standard Score. This is consistent with the previous findings that showed either intermediate or similar to ASD pattern of brain activity in the first-degree relatives of individuals with ASD [95, 96] as well as lower language and communication skills in comparison to neurotypical population [10, 11]. The findings supported the hypothesis of broader autism phenotype [97], pointing to a highly heritable nature of language processing in autistic individuals. To understand the complexity of the relationship between gamma power and language skills in the US group, we also provided mediation modeling. The results demonstrated that EEG spectral power at the gamma frequency range in response to speech stimuli was related to language skills via nonverbal IQ. It is important to note that the US individuals from our study had both normal language skills and nonverbal IQ. Previous studies with autistic individuals have shown a relationship between nonverbal IQ and language [2] as well as nonverbal IQ and gamma power [29], but this is the first study that identified the mediation role of nonverbal IQ. These findings highlight the specific phenotypical characteristic of US individuals, contributing to better understanding of variability in functioning in this population.
Limitations
The findings of the study should be considered within the context of several limitations. First, we want to highlight that the study focused on one of the possible neural mechanisms of language functioning in ASD and the broader autistic phenotype and did not include the analysis of other types of brain responses. Future studies would benefit from addressing the neural activity at all frequency bands and regional specificity of these patterns. This could contribute to understanding of the complex neural system underlying language variability in families with child with autism. Second, the US group consisted of smaller number of participants in comparison to ASD or TD groups (US = 40 youth, ASD = 125 youth, TD = 121 youth); thus, given the low statistical power of the mediation analysis the replication is needed. Finally, due to the timing issue in EEG system we have provided only non-phase-locked analysis. It is important to note, however, that we have adjusted the timing window as much as it was possible during the post-acquisition processing to address the error as it manifested in the systems we used. Following the disclosure of the timing error by EGI we incorporated StimTracker event markers, which use auditory and photodiode sensors and 5v inputs to the digital inputs of the amplifiers to assess ground-truth timing accuracy. These analyses revealed (1) that the timing drift was not present at all sites; (2) in those recording sessions where there was drift, it was no larger than 25 ms across a 45 min recording session. However, as we cannot confirm the specific timing error for each individual nor the stability of the delay across the whole of the recording, we adjusted our analysis window such that we are able to expect that 90% of the 1 s trial reflects brain activity during the perception of the pseudoword. Thus, future studies using the same EEG system should address this issue and check the timing drift for providing as much precise analysis as possible.
Conclusions
To conclude, the study demonstrated, first, an elevated EEG spectral power at the gamma frequency range in response to speech stimuli in autistic youth and the intermediate pattern of activity in unaffected siblings (US) of youth with ASD. These results may support the hypothesis of E/I imbalance in autistic individuals. However, there can be alternative interpretations of our results, such as, for example, more involvement of attention of autistic individuals in the task and increased gamma power due to increased attention. Second, the findings revealed that elevated gamma power was related to lower language skills. Finally, the phenotypic analysis of the US group showed that the link between gamma activity and language skills was mediated by nonverbal IQ.
Notes
An advisory notice related to timing offsets for the GES 300 systems with firewire cameras was released in 2016. Due to the potential impact on some of our data acquired, we do not provide stimulus locked results.
https://www.egi.com/knowledge-center/item/63-ges-300-and-firewire-cameras
Abbreviations
- ASD:
-
Autism Spectrum Disorder
- EEG:
-
Electroencephalography
- MEG:
-
Magnetoencephalography
- GABA/GABAergic:
-
Gamma-aminobutyric acidergic
- PV:
-
Parvalbumin
- TD:
-
Typically developing
- US:
-
Unaffected siblings
- ADOS-2:
-
Autism diagnosis observation schedule-second edition
- DAS-II:
-
Differential ability scales-second edition
- SRS-2:
-
Social responsiveness scale-second edition
- Vineland-II:
-
Vineland adaptive behavior scales-second edition
- CELF-4:
-
Clinical evaluation of language fundamentals-fourth edition
- PSD:
-
Power spectral density
- BEAPP:
-
Batch EEG automated processing platform
- HAPPE:
-
Harvard automated preprocessing pipeline for EEG
- ICA:
-
Independent component analysis
- ROI(s):
-
Region(s) of interest
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Washington, DC; London: American Psychiatric Publication, 2013.
Arutiunian V, Lopukhina A, Minnigulova A, Shlyakhova A, Davydova E, Pereverzeva D, et al. Language abilities of Russian primary-school-aged children with autism spectrum disorder: evidence from comprehensive assessment. J Autism Dev Disord. 2022;52:584–99.
Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: implications for genetic subgroups. Lang Cognit Process. 2001;16:287–308.
Pickles A, Anderson DK, Lord C. Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J Child Psychol Psychiatry. 2014;55:1354–62.
Tager-Flusberg H, Kasari K. Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Res. 2013;6:468–78.
Tager-Flusberg H. Risk factors associated with language in autism spectrum disorder: clues to underlying mechanisms. J Speech Lang Hear Res. 2016;59:143–54.
Roberts TPL, Matsuzaki J, Blaskey L, Bloy L, Edgar JC, Kim M, et al. Delayed M50/M100 evoked response component latency in minimally verbal/nonverbal children who have autism spectrum disorder. Molecular Autism. 2019;10:34.
Schwartz S, Wang L, Shinn-Cunningham BG, Tager-Flusberg H. Atypical perception of sounds in minimally and low verbal children and adolescents with autism as revealed by behavioral and neural measures. Autism Res. 2020;13:1718–29.
Wilkinson CL, Levin AR, Gabard-Durnam LJ, Tager-Flusberg H, Nelson CA. Reduced frontal gamma power at 24 months is associated with better expressive language in toddlers at risk for autism. Autism Res. 2019;12:1211–24.
Lindgren KA, Folstein SE, Tomblin JB, Tager-Flusberg H. Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Res. 2009;2:22–38.
Pisula E, Ziegart-Sadowska K. Broader autism phenotype in siblings of children with ASD—a review. Int J Mol Sci. 2015;16:13217–58.
Agetsuma M, Hamm JP, Tao K, Fujisawa S, Yuste R. Parvalbumin-positive interneurons regulate neuronal ensembles in visual cortex. Cereb Cortex. 2018;28:1831–45.
Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–8.
Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for the NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–48.
Espinoza C, Guzman SJ, Zhang X, Jonas P. Parvalbumin+ interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat Commun. 2018;9:4605.
Ferguson BR, Gao W-J. PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits. 2018;12:37.
Magueresse CL, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405.
Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiat. 2010;68:1100–6.
Rojas DC, Wilson LB. γ-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8:353–68.
Contractor A, Ethell IM, Portera-Cailliau C. Cortical interneurons in autism. Nat Neurosci. 2021;24:1648–59.
Ford TC, Abu-Akel A, Crewther DP. The association of excitation and inhibition signaling with the relative symptom expression of autism and psychosis-proneness: Implications for psychopharmacology. Progress Neuropsychopharmacol Biol Psychiatry. 2019;88:235–42.
Ford TC, Woods W, Enticott PG, Crewther DP. Cortical excitation-inhibition ratio mediates the effect of pre-attentive auditory processing deficits on interpersonal difficulties. Progress Neuropsychopharmacol Biol Psychiatry. 2020;98: 109769.
Levin R, Nelson CA. Inhibition-based biomarkers for autism spectrum disorder. Neurotherapeutics. 2015;12:546–52.
Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67.
Sohal VS, Rubenstein JLR. Excitation-inhibition balance as a framework for investigating mechanisms of neuropsychiatric disorders. Mol Psychiatry. 2019;24:1248–57.
Tang X, Jaenisch R, Sur M. The role of GABAergic signalling in neurodevelopmental disorders. Nat Rev Neurosci. 2021;22:290–307.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8.
Cao W, Lin S, Xia Q-Q, Du Y-I, Yang Q, Zhang M-Y, et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron. 2018;97:1253–60.
Manyukhina VO, Prokofyev AO, Galuta IA, Goiaeva DE, Obukhova TS, Schneiderman JF, et al. Globally elevated excitation-inhibition ratio in children with autism spectrum disorder and below-average intelligence. Mol Autism. 2022;13:20.
Arutiunian V, Arcara G, Buyanova I, Davydova E, Pereverzeva D, Sorokin A, et al. Neuromagnetic 40 Hz auditory steady-state response in the left auditory cortex is related to language comprehension in children with Autism Spectrum Disorder. Progress Neuropsychopharmacol Biol Psychiatry. 2023;122: 110690.
Braeutigam S, Swithenby SJ, Bailey AJ. Contextual integration the unusual way: a magnetoencephalographic study of responses to semantic violation in individuals with autism spectrum disorders. Eur J Neurosci. 2008;27:1026–36.
McFadden KL, Hepburn S, Winterrowd E, Schmidt G, Rojas DC. Abnormalities in gamma-band responses to language stimuli in first-degree relatives of children with autism spectrum disorder: an MEG study. BMC Psychiatry. 2012;12:213.
Ortiz-Mantilla S, Cantiani C, Shafer VL, Benasich AA. Minimally-verbal children with autism show deficits in theta and gamma oscillations during processing of semantically-related visual information. Sci Rep. 2019;9:5072.
Roberts TPL, Bloy L, Liu S, Ku M, Blaskey L, Jackel C. Magnetoencephalography studies of the envelope following response during amplitude-modulated sweeps: diminished phase synchrony in autism spectrum disorder. Front Hum Neurosci. 2021;15: 787229.
Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66.
Rojas DC, Teale PD, Maharajh K, Kronberg E, Youngpeter K, Wilson LB, et al. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Molecular Autism. 2011;2:11.
Wang X, Delgado J, Marchesotti S, Kojovic N, Sperdin HF, Rihs TA, et al. Speech reception in young children with autism is selectively indexed by a neural oscillation coupling anomaly. J Neurosci. 2023;43:6779–95.
Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiat. 2007;62:192–7.
Romeo RR, Choi B, Gabard-Durnam LJ, Wilkinson CL, Levin AR, Rowe ML, et al. Parental language input predicts neuroscillatory patterns associated with language development in toddlers at risk of autism. J Autism Dev Disord. 2022;52:2717–31.
Wilkinson CL, Nelson CA. Increased aperiodic gamma power on young boys with Fragile X Syndrome is associated with better language ability. Molecular Autism. 2021;12:17.
Giraud A-L, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–7.
Neuhaus E, Kang VY, Kresse A, Corrigan S, Aylward E, Bernier R, et al. Language and aggressive behaviors in male and female youth with autism spectrum disorder. J Autism Dev Disord. 2022;52:454–62.
Payne TW, Lynn R. Sex differences in second language comprehension. Personality Individ Differ. 2011;50:434–6.
Peterson J. gender differences in verbal performance: a meta-analysis of United States state performance assessments. Educ Psychol Rev. 2018;30:1269–81.
Rea HM, Øien RA, Shic F, Webb SJ, Ratto AB. Sex differences on the ADOS-2. J Autism Dev Disord. 2023;53:2878–90.
Simpson EA, Nicolini Y, Shetler M, Suomi SJ, Ferrari PF, Paukner A. Experience-independent sex differences in newborn macaques: Females are more social than males. Sci Rep. 2016;6:19669.
Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, second edition (ADOS-2) manual (part I): modules 1–4. Torrance, CA: Western Psychological Services, 2012.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: Author; 2000.
Elliott CD. Differential ability scales. 2nd ed. San Antonio, TX: The Psychological Corporation; 2007.
Constantino JN. Social Responsiveness Scale, second edition (SRS-2). Torrance, CA: Western Psychological Services, 2012.
Rutter ML, Bailey A, Lord C. Social Communication Questionnaire. Torrance, CA. Western Psychological Services, 2003.
Sparrow S, Cicchetti D, Balla D. Vineland adaptive behavior scales, second edition (Vineland-II). Circle Pines, MN: American Guidance Service; 2005.
Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals, 4th edition (CELF-4). Toronto: The Psychological Corporation/A Harcourt Assessment Company; 2003.
Arnett AB, Hudac CM, DesChamps TD, Cairney BM, Gerdts J, Wallace AS, et al. Auditory perception is associated with implicit language learning and receptive language ability in autism spectrum disorder. Brain Lang. 2018;187:1–8.
Liu J, Tsang T, Ponting C, Jackson L, Jeste SS, Bookheimer SY, Dapretto M. Lack of neural evidence for implicit language learning in 9-month-old infants at high risk for developing autism. Dev Sci. 2021;24: e13078.
McNealy K, Mazziotta JC, Dapretto M. Age and experience shape developmental changes in the neural basis of language-related learning. Dev Sci. 2011;14:1261–82.
McNealy K, Mazziotta JC, Dapretto M. The neural basis of speech parsing in children and adults. Dev Sci. 2010;13:385–406.
Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-months-old infants. Science. 1996;274:1926–8.
Scott-Van Zeeland AA, McNealy K, Wang AT, Sigman M, Bookheimer SY, Dapretto M. No neural evidence of statistical learning during exposure to artificial languages in children with autism spectrum disorders. Biol Psychiat. 2010;68:345–51.
Levin AR, Méndez Leal AS, Gabard-Durnam LJ, O’Leary HM. BEAPP: The Batch Electroencephalography Automated Processing Platform. Front Neurosci. 2018;12:513.
Gabard-Durnam LJ, Méndez Leal AS, Wilkinson CL, Levin AR. The Harvard automated processing pipeline for electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front Neurosci. 2018;12:97.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, 2019. URL. https://www.R-project.org/
Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
Fox J. Structural equation modeling with the sem package in R. Struct Equ Model. 2006;13:465–86.
Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48:1–36.
Wickham H. ggplot 2: elegant graphics for data analysis. New York: Springer-Verlag; 2016.
Epskamp S. semPlot: unified visualizations of structural equation models. Struct Equ Model. 2015;22:474–83.
Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng. 2007;9:90–5.
Aoyagi M, Kiren T, Furuse H, Fuse T, Fuse T, Suzuki Y, et al. Effects of aging on amplitude-modulation following response. Acta Otolaryngol. 1994;114(sup511):15–22.
Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen C-MA., Lewis DA. Development of Sensory Gamma Oscillations and Cross-Frequency Coupling from Childhood to Early Adulthood. Cerebral Cortex 2015; 25: 1509–1518.
Edgar JC, Fisk CL 4th, Liu S, Pandey J, Herrington JD, Schultz RT, Roberts TPL. Translating adult electrophysiology findings to younger patient populations: difficulty measuring 40-Hz auditory steady-state response in typically developing children and children with autism spectrum disorder. Dev Neurosci. 2016;38:1–14.
Neuhaus E, Lowry SJ, Santhosh M, Kresse A, Edwards LA, Keller J, et al. Resting state EEG in youth with ASD: age, sex, and relation to phenotype. J Neurodev Disord. 2021;13:33.
Rojas DC, Maharajh K, Teale PD, Kleman MR, Benkers TL, Carlson JP, Reite ML. Development of the 40 Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin Neurophysiol. 2006;117:110–7.
Seymour RA, Rippon G, Gooding-Williams G, Sowman PF, Kessler K. Reduced auditory steady state responses in autism spectrum disorder. Molecular Autism. 2020;11:56.
Kayarian FB, Jannati A, Rotenberg A, Santarnecchi E. Targeting gamma-related pathophysiology in autism spectrum disorder using transcranial electrical stimulation: opportunities and challenges. Autism Res. 2020;13:1051–71.
Korisky A, Gordon I, Goldstein A. Oxytocin impact top-down and bottom-up social perception in adolescents with ASD: a MEG study of neural connectivity. Molecular Autism. 2022;13:36.
Safar K, Yuk V, Wong SM, Leung RC, Anagnostou E, Taylor MJ. Emotional face processing in autism spectrum disorder: effects in gamma connectivity. Biol Psychol. 2020;149: 107774.
Gabard-Durnam LJ, Wilkinson C, Kapur K, Tager-Flusberg H, Levin AR, Nelson CA. Longitudinal EEG power in the first postnatal year differentiates autism outcome. Nature Communication. 2019;10:4188.
Snijders TM, Milivojevic B, Kemner C. Atypical excitation-inhibition balance in autism captured by the gamma response to contextual modulation. NeuroImage Clin 2013; 3: 65–72.
Zikopoulos B, Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci. 2013;7:609.
Besag FMC. Epilepsy in patients with autism: links, risks, and treatment challenges. Neuropsychiatr Dis Treat. 2018;14:1–10.
Gonçalves J, Violante IR, Sereno J, Leitão RA, Cai Y, Abrunhosa A, et al. Testing the excitation/inhibition imbalance hypothesis in a mouse model of the autism spectrum disorder: in vivo neurospectroscopy and molecular evidence for regional phenotypes. Molecular Autism. 2017;8:47.
Lee E, Lee J, Kim E. excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiat. 2017;81:838–47.
Vaccarino FM, Grigorenko EL, Smith KM, Stevens HE. Regulation of cerebral cortical size and neuron number by fibroblast growth factors: implications for autism. J Autism Dev Disord. 2009;39:511–20.
Giraud A-L, Kleinschmidt A, Poeppel D, Lund TE, Frackwiak RSJ, Laufs H. Endogenous cortical rhythms determine cerebral specialization for speech perception and production. Neuron. 2007;56:1127–34.
Moon J, Orlandi S, Chau T. A comparison and classification of oscillatory characteristics in speech perception and covert speech. Brain Res. 2022;1781: 147778.
Poeppel D, Assaneo MF. Speech rhythms and their neural foundations. Nat Rev Neurosci. 2020;21:322–34.
Hämäläinen JA, Rupp A, Soltész F, Szücs D, Goswami U. Reduced phase locking to slow amplitude modulation in adults with dyslexia: An MEG study. Neuroimage. 2012;59:2952–61.
Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as ‘asymmetric sampling in time.’ Speech Commun. 2003;41:245–55.
Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–39.
Ben-Ari Y. The GABA excitatory/inhibitory sequence: a personal journey. Neuroscience. 2014;279:187–219.
Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–9.
Orekhova EV, Sysoeva OV, Schneiderman JF, Lundström S, Galuta IA, Goiaeva DE, et al. Input-dependent modulation of MEG gamma oscillations reflects gain control in the visual cortex. Sci Rep. 2018;8:8451.
Murty DVPS, Manikandan K, Kumar WS, Ramesh RG, Purokayastha S, Javali M, et al. Gamma oscillations weaken with age in healthy elderly in human EEG. Neuroimage. 2020;215: 116826.
Chien Y-L, Chen Y-J, Tseng W-L, Hsu Y-C, Wu C-S, Tseng W-YI, Gai SS-F. Differences in white matter segments in autistic males, non-autistic siblings, and non-autistic participants: An intermediate phenotype approach. Autism 2023; 27: 1036–1052.
Shephard E, Milosavljevic B, Mason L, Elsabbagh M, Tye C, Gliga T, Jones EJH, et al. Neural and behavioural indices of face processing in siblings of children with autism spectrum disorder (ASD): a longitudinal study from infancy to mid-childhood. Cortex. 2020;127:162–79.
Gerdts J, Bernier R. The broader autism phenotype and its implications on the etiology and treatment of autism spectrum disorders. Autism Res Treat. 2011;2011: 545901.
Acknowledgements
We wish to thank the families, parents, and children who participated in our study at our four data collection sites. The ACE GENDAAR Network additionally included contributions from: Katy Ankenman MSW, Elizabeth Aylward PhD, Veronica Kang PhD, Erin J. Libsack PhD, and Désirée Lussier-Lévesque PhD who were formerly at Seattle Children’s Research Institute; Sarah Corrigan MA and Waylon Howard PhD who are currently at Seattle Children’s Research Institute; Laura A. Edwards PhD and Jack Keller who were formerly at Boston Children’s Hospital; Rachael Tillman Ph.D who was formerly at Yale Child Study Center; Scott Huberty PhD who was formerly at UCLA; Zachary Jacokes who is currently at University of Virginia; Carinna Torgerson who is currently at USC; and Charles Nelson who is currently at Boston Children’s Hospital and Harvard Medical School.
Funding
Funding was provided by the R01MH10028 (NIMH ACE Network, Pelphrey), R01MH117982 (Dapretto/Pelphrey), and the University of Washington Intellectual and Developmental Disabilities Research Center (U54HD083091).
Author information
Authors and Affiliations
Contributions
VA, MS, EN, HB, CT, RAB, SYB, MD, ARG, AJ, SJ, JCM, AN, JDVH, KAP, and SJW participated in project conceptualization and writing, including editing and final approval. MS, HB, SJ, AN, and SJW were involved in EEG data acquisition. VA, MS, CT, HB, and SJW completed the analysis. VA and SJW drafted the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Yale Institutional Review Board, the UCLA Office of Human Research Protection Program, Boston Children’s Hospital Institutional Review Board, USC Office for the Protection of Research Subjects, and the University of Virginia Institutional Review Board for Health Sciences Research. All procedures performed were in accordance with the Declaration of Helsinki. All minor children provided verbal assent to participate in the study and were informed that they can withdraw from the study at any time during the experiment. A written consent form was obtained from a parent of each child participating in the study.
Consent for publication
Not applicable.
Availability of data and materials
The behavioral and EEG data from the current study are available via the National Institute of Mental Health Data Archive Data Collection #2021.
Competing interests
James C. McPartland consults with Customer Value Partners, Bridgebio, Determined Health, and BlackThorn Therapeutics, has received research funding from Janssen Research and Development, serves on the Scientific Advisory Boards of Pastorus and Modern Clinics, and receives royalties from Guilford Press, Lambert, Oxford, and Springer. The remaining authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arutiunian, V., Santhosh, M., Neuhaus, E. et al. The relationship between gamma-band neural oscillations and language skills in youth with Autism Spectrum Disorder and their first-degree relatives. Molecular Autism 15, 19 (2024). https://doi.org/10.1186/s13229-024-00598-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13229-024-00598-1