Abstract
Purpose
Determine whether variable extrathoracic airflow limitation (VEAL) is observed in patients with negative methacholine challenge tests (MCT).
Methods
Electronic medical records of patients undergoing MCT at Jesse Brown VA Medical Center between January 2017 and December 2019 were reviewed. Only patients with negative MCT were selected. Pertinent demographic, clinical, and pulmonary function tests (PFT) and MCT data were abstracted from each record. Spirometric flow-volume loops recorded during each test were inspected by one co-author to determine the first inhaled methacholine concentration at which FEF50/FIF50 was either > 1 or further increased if baseline FEF50/FIF50 after nebulized saline (vehicle) already exceeded 1. Student’s t-test was used for statistical analysis. P < 0.05 was considered statistically significant.
Results
One hundred and twenty-seven consecutive patients with normal baseline PFT and negative MCT were identified. Thirteen patients (10.2%) had negative MCT and FEF50/FIF50 > 1 after testing. They were predominately obese (BMI, 31.3 ± 6.6), non-smoking (10), White (8) males (9) aged 51.3 ± 14.1 years (mean ± SD) referred for symptoms suggestive of asthma (n = 7) or for chronic cough (n = 6). Five had obstructive sleep apnea, three gastroesophageal reflux disease, and two chronic rhinosinusitis. FEF50/FIF50 increased significantly from 0.72 ± 0.21 after nebulized saline (vehicle) to 1.21 ± 0.13 after inhaled methacholine (p < 0.001). Median inhaled methacholine concentration eliciting these responses was 1.0 mg/mL (range, 0.25–16 mg/mL).
Conclusions
VEAL is observed in a subset of patients with a negative MCT. This phenomenon should be recognized and reported to the referring healthcare providers and its clinical significance addressed as indicated.
Similar content being viewed by others
Introduction
Methacholine challenge testing (MCT) is a form of bronchoprovocation testing, which uses the acetylcholine derivative methacholine to induce bronchoconstriction. In this test, methacholine is administered via nebulization in increasing concentrations ranging from 0.016 to 16 mg/mL, in two to four-fold dilutions. Forced expiratory volume in 1 s (FEV1) is measured after each successive dose and the test is stopped and considered positive when the FEV1 drops by more than 20% from baseline—considered the provocation dose (PC20). A negative MCT, is defined by a no response to the highest concentration of methacholine administered [1]. Current guidelines on performance of methacholine challenge test (MCT) in adults are limited to the expiratory portion of the flow-volume curve recorded during spirometry [1, 2]. However, previous studies have shown that inhaled methacholine could concomitantly affect the inspiratory portion of the flow-volume curve suggesting the presence of variable extrathoracic airflow limitation (VEAL) [3,4,5]. Whether this response is also observed and reported in patients with negative MCT is uncertain.
Conceivably, isolated inspiratory flow limitation as assessed by maximum expiratory to inspiratory flows at 50% of forced vital capacity ratio (FEF50/FIF50) observed during a negative MCT could guide healthcare providers to consider alternative upper airway disorders associated with laryngeal hyperresponsiveness. These conditions, such as obstructive sleep apnea, reflux disease, inducible laryngeal obstruction and chronic rhinosinusitis, could then be treated accordingly [6,7,8].
Therefore, the purpose of this study was to begin to address this issue by determining whether VEAL is observed and reported in patients with negative MCT at our facility.
Methods
The electronic health records (EHR) of patients undergoing MCT at Jesse Brown VA Medical Center (JBVAMC), Chicago, Illinois, between January 2017 and December 2019 were reviewed. Only patients with negative MCT according to the American Thoracic Society guidelines were selected [1].
Pertinent demographic, clinical, pulmonary function tests (PFT) and MCT data were abstracted from each record. All PFT and MCT data were reviewed by one co-author (ZZE). Spirometric flow-volume loops recorded during each test were inspected to determine the first inhaled methacholine concentration at which FEF50/FIF50 was either > 1 or further increased if baseline FEF50/FIF50 after nebulized saline (vehicle) already exceeded 1.
Data and statistical analyses
Data are reported as means and standard deviation where appropriate. Student’s t-test was used for statistical analysis. P < 0.05 was considered statistically significant.
Results
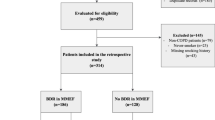
A total of 139 MCTs were performed during the 3-year study period of which 127 were negative. Thirteen patients (10.2%) with negative MCTs had FEF50/FIF50 > 100% post-MCT (Table 1). In twelve patients, FEF50/FIF50 exceeded 1 after testing while in one patient with baseline FEF50/FIF50 > 1 it further increased after testing (Fig. 1). These findings were not noted in the report sent to the referring healthcare providers.
Patients were predominately obese (BMI, 31.3 ± 6.6), non-smoking (n = 10), White (n = 8) males (n = 9) aged 51.3 ± 14.1 years who were referred for evaluation of symptoms suggestive of asthma (n = 7) or for chronic cough (n = 6). Five had physician-diagnosed obstructive sleep apnea (OSA), three gastroesophageal reflux disease, and two chronic rhinosinusitis (Tables 1 and 2). In three patients with a negative MCT, a presumptive diagnosis that may underlie VEAL was not established.
Mean FEF50/FIF50 increased significantly from 0.72 ± 0.21 after nebulized saline (vehicle) to 1.21 ± 0.13 after inhaled methacholine (Fig. 1). The median inhaled methacholine concentration eliciting this response was 1.0 mg/mL (range, 0.25–16 mg/mL) (Table 2). Using Kelso et al. definition [3], we found that 11 out of 13 patients had a clinically significant decrease in the FIF50 > 20% after MCT.
Discussion
The new finding of this study is that VEAL observed in a small proportion of patients with negative MCT at our facility was not interpreted nor reported to the referring healthcare providers. Conceivably, these spirometric data could indicate the presence of alternative disorders associated with laryngeal hyperresponsiveness that should then be pursued and treated accordingly [6,7,8,9,10,11]. Hence, we propose that FEF50/FIF50 recorded during MCT should be interpreted and reported to the referring healthcare providers and its clinical implications addressed as indicated.
Kelso et al. [3] showed that in fourteen of seventy-six consecutive patients with negative MCT (18%) FIF50 decreased by 20 to 35% from baseline suggesting the presence of VEAL. However, baseline and post-MCT FEF50/FIF50 data were not reported. Moreover, criteria for a positive inspiratory challenge during MCT of ≥ 20% fall in FIF50 from baseline chosen by these authors have not been published so far [1, 2].
To the best of our knowledge, VEAL reported in our patients with OSA and a negative MCT has not been previously described in the literature. To that end, Lin et al. [12] found positive MCT in four of sixteen patients with OSA but did not report FEF50/FIF50 data in those with negative MCT. Whether patients with OSA and VEAL observed during negative MCTs represent a distinct phenotype of upper airway dysfunction in this disorder remains to be determined. To that end, obesity, a distinct feature in patients with OSA, is associated with tidal flow limitation due to reduced functional residual capacity and expiratory reserve volume [13]. Conceivably, this phenomenon could result in higher nebulized methacholine dose delivered to the upper airway of obese patients leading to local, non-selective muscarinic receptor activation and VEAL. Further studies are warranted to support or refute this hypothesis.
Several limitations of this study are notable. It was a small, retrospective, single site study comprised predominantly of white obese males. Hence, generalizability of our observations is limited. Therefore, we propose that a larger, prospective, multi-site study should be conducted to determine the prevalence of VEAL in patients with negative MCT and to unravel upper airway disorders associated with this phenomenon.
In summary, we found that VEAL is observed in some patients with a negative MCT. This phenomenon should be interpreted and reported to the referring healthcare providers and its clinical implications addressed as indicated.
Availability of data and materials
The datasets during and/or analyzed during the current study and available from the corresponding author on reasonable request.
References
Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–29.
Coates AL, Wanger J, Cockcroft DW, Culver BH, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017;49:1601526.
Kelso JM, Enright PL, Scanlon PD, O’Connell EJ, Sachs MI. Effect of inhaled methacholine on inspiratory flow. Chest. 1990;98:1426–9.
Perkins PJ, Morris MJ. Vocal cord dysfunction induced by methacholine challenge testing. Chest. 2002;122:1988–93.
Guss J, Mirza N. Methacholine challenge testing in the diagnosis of paradoxical vocal fold motion. Laryngoscope. 2006;116:1558–61.
Miller RD, Hyatt RE. Evaluation of obstructing lesions of the trachea and larynx by flow-volume loops. Am Rev Respir Dis. 1973;108:475–81.
Das AK, Davanzo LD, Poiani GJ, Zazzali PG, Scardella AT, Warnock ML, et al. Variable extrathoracic airflow obstruction and chronic laryngotracheitis in Gulf War veterans. Chest. 1999;115:97–101.
Sterner JB, Morris MJ, Sill JM, Hayes JA. Inspiratory flow-volume curve evaluation for detecting upper airway disease. Respir Care. 2009;54:461–6.
Ryan NM, Vertigan AE, Gibson PG. Chronic cough and laryngeal dysfunction improve with specific treatment of cough and paradoxical vocal fold movement. Cough. 2009;5:4.
Good JT Jr, Rollins DR, Kolakowski CA, Stevens AD, Denson JL, Martin RJ. New insights in the diagnosis of chronic refractory cough. Respir Med. 2018;141:103–10.
Famokunwa B, Walsted ES, Hull JH. Assessing laryngeal function and hypersensitivity. Pulm Pharmacol Ther. 2019;56:108–15.
Lin CC, Lin CY. Obstructive sleep apnea syndrome and bronchial hyperreactivity. Lung. 1995;173:117–26.
Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol. 1985;1998(85):1236–43.
Acknowledgements
The authors thank Ms. Karen Turner, RRT, MBA, for technical assistance. This material is the result of work supported with resources and the use of facilities at the Jesse Brown VA Medical Center, Chicago, Illinois, USA. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Funding
None.
Author information
Authors and Affiliations
Contributions
IR, ZE and SZ: conceptualization, methodology, data curation, analysis, writing—original draft preparation, writing—reviewing and editing, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The JBVAMC Institutional Review Board determined that this study did not constitute human subjects research.
Consent for publication
The JBVAMC Institutional Review Board determined that this study did not constitute human subjects research and can be published.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elfessi, Z.Z., Zavala, S. & Rubinstein, I. Presence of variable extrathoracic airflow limitation in patients with a negative methacholine challenge test. Allergy Asthma Clin Immunol 19, 101 (2023). https://doi.org/10.1186/s13223-023-00860-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-023-00860-w