Abstract
Background
Reports of allergic reactions to the COVID-19 vaccines have been documented, which may also contribute to hesitancy. Despite the low likelihood that the COVID-19 vaccine will trigger an allergic reaction, we and others have reported that families with allergy remain vaccine hesitant due to concerns of COVID-19-vaccine-triggered anaphylaxis.
Objective
To present our scoping review protocol, that will inform a forthcoming living scoping review in which we will investigate the peer-reviewed and grey literature on COVID-19 vaccine hesitancy and allergic disease and/or allergic reactions following a COVID-19 vaccine.
Methods
Informed by Arksey and O’Malley framework for methodological review, we have developed a search strategy with content and methodological experts, and which has undergone Peer Review of Electronic Search Strategies review. A search of four scientific databases, as well as gray literature, will be performed without restriction to articles by type of COVID-19 vaccine, or country of study, and will include publications in the ten languages our team can handle. Bi-monthly search alerts based on the search strategy will be generated.
Results
The first search will result in a stand alone peer reviewed scoping review. Bi-monthly updates will be posted on a pre-print server. Depending on the volume of literature, these updates will be synthesized and submitted for peer-review at 6 and/or 12 months.
Conclusion
COVID-19 vaccine hesitancy amongst individuals with allergy persists, despite very low risk of serious adverse reactions. Our living scoping review, which includes multiple forms of knowledge translation, will be a rigorous way to address hesitancy.
Similar content being viewed by others
Background
The COVID-19 pandemic has impacted families worldwide. In the early weeks of the pandemic, we reported elevated rates of maternal anxiety and depression relative to population norms [1]. Yet, anxiety was even more pronounced amongst mothers with a child with food allergy [2] as well as those who consumed more COVID-19-related news [3]. Indeed, the sheer volume and, the misinformation about COVID-19, has contributed to the World Health Organization’s description of an “infodemic” [4]. Unfortunately, research suggests that the propagation and consumption of misinformation online is associated with greater vaccine hesitancy [5], which has been described as “the next challenge in the fight against COVID-19” [6]. Moreover, reports of allergic reactions to the COVID-19 vaccines have been documented [7], which may also contribute to hesitancy [8]. When reports of anaphylactic reactions triggered by the mRNA COVID-19 vaccine were first reported by patients, it was suspected, but not confirmed, that one of the triggers for these reactions was polyethylene glycol, or PEG [7, 9]. Polyethylene glycols consist of several polymers which are present in many medical and industrial products, including pharmaceuticals, cosmetics, and food products [10]. It should be noted, however, that PEG compounds are not uniform as they differ in molecular weight across products. PEG compounds can also differ in their structure as many drugs and medical devices contain PEG-derivatives like polysorbates, poloxamers, and PEG castor oils [10]. Although PEG is nearly ubiquitous in personal hygiene products and medications, pre-COVID reports of PEG-triggered anaphylaxis are rare, at 37 cases globally between 1977 and 2016 [10]. As more evidence is accumulated, the role of PEG is minor, if any, in the development of allergic reactions to vaccines [11, 12]. If PEG allergy remains a concern, despite the rarity of reactions, viral vector COVID-19 vaccines that do not contain PEG are available, such as the Johnson & Johnson vaccine and the AstraZeneca vaccine [13, 14].
Despite the low likelihood that the COVID-19 vaccine will trigger an allergic reaction [15,16,17], we [18] and others [19, 20] have reported that families with allergy remain vaccine hesitant due to concerns of COVID-19-vaccine-triggered anaphylaxis. A meta-analysis published in January 2022, of highly heterogeneous studies on the association between anaphylaxis and COVID-19 vaccines provides very modest evidence of a slight increase in odds of patient-reported anaphylactic reactions post-administration of an authorized vaccine amongst those aged 18–85 years, and particularly amongst females [21]. Notably, mRNA vaccines were associated with the lowest risk of anaphylaxis. The reported increase of anaphylaxis amongst women was attributed to hormonal regulation [21], an observation that may align with sex-specific reactions to other vaccines described elsewhere [22]. Whereas any type of vaccination carries risk, the authors of this recent meta-analysis failed to contextualize the risk of anaphylaxis associated with the COVID-19 vaccine, or the minimal increased risk of anaphylaxis in relation to the risk of COVID-19 complications. Of equal concern is the role of gender. Women, compared to men, make between 80 and 90% of healthcare decisions for their families, and are more likely to act as caregivers [23,24,25]. This caregiver role has been described amongst families managing a child’s food allergy, with 14% of mothers but no fathers reporting career limitations due to food allergy [26]. The increase in risk of anaphylaxis among women unequivocally affects the way mothers provide care and management to their children’s allergies. These observations, coupled with the COVID-19 infodemic, has underscored the need for a comprehensive, regularly updated review of literature surrounding COVID-19 vaccine hesitancy amongst those managing allergy.
The synthesis of individual research studies and interpreting them in a global context is an essential component of knowledge creation, and in turn, will contribute to knowledge translation [27]. To this end, the first objective of this scoping review is to investigate the peer-reviewed and grey literature on COVID-19 vaccine hesitancy and allergic disease and/or allergic reactions following a COVID-19 vaccine, that will be regularly maintained and updated as new evidence is published. The second objective is to inform knowledge translation activities resulting from this scoping review.
Methods and analysis
For the aims of the proposed project, a scoping review is preferable to a systematic review as the former is useful when examining emerging evidence when there is a lack of clarity as to what more specific questions may be valuably addressed in a systematic review [28], and in which we have documented expertise [29,30,31,32].
Inclusion and exclusion criteria
We will not restrict articles by type of COVID-19 vaccine or by country of study. Grey literature searches will be restricted to those published languages spoken by the research team (Table 1).
Concept
In keeping with the Arksey and O’Malley framework for methodological reviews [33], searches and screening will be independently but concurrently performed by trained staff (initials blinded for review). Articles will be included based on consensus. In the event of conflicts, the study lead (initials blinded for review) will guide a decision.
Information sources
Four scientific databases (CINAHL, PsycINFO, Medline, Embase) will be searched, but without restrictions to patient age or language of publication. Due to the nature of the topic, the literature will be exclusively from 2020 onward.
Grey literature sites will include, but are not limited to websites of the Canadian Society of Allergy and Clinical Immunology, American Academy of Allergy, Asthma and Immunology, European Academy of Allergy and Clinical Immunology, and World Health Organization.
Initial search strategy
The original search will be guided by (initials blinded for review), approved by the study lead (blinded for review), then peer-reviewed by a librarian colleague, per Peer Review of Electronic Search Strategies guidelines [34]. Sample scoping review search terms are presented in Table 2.
Screening
After the initial search, all citations will be uploaded into Rayyan [35]. Trained student research assistants will de-duplicate the search. Thereafter, they will independently perform title and abstract screening, noting which studies are to be excluded or included from the full text screening. This process will be blinded, such that each screener does not have access to whether the other screener has decided to include or exclude an article based on the title/abstract.
After the title and abstract screening is complete, full texts will be uploaded to Rayyan [35]. Full text screening will follow the same process as for the title and abstract screening described above. Decisions whether to exclude (and the reason for exclusion) or include the full text in the review will be based on the inclusion criteria. Once screeners have independently screened the full texts, the results will be unblinded. Screeners will meet to discuss any conflicts, and in the event they cannot agree whether an article should be included, they will defer to the study lead or her designate.
For articles that are published in languages other than English, the decision to exclude or include an article will be at the discretion of the team member(s) who comprehends that language. Screeners will direct the article(s) to team members who read the particular language.
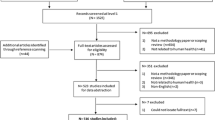
For all included articles, data will be extracted into tables. The tables, at a minimum, will include the study’s title, authors, year of publication, country of the participants, aims, sample size, study methodology, outcome measures, and key findings. At the point of extraction, the student research assistants will be asked to extract all information that may be relevant to the review. A flow chart of the search process will also be included in the initial scoping review, per the structure provided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews [36].
Bi-monthly update
A bi-monthly search alert based on the search strategy will be generated by health sciences librarian (initials blinded for review). The student research assistants assigned to this project will be tasked with screening these articles and assessing if they should be excluded or included. Conflicts between the student research assistants regarding the inclusion of particular articles will be solved through discussion. If the student research assistants cannot agree, the study lead or her designate will be asked to make the final decision.
Patient engagement
Patient partners will be consulted at all stages of this study, from design to interpretation of the results. Our patient partners are also committed to sharing the findings of this review within their patient partner network. Additionally, the results of this review will be shared on social media platforms, including our lab website; and will be used to inform future webinars hosted by our research group.
Ethics
As this is a scoping review of existing literature, no ethical approval is required. However, the overarching study has been approved by the University of Manitoba Health Research Ethics Board.
Knowledge translation
Following preparation of the manuscript, we will post it on a pre-print server, and submit for publication. Per conditions of funding of COVID grants, this—and all resulting publications from this project—must be open access. These updates will follow the same process as described above. Bi-monthly updates will be posted on a pre-print server. Depending on the volume of literature, these updates will be synthesized and submitted for peer-review at 6 and/or 12 months.
Plain language summaries, in the form of infographics, will be posted on the study lead’s website (URL blinded for peer review) and shared widely on social media.
Availability of data and materials
Not applicable.
Abbreviations
- PEG:
-
Polyethylene glycol
References
Cameron EC, Joyce K, Delaquis C, Reynolds K, Protudjer JLP, Roos L. Maternal psychological distress & mental health services use during the COVID-19 pandemic. J Affect Disord. 2020;276:765–74.
Protudjer JLP, Golding MA, Salisbury MR, Abrams EM, Roos LE. High anxiety and health-related quality-of-life in families with children with food allergy during COVID-19. Ann Allergy Asthma Immunol. 2021;126(1):83–8.
Golding M, Salisbury MR, Reynolds K, Roos LE, Protudjer JL. COVID-19-related media consumption and parental mental health. Can J Behav Sci. 2021. https://doi.org/10.1037/cbs0000280.
World Health Organization. Managing the COVID-19 infodemic: Promoting healthy behaviours and mitigating the harm from misinformation and disinformation. https://www.who.int Accessed 23 Sep 2020
Puri N, Coomes EA, Haghbayan H, Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Hum Vacc Immunotherap. 2020;11:1–8.
Dror AA, Eisnenbach N, Taiber S, Morozov NG, Zigron A, Srouji S, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Int J Environ Res Public Health. 2020;17(16):5893.
Pitlick MM, Sitek AN, Kinate SA, Joshi AY, Park MA. Polyethylene glycol and polysorbate skin testing in the evaluation of COVID-19 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021. https://doi.org/10.1016/j.anai.2021.03.012.
Carrieri V, Madio L, Principe F. Vaccine hesitancy and (fake) news: quasi-experimental evidence from Italy. Health Econ. 2019;28(11):1377–82.
Cabanillas B, Akdis C, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2020. https://doi.org/10.1111/all/14711.
Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–22.
Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126(3):e106–8. https://doi.org/10.1016/j.bja.2020.12.020.
Abrams EM, Greenhawt M, Shaker M, Kosowan L, Singer AG. Primary care provider-reported prevalence of vaccine and polyethylene glycol allergy in Canada. Ann Allergy Asthma Immunol. 2021;127(4):446-450.e1. https://doi.org/10.1016/j.anai.2021.05.011.
AstraZeneca Canada. Product monograph: VaxzevriaTM COVID-19 Vaccine (ChAdOx1-S [recombinant]). https://covid-vaccine.canada.ca/info/pdf/astrazeneca-covid-19-vaccine-pm-en.pdf Accessed 19 Aug 2022
Janssen Inc. Product monograph: JCOVDENTM COVID-19 Vaccine (Ad26.COV2-S [recombinant]). https://covid-vaccine.canada.ca/info/pdf/janssen-covid-19-vaccine-pm-en.pdf Accessed 19 Aug 2022
Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approaches. J Allergy Clin Immunol Pract. 2020;S2213–98(20):31411–2.
Canadian Society of Allergy and Clinical Immunology. COVID-19 resources 2021. https://www.csaci.ca/covid19-resources/. Accessed 2 Dec 2021.
CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR. 2021;70(2):46–51.
Gooding G, Gabrielli S, Protudjer JLP, Ben-Shoshan M. COVERS: COVID vaccine evaluation of resources and solutions (under review).
Turner PJ, Larson H, Dubé È, Fisher A. Vaccine hesitancy: drivers and how the allergy community can help. J Allergy Clin Immunol Pract. 2021;S2213–98(21):763–7.
Erdeljic TV. Anaphylaxis associated with the mRNA COVID-19 vaccines: approach to allergy investigation. Clin Immunol. 2021;227: 108748.
Sobczak M, Pawliczak R. The risk of anaphylaxis behind authorized COVID-19 vaccines: a meta-analysis. Clin Mol Allergy. 2022;20:1.
Tramontana F, Battisti S, Napoli N, Strollo R. Immuno-endocrinology of COVID-19: the key role of sex hormones. Front Endocrinol. 2021. https://doi.org/10.3389/fendo.2021.726696.
U.S. Department of Labor. General facts on women and job based health. Washington, DC: U.S. Department of Labor, 2005. www.dol.gov/ebsa/newsroom/fshlth5.html
Zimmerman MK, Hill SA. Health care as a gendered system. In: Saltzman-Chafetz J, editor. Handbook of the sociology of gender. New York: Plenum Publishers; 1999. p. 483–518.
Grant KR, Amaratunga C, Armstrong P, et al editors. Caring for/caring about: women, homecare, and unpaid caregiving. Aurora: Garamond Press Ltd.; 2004.
Frykas TLM, Golding M, Abrams EM, Simons E, Protudjer JLP. Mothers of children with food allergies report poorer perceived life status which may be explained by limited career choices. Allergy Asthma Clin Immunol. 2021;17(1):12. https://doi.org/10.1186/s13223-021-00515-8.
Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell C, Robinson N. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24. https://doi.org/10.1002/chp.47.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Method. 2018. https://doi.org/10.1186/s12874-018-0611-x.
Golding MA, Gunnarsson NV, Middelveld R, Ahlstedt S, Protudjer JLP. A scoping review of the caregiver burden of pediatric food allergy. Ann Allergy Asthma Immunol. 2021;S1081–1206(21):00342–52.
Hildebrand HV, Arias A, Simons E, Gerdts J, Povolo B, Rothney J, Protudjer JLP. Adult and pediatric food allergy to chickpea, pea, lentil, and lupine: a scoping review. J Allergy Clin Immunol Pract. 2021;9(1):290-301.e2.
Protudjer JLP, Mikkelsen A. Veganism and paediatric food allergy: two increasingly prevalence dietary issues that are challenging when co-occurring. BMC Pediatr. 2020;20(1):341.
Merrill KA, William TNN, Joyce KM, Roos LE, Protudjer JLP. Potential psychosocial impact of COVID-19 on children a scoping review of pandemics and epidemics. J Global Health Rep. 2020. https://doi.org/10.29392/001c.18229.
Arkey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Social Res Method. 2005;8(1):19–32.
McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L, Hempel S, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850.
Acknowledgements
Not applicable
Funding
Funding for this project was provided by the Canadian Institutes of Health Research, COVID-19 Vaccine Confidence—Operating Grant (Nominated Principal Applicant J Protudjer). The funder played no role in the design of the study and collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Contributions
MAG contributed to the development of the search terms, and co-wrote the first draft of the manuscript. NA developed the search strategy. ALRB and KAM are leading the search, and approved the manuscript. EMA, PB, MB-S, EL, LER, VP contributed to the study design and approved the manuscript. JLPP contributed to the development of the search terms, and co-wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable; this is a scoping review protocol and thus does not require ethical approval.
Consent for publication
Not applicable.
Competing interests
MA declares no competing interests. NA declares no competing interests. AB declares no competing interests. KM declares no competing interests. EA is a member of Food Allergy Canada’s Medical Advisory Board, and does research with DBV Technologies. PB reports personal fees from Novartis, Pfizer, Sanofi, DBV Technologies, ALK, and Aralez outside the submitted work and research support from Novartis, Regeneron, and Sanofi outside the submitted work. MB-S a Member of the Board, Canadian Society of Allergy and Clinical Immunology, and on the steering committee for Canada’s National Food Allergy Action Plan. EL is the co-founder and director of Science for All Audiences (SciFAA). LR declares no competing interests. VP declares no competing interests. JP is Section Head, Allied Health and Member of the Board, Canadian Society of Allergy and Clinical Immunology; sits on the steering committee for Canada’s National Food Allergy Action Plan; reports research support from CIHR; Canadian Allergy, Asthma Immunology Foundation; Research Manitoba; Children’s Hospital Research Institute of Manitoba; Manitoba Medical Services Foundation; George and Fay Yee Centre for Healthcare Innovation; and, the University of Manitoba outside the submitted work; and, personal fees from Novartis outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Golding, M.A., Askin, N., Batac, A.L.R. et al. vACcine COnfidence amongst those living with alleRgy during the COVID pandemic (ACCORD): a scoping review protocol. Allergy Asthma Clin Immunol 18, 83 (2022). https://doi.org/10.1186/s13223-022-00723-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-022-00723-w