Abstract
Background
Allergen immunotherapy (AIT) is the only treatment that has modified the natural history of allergic diseases. However, since its overall effect on the immune system has not been elucidated, AIT is either absolutely or relatively contraindicated in patients with rheumatic autoimmune diseases (RADs). Therefore, there have been no long-term observations of patients with RADs receiving AIT; thus, the effectiveness and safety of AIT in these patients remain unclear.
Methods
This was a single-center retrospective observational study. RAD patients receiving AIT for allergic rhinitis at our institution were selected. Changes in the activity of RAD patients were investigated for 2 years from baseline, including those who discontinued AIT. The effectiveness of AIT was also investigated using the Japan Allergic Rhinitis Standard Quality of Life Questionnaire.
Results
Thirteen patients with RADs were enrolled in the study. All patients received sublingual immunotherapy, of which four discontinued AIT owing to adverse events. Among all patients, the symptoms of RADs in three patients worsened during the observation period; however, none of them were causally related to AIT. Most of the adverse events associated with AIT were mild, in which only one patient required drug intervention due to worsening rhinitis symptoms. In the nine patients who were able to continue AIT, their eye and nasal symptom scores showed a significant improvement from 1.67 (1.5–2.0) at baseline to 0.67 (0–1.17) in the 2nd year of treatment (p = 0.0141).
Conclusions
AIT is a safe and effective treatment modality for patients with allergic rhinitis complicated by RADs.
Similar content being viewed by others
Introduction
Allergic rhinitis is a highly common allergic disease characterized by IgE-mediated inflammation of the upper respiratory tract [1]. In Japan, allergic rhinitis is described as a national malady. In particular, cedar pollinosis affects more than one-third of the population [2]. Allergic rhinitis not only impairs the quality of life of patients due to symptoms such as rhinorrhea, nasal obstruction, sneezing, and itching, but also causes a significant socio-economic loss due to decreased labor productivity caused by the presenteeism brought about by these symptoms [1, 3]. Therefore, overcoming allergic rhinitis has extremely important public health implications.
Currently, allergic rhinitis is mainly treated with antihistamines, leukotriene receptor antagonists, and intranasal corticosteroids [1]. Advances in these therapeutic agents have greatly improved the clinical outcome of allergic rhinitis. However, these medications are symptomatic and do not radically cure allergic diseases. Meanwhile, allergen immunotherapy (AIT), which aims to develop tolerance through the continuous administration of small amounts of allergens, is the only causative treatment that modifies the natural history of allergic diseases and can be expected to lead to a complete cure [4]. In Japan, sublingual immunotherapy for allergic rhinitis caused by cedar pollen and mites has been widely conducted [5]. However, in many guidelines, AIT was considered as an absolute or relative contraindication for patients with allergic rhinitis concomitant with rheumatic autoimmune diseases (RADs) [6, 7]. Furthermore, there are no studies that have comprehensively evaluated the impact of the biological process of AIT on the pathogenesis of RADs; there is no solid evidence that AIT does not aggravate RADs [8]. However, there are several case reports on the development of RADs after AIT, although the possibility is empirically estimated to be low [8]. On the other hand, in long-term observational studies comparing AIT and conventional anti-allergy therapy for allergic patients over 10 years, there was no significant difference in the incidence of RADs between the AIT and conventional therapy groups [9, 10]. In addition, AIT has been reported to be effective and safe in patients with allergic rhinitis complicated by acquired immune deficiency syndrome, which also causes immune abnormalities [11]. Based on these findings, AIT has gradually been extended to patients with allergic rhinitis concomitant with RADs. In fact, in an electronic survey of physicians affiliated with the European Academy of Allergy and Clinical Immunology, 29.4% answered that they had experience in administering AIT to patients with allergic diseases complicated by RADs [12].
However, it should be noted that the aforementioned safety data for AIT were estimated only in average allergic patients but not in those with RADs. Therefore, in allergic patients with RADs, the possibility that AIT negatively affects RADs cannot be excluded. Thus, it has not yet been shown whether AIT alters the activity of concomitant RADs or whether AIT has equivalent effectiveness in patients with RADs who are receiving immunosuppressive therapy. Therefore, this observational study aimed to investigate the effectiveness and safety of AIT in patients with allergic rhinitis complicated by RADs.
Methods
Study design
This was a single-center observational case series. We retrospectively examined the clinical data of patients with allergic rhinitis concomitant with RADs who received AIT and attended the Department of Rheumatology and Allergology at Kyoto Prefectural University of Medicine between December 2016 and December 2021. In all patients, these clinical data were examined for 2 years after the initiation of AIT, including those who discontinued AIT during treatment. The AIT protocol was performed in accordance with the manufacturers’ instructions.
Patients
Eligible patients were defined as those aged ≥ 20 years who voluntarily expressed their intention to receive AIT. The decision on the application of AIT was not made for this study; it was made by the attending physician who judged the necessity of AIT during regular medical practice. The diagnosis and treatment of allergic rhinitis with concomitant RADs as well as the monitoring of their symptoms were performed by a physician specializing in both rheumatology and allergy.
The diagnosis of allergic rhinitis was based on the following: rhinitis symptoms during the relevant allergen dispersal period, the presence of allergen-specific IgE antibodies, or a positive reaction to the skin prick test using standardized relevant allergen extracts (Torii Pharmaceutical, Tokyo).
Outcome measurements
The primary endpoint of this study was the proportion of patients who had worsened activity of concomitant RADs within 2 years post-AIT initiation. The clinical data used to evaluate the effect of AIT on RADs were collected at regular visits during usual medical practice.
The following indices were used to evaluate the activity of each RADs: Simplified Disease Activity Index (SDAI) for rheumatoid arthritis (RA) [13], Disease Activity Score 28-CRP (DAS28-CRP) for peripheral spondyloarthritis [14], Birmingham Vasculitis Activity Score (BVAS) for eosinophilic granulomatosis with polyangiitis (EGPA) [15], modified Rodnan total skin thickness score (m-Rodnan TSS) for systemic sclerosis (SSc) [16], serum creatine kinase (CK) level for polymyositis (PM), serum C-reactive protein (CRP) level for diffuse fasciitis, and EULAR Sjögren's syndrome disease activity index (ESSDAI) for Sjögren’s syndrome (SjS) [17].
The secondary endpoints were the effectiveness of AIT for rhinitis symptoms and the occurrence of adverse events, except for the exacerbation of RADs. Effectiveness was assessed based on the Japan Allergic Rhinitis Standard Quality of Life Questionnaire (JRQLQ No. 1) [18]. The JRQLQ was filled out by patients before the start of AIT, during the relevant allergen dispersal period in the second year of treatment for seasonal allergic rhinitis, and 2 years after the start of AIT for perennial allergic rhinitis.
The JRQLQ consists of 24 questions in eight domains: nasal and eye symptoms, usual activities, outdoor activities, social functioning, sleep problems, general physical and emotional functioning, and overall quality of life. The scores at baseline and at the second year of treatment were compared for the eight domains.
Statistical analysis
Categorical variables were described using absolute numbers and percentages, whereas quantitative variables were described using medians and interquartile ranges. The disease activity of RADs and JRQLQ scores before and after the start of AIT were compared using the Wilcoxon signed-rank test. For all statistical tests, the significance level was set at 0.05. All statistical analyses were performed using EZR version 1.53 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R [19].
Results
Patient demographics and baseline characteristics
Thirteen patients with RADs treated with AIT were enrolled in this study. Among them, 11 had cedar pollinosis and two had perennial allergic rhinitis caused by mites. The AIT type was sublingual immunotherapy (SLIT) in all cases. The background RADs were RA in eight cases, PM with SSc in one case, SjS in one case, EGPA in one case, peripheral spondyloarthritis in one case, and diffuse fasciitis in one case. Immunosuppressive therapy for RADs was administered in 12 patients. Glucocorticoids were used in three patients; the median dose regimen was 1(0.88–2.5) mg/day of prednisolone equivalent. As immunosuppressive agents or immunomodulators, methotrexate was used in four patients (30.7%); salazosulfapyridine, bucillamine, tacrolimus, and azathioprine were also used. Biologics were used in six patients (46.1%); all of whom had RA. The details of the data are described in Table 1. Nine of the 13 patients were able to continue AIT for more than 2 years. However, four patients (three RA and one SjS) were discontinued due to the occurrence of adverse events.
Clinical course of RADs
The baseline disease activities of all eight RA patients were under 11.0 on the SDAI score, thereby indicating remission or low disease activity. There was no significant worsening of the SDAI score or matrix metalloproteinase-3 (MMP-3) values compared to baseline at all timepoints within 2 years post-AIT initiation, including cases of AIT discontinuation (Fig. 1A, B). Only the CRP level worsened significantly from 0.035 (0.01–0.0625) at baseline to 0.075 (0.0175–0.095) within 18 months. However, both were within normal limits and the change was not clinically meaningful (Fig. 1C). However, two patients showed an elevation in the SDAI score during the observation period. In the first case, although the SDAI score deteriorated during AIT, there were no obvious inflammatory findings on blood tests or joint ultrasonography; the symptoms spontaneously improved without any intensified treatment. Therefore, it was concluded that the symptoms were caused by temporary environmental stress and thus did not change the activity of RA (Fig. 1A, arrow a). The second case was also judged to have deteriorated due to factors other than AIT; the patient had discontinued AIT 1 week post-initiation. Furthermore, the deterioration may have occurred immediately after extending the administration interval of the biological agent (Fig. 1A, arrow b).
Changes in rheumatoid arthritis-related parameters after the initiation of allergen immunotherapy (AIT). A simplified disease activity score (SDAI). B matrix metaroproteinase-3 (MMP-3). C c-reactive protein (CRP). For all parameters, comparisons were made between baseline and each of the following time points with the Wilcoxon signed rank test: 1 month, 2 months, 3 months, 6 months, 9 months, 12 months, 15 months, 18 months, 21 months, and 24 months. Each gray line corresponds to an individual value; the median and interquartile ranges are indicated by bold black lines. Two patients had worsening disease activity, which is indicated by the arrows
The other patients with RADs did not show any change in disease activity during the observation period, except for one patient with Sjogren's syndrome who experienced a temporary worsening of disease activity (lymphadenopathy). However, it was judged that there was no causal relationship between AIT and lymphadenopathy in this case because of the following reasons: AIT was discontinued a few days after the start of AIT, the timing of the appearance of lymphadenopathy was only 2 months later, and similar symptoms had appeared before the start of AIT. The course of these cases is shown in the Fig. 2.
Changes in activity assessment parameters of rheumatic autoimmune diseases other than rheumatoid arthritis after initiation of allergen immunotherapy (AIT). A Polymyositis with systemic sclerosis. Black and broken lines represent creatinine kinase (CK) value and modified Rodnan total skin score (m-Rodnan TSS), respectively. B Sjögren's syndrome. C Eosinophilic granulomatosis with polyangiitis. D Peripheral spondyloarthritis. E Diffuse fasciitis without eosinophilia. ESSDAI, EULAR Sjögren's syndrome disease activity index; BVAS, Birmingham vasculitis activity score; DAS28, disease activity score 28; CRP, C-reactive protein
Safety profile
During the observation period, 11 adverse events were considered to be related to AIT (Table 2). There were no serious adverse events of grade 3 or higher according to the grade classification of Common Terminology Criteria for Adverse Events (CTCAE—version 5.0); most of them were mild [20]. Only one case (7.7%) of nasal discharge requiring antihistamine administration was observed as a moderate adverse effect. Although there was a case of asthma complication, in which asthma symptoms worsened during AIT, it was only transient and mild, thus not requiring therapeutic intervention and not leading to the discontinuation of AIT. The most common adverse reaction was oral pruritus in three patients (23.1%); it was not severe. However, AIT was self-discontinued in four patients due to these adverse events; three discontinued within 1 week and the other discontinued at 6 months after initiation due to oral pruritus or laryngeal discomfort.
Effectiveness for rhinitis
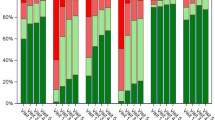
In nine patients (five with RA, one with PM and SSc, one with EGPA, one with peripheral SpA, and one with diffuse fasciitis) who were able to continue AIT for more than 2 years, allergic rhinitis symptoms showed significant improvement after the start of AIT as evaluated by the JRQLQ questionnaire. The median score for eye and nose symptoms improved from 1.67 (1.5–2.0) at baseline to 0.67 (0–1.17) at the second year of treatment, thus showing a statistically significant difference. In QOL-related items, the score for outdoor activity improved from 1.0 (0.5–2.0) at baseline to 0 (0–0.5) at the second year of treatment, thus also showing a statistically significant difference. There were no statistically significant differences in the other domain scores; however, there was a downward trend in all domains. The overall index of the general state also improved from 4.0 (3.8–4.0) at baseline to 2.0 (1.0–2.0) at the second year of treatment, thus showing a statistically significant difference. Changes in each QOL-related item are presented in Table 3.
Discussion
This was a two-year observational study investigating whether AIT affects the activity of concomitant RADs and whether AIT is effective in patients with allergic rhinitis complicated by RADs. As far as studies evaluating the impact of AIT on underlying RADs were concerned, there was only one case report showing that AIT was safe for RA after a 1-year observation [21]. The reason for the lack of studies was speculated to be that allergists are generally reluctant to perform AIT in patients with allergic rhinitis complicated by RADs [6]. Certainly, there are several reports on the appearance of autoimmune diseases such as rheumatoid arthritis [22], systemic lupus erythematosus [23], Sjogren’s syndrome [24], and vasculitis after AIT [25, 26].
The reason for the development of these RADs is not clear. It was speculated that some of these reports were relatively old and the allergen extracts used for AIT were not well-purified, which might lead to the formation of immune complexes and the development of the disease [8]. The quality of allergen extracts has been improving due to the progress of purification and standardization technologies, thereby indicating that this risk may have been relatively reduced in recent years [27]. In this study, many patients with RADs were able to continue AIT and did not experience worsening of RAD activity. Only three patients experienced a temporary worsening of RAD activity; however, their causal relationship to AIT was denied by the attending physician, either because AIT had already been discontinued or because of other clear-cut factors. In addition, most adverse events other than those related to RADs were minimal, thereby suggesting that AIT may be performed without major problems, even in patients with RADs.
In general, AITs may have an unfavorable effect on RADs because of their mechanism. Although not fully understood, it is mainly due to the suppression of the Th2-type immune response by Treg induction [28], allergen-specific immune deviation from Th2 to Th1 represented by interferon-γ (IFN-γ) production [29, 30], and antigen-specific IgG4 antibody production [31]. Of these, Th1-type cytokines, such as IFN-γ, have been reported to contribute to the pathogenesis of RADs. A number of reports suggest that IFN-γ-producing CD4 + T cells function as effector cells at the site of inflammation in RA [32]. A meta-analysis of microarray data from RA patients has shown that IFN-γ is a potent upstream regulator of RA synovial biology [33]. In systemic lupus erythematosus, IFN-γ signaling promotes germinal center responses and the production of autoreactive B cells, thereby leading to disease progression [34]. In fact, some patients treated with IFN-γ have been reported to develop lupus-like symptoms [35, 36]. Therefore, the possibility of RAD deterioration under IFN-γ-induced conditions cannot be ruled out. However, although IFN-γ is used as a treatment for renal cancer, idiopathic pulmonary fibrosis, and chronic granulomatous diseases, it has not been reported that autoimmune diseases occur frequently in these trials [37,38,39]. In this study, worsening of RADs due to AIT was not observed. Although caution should be exercised regarding the worsening of symptoms when AIT is administered to patients with autoimmune diseases, it is presumed that this possibility is low.
On the other hand, the present study also investigates the effectiveness of AIT. The symptoms and QOL scores in the second year of treatment showed significant improvement compared to baseline. Even if statistically significant differences were not detected, most scores decreased. In a phase III trial evaluating the efficacy of SLIT in cedar pollinosis, the improvement in the general state index of JRQLQ was about 20% in the 2nd year compared with the placebo; the result of this study was not inferior to this result, even though direct comparison was difficult [40]. Since AIT has been reported to improve treatment outcomes with the passage of time, further improvements in efficacy can be expected in the future [41].
In this study, most of the enrolled patients were using glucocorticoids, immunosuppressive agents, or biologics; thus, there was a concern that these drugs might impair the effectiveness of AIT. Furthermore, the immunogenicity of the vaccine has been reported to decrease when administered to patients with RADs receiving immunosuppressive therapies, such as glucocorticoids, methotrexate, rituximab, and abatacept [42]. On the other hand, it has been reported that short-term glucocorticoids or anti-cytokine therapy targeting TNF-α and IL-6 do not impair the immunogenicity of the vaccine [43, 44]. Additionally, the negative effects of glucocorticoids and methotrexate on immunogenicity were noted to be dose-dependent [45]. This study enrolled patients who used these drugs at low doses, which might explain why the effectiveness of AIT did not disappear. It is also possible that the immunogenicity of AIT does not completely disappear even if it is affected; thus, the effect of AIT may have appeared.
This study has several limitations. First, this was a small, single-center, retrospective study that could not eliminate unintentional selection bias. Most of the concomitant RADs had low activity levels; patients with moderately or highly active RADs were not included. If these patients had been treated with AIT, it is possible that their outcomes would have been different. Second, we could not observe the details of the immunological effects of AIT due to the lack of immunological data, such as Treg and antigen-specific IgG4, as well as rheumatoid factor and anti-CCP antibody trends. Third, to accurately evaluate the safety and effectiveness of AIT in patients with RADs, it is desirable to compare the results from allergic rhinitis patients without RADs who underwent AIT. Therefore, the results of this study should be validated in a prospective multicenter study using such allergic rhinitis patients as controls.
In conclusion, our results suggest that AIT is safe and effective in patients with allergic rhinitis complicated by RADs with low activity level. AIT is expected to become a potential therapeutic option for patients with allergic rhinitis complicated by RADs who have failed to achieve satisfactory improvement with conventional pharmacotherapy.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AIT:
-
Allergen immunotherapy
- RADs:
-
Rheumatic autoimmune diseases
- RA:
-
Rheumatoid arthritis
- SDAI:
-
Simplified disease activity index
- DAS28:
-
Disease activity score 28
- BVAS:
-
Birmingham Vasculitis Activity Score
- EGPA:
-
Eosinophilic granulomatosis with polyangiitis
- m-Rodnan TSS:
-
Modified Rodnan total skin thickness score
- SSc:
-
Systemic sclerosis
- PM:
-
Polymyositis
- ESSDAI:
-
EULAR Sjögren's Syndrome Disease Activity Index
- SjS:
-
Sjogren’s syndrome
- JRQLQ:
-
Japan allergic rhinitis standard quality of life questionnaire
- MMP-3:
-
Matrix metalloproteinase-3
- CCP:
-
Cyclic citrullinated peptide
- CK:
-
Creatine kinase
- CRP:
-
C-reactive protein
References
Small P, Keith PK, Kim H. Allergic rhinitis Allergy Asthma. Clin Immunol. 2018;14:51 (aacijournal.biomedcentral.com).
Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: the national affliction. J Allergy Clin Immunol. 2014;133:632-9.e5.
Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, Muraro A, Postma MJ, Kocks JWH, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437–53.
Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future allergy asthma. Immunol Res. 2016;8:191–7 (synapse.koreamed.org).
Masuyama K, Matsuoka T, Kamijo A. Current status of sublingual immunotherapy for allergic rhinitis in Japan. Allergol Int. 2018;67:320–5 (jstage.jst.go.jp).
Pitsios C, Demoly P, Bilò MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909.
Pitsios C, Tsoumani M, Bilò MB, Sturm GJ, Rodríguez Del Río P, Gawlik R, et al. Contraindications to immunotherapy: a global approach. Clin Transl Allergy. 2019;9:45.
Linneberg A, Madsen F, Skaaby T. Allergen-specific immunotherapy and risk of autoimmune disease. Curr Opin Allergy Clin Immunol. 2012;12:635–9.
Linneberg A, Jacobsen RK, Jespersen L, Abildstrøm SZ. Association of subcutaneous allergen-specific immunotherapy with incidence of autoimmune disease, ischemic heart disease, and mortality. J Allergy Clin Immunol. 2012;129:413–9.
Bozek A, Kozłowska R, Jarzab J. The safety of specific immunotherapy for patients allergic to house-dust mites and pollen in relation to the development of neoplasia and autoimmune disease: a long-term, observational case-control Study. Int Arch Allergy Immunol. 2014;163:307–12.
Iemoli E, Borgonovo L, Fusi A, Magni C, Ricci ED, Rizzardini G, et al. Sublingual allergen immunotherapy in HIV-positive patients. Allergy. 2016;71:412–5.
Rodríguez del Rio P, Pitsios C, Tsoumani M, Pfaar O, Paraskevopoulos G, Gawlik R, et al. Physicians’ experience and opinion on contraindications to allergen immunotherapy: The CONSIT survey. Ann Allergy Asthma Immunol. 2017;118:621–8.
Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42:244–57.
van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50.
Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68:1827–32.
Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5.
Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69:1103–9.
Okuda M, Ohkubo K, Goto M, Okamoto H, Konno A, Baba K, et al. Comparative study of two Japanese rhinoconjunctivitis quality-of-life questionnaires. Acta Otolaryngol. 2005;125:736–44.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
U.S. Department Of Health And Human Services. 2017 Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 2022 Apr 22
Fiorillo A, Fonacier L, Diola C. Safety of allergenic immunotherapy in systemic lupus erythematosus. J Allergy Clin Immunol. 2006;117:S264.
Ghoreschi K, Fischer J, Biedermann T. Manifestation of rheumatoid arthritis during subcutaneous allergen-specific immunotherapy with bee venom. J Allergy Clin Immunol. 2012. https://doi.org/10.1016/j.jaci.2012.08.052.
Mukherjee S, Samajdar SS, Basu K, Moitra S, Tripath SK. Patient on allergen immunotherapy developed systemic lupus erythematosus?– A clinico-pharmacological look out. IP International Journal of Orthopaedic Rheumatology. 2022;7:90–2 (Innovative Publication Pvt Ltd).
Turkcapar N, Kinikli G, Sak SD, Duman M. Specific immunotherapy-induced Sjögren’s syndrome. Rheumatol Int. 2005;26:182–4.
Phanuphak P, Kohler PF. Onset of polyarteritis nodosa during allergic hyposensitization treatment. Am J Med. 1980;68:479–85.
Taylor RJ. Hypersensitivity vasculitis occurring in a patient receiving immunotherapy. J Allergy Clin Immunol. 1991;87:889–90.
Codina R, Crenshaw RC, Lockey RF. Considerations about pollen used for the production of allergen extracts. J Allergy Clin Immunol Pract. 2015;3:676–82.
Terada T, Matsuda M, Inaba M, Hamaguchi J, Takemoto N, Kikuoka Y, et al. Sublingual immunotherapy for 4 years increased the number of Foxp3+ Treg cells, which correlated with clinical effects. Inflamm Res. 2021;70:581–9.
Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-γ. J Allergy Clin Immunol Elsevier. 1996;97:1356–65.
Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13.
Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71.
Chemin K, Gerstner C, Malmström V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation—Lessons From Rheumatoid Arthritis. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.00353 (frontiersin.org).
Lee EJ, Lilja S, Li X, Schäfer S, Zhang H, Benson M. Bulk and single cell transcriptomic data indicate that a dichotomy between inflammatory pathways in peripheral blood and arthritic joints complicates biomarker discovery. Cytokine. 2020;127:154960.
Chodisetti SB, Fike AJ, Domeier PP, Singh H, Choi NM, Corradetti C, et al. Type II but not type I IFN signaling is indispensable for TLR7-promoted development of autoreactive B cells and systemic autoimmunity. J Immunol. 2020;204:796–809.
Machold KP, Smolen JS. Interferon-gamma induced exacerbation of systemic lupus erythematosus. J Rheumatol. 1990;17:831–2.
Wandl UB, Nagel-Hiemke M, May D, Kreuzfelder E, Kloke O, Kranzhoff M, et al. Lupus-like autoimmune disease induced by interferon therapy for myeloproliferative disorders. Clin Immunol Immunopathol. 1992;65:70–4.
Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma canadian urologic oncology group. N Engl J Med. 1998;338:1265–71.
Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–33 (Massachusetts Medical Society).
Marciano BE, Wesley R, De Carlo ES, Anderson VL, Barnhart LA, Darnell D, et al. Long-term interferon-γ therapy for patients with chronic granulomatous disease. Clin Infect Dis Oxford Academic. 2004;39:692–9.
Okamoto Y, Okubo K, Yonekura S, Hashiguchi K, Goto M, Otsuka T, et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2015;166:177–88.
Yonekura S, Gotoh M, Kaneko S, Kanazawa K, Takeuji Y, Okubo K, et al. Treatment duration-dependent efficacy of Japanese cedar pollen sublingual immunotherapy: evaluation of a phase II/III trial over three pollen dispersal seasons. Allergol Int. 2019;68:494–505.
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–8.
Yang J, Ko J-H, Baek JY, Hong J, Ha S, Lee B, et al. Effects of short-term corticosteroid use on reactogenicity and Immunogenicity of the first dose of ChAdOx1 nCoV-19 vaccine. Front Immunol. 2021;12: 744206.
Day AL, Winthrop KL, Curtis JR. The effect of disease-modifying antirheumatic drugs on vaccine immunogenicity in adults. Cleve Clin J Med. 2020;87:695–703.
Bugatti S, De Stefano L, Balduzzi S, Greco MI, Luvaro T, Cassaniti I, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021;80:1635–8.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
No specific financial support or other benefits were received for the study described in this manuscript.
Author information
Authors and Affiliations
Contributions
KF, AK, WF, TS, and YK conceived and designed the study. KF, AK, WF, TS, and MW contributed to the data collection. KF and TK performed the statistical analyses. KF, TK, WF, TS, and MK interpreted the data. KF, TS and YK wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Kyoto Prefectural University of Medicine (ERB-C-1044).
Consent for publication
All authors have read and approved the contents of the manuscript, and agreed with publication.
Competing interests
KF is not considered to have any competing interests related to this study, but declares as a potential competing interest that KF has received research grants from GlaxoSmithKline. WF is not considered to have any competing interests related to this study, but declares as potential competing interests that WF has received research grants from Boehringer Ingelheim, GlaxoSmithKline, and Takeda. YK is not considered to have any competing interests related to this study, but declares as potential competing interests that YK has received research grants from AbbVie, Asahi Kasei, Astellas, Ayumi, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi Tanabe, Pfizer, Takeda, Teijin, GlaxoSmithKline, and Gilead Sciences. The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fujioka, K., Kasahara, A., Kida, T. et al. Effectiveness and safety of allergen immunotherapy in patients with allergic rhinitis complicated by rheumatic autoimmune diseases: a case series study. Allergy Asthma Clin Immunol 18, 63 (2022). https://doi.org/10.1186/s13223-022-00703-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-022-00703-0