Abstract
Background
To investigate the role of microRNA-29 (miR-29) in mice with allergic rhinitis (AR) and its underlying mechanism.
Methods
AR model was established in BALB/c mice by intraperitoneal sensitization and intranasal challenge with ovalbumin (OVA). miRNA expression was examined in the nasal mucosa tissues of mice and patients with AR, and miRNA-29 was found to be downregulated. To unveil the role of miRNA-29 in AR, it was overexpressed in the nasal mucosa of AR mice by intranasal administration of miRNA-29 agomir. The symptoms of nasal rubbing and sneezing were recorded and evaluated. miR-29 expression, OVA-specific immunoglobulin E (IgE) concentration, pro-inflammatory cytokines levels, eosinophils number, and cleaved caspase-3 and CD276 expression were examined in nasal mucosa tissues and nasal lavage fluid (NALF) by qRT-PCR, ELISA, hematoxylin and eosin staining, western blotting, or immunohistochemistry, respectively. TUNEL assay was used to analyze nasal mucosa cells apoptosis.
Results
Decreased expression of miR-29 was observed in AR, the symptoms of which were alleviated by overexpressing miR-29. In addition, overexpression of miR-29 markedly reduced the concentration of OVA-specific IgE, the levels of IL-4, IL-6, IL-10, and IFN-γ, the pathological alterations and eosinophils infiltration in the nasal mucosa. Furthermore, restoration of miR-29 expression reduced nasal mucosa cell apoptosis. Moreover, overexpression of miR-29 significantly attenuated CD276 mRNA and protein levels in nasal mucosa cells.
Conclusion
MiR-29 mediated antiallergic effects in OVA-induced AR mice by decreasing inflammatory response, probably through targeting CD276. MiRNA-29 may serve as a potential novel therapeutic target for the treatment of AR.
Similar content being viewed by others
Background
Allergic rhinitis (AR) is an inflammatory condition of the nasal mucosa that is mediated by an IgE-associated response to indoor and outdoor environmental allergens [1]. AR is a common disease and a risk factor for the development of other diseases, such as asthma. AR not only affects the social life, sleep, and work of the inflicted individual, but also causes family and socioeconomic burden [2]. AR mainly refers to the inflammation of the nasal mucosa due to the inhalation of allergens and the release of inflammatory mediators [3]. The central clinical symptoms of AR include stuffy nose, runny nose, nasal itching, sneezing, and olfactory disorders [4]. Current pharmacotherapy options for AR include corticosteroids, leukotriene receptor antagonists, mast cell stabilizers, and anticholinergics, but these treatments are known to exert potentially serious side effects and do not show sufficient subjective or objective improvements in about 20% of AR patients [5, 6]. Therefore, it is still necessary to identify a more effective treatment strategy for AR patients.
It has been well recognised that inflammatory mechanisms play a key role in the development of AR [7, 8]. The inflammatory response is associated with an increased number of eosinophils and upregulated levels of proinflammatory cytokines, such as interleukin (IL)-4, IL-6, IL-10, and interferon (IFN)-γ [9]. MicroRNAs (miRNAs) are small, single-stranded RNAs that bind to the 3′-untranslated region (UTR) of target messenger RNAs to regulate their expression [10,11,12]. Accumulating evidence has suggested that miRNAs are involved in the development of tumors, autoimmune diseases, and other systemic diseases [13, 14]. Teng et al. [1] observed that miRNA-143 was significantly downregulated in the nasal mucosal tissues of AR patients and inhibited the production of inflammatory cytokines and mucus by nasal epithelial cells. MiRNA-let-7e has been shown to regulate the progression and development of AR via its anti-inflammatory effects [15]. Together, these reports have demonstrated that miRNAs play a significant role in the regulation of AR.
CD276 (also known as B7-H3) is a member of the B7/CD28 immunoglobulin superfamily that provides crucial co-stimulatory signals that regulate T cell functions involved in tumor surveillance, infection response, and autoimmune diseases [16]. CD276 is overexpressed in tumor and tumor-associated cells, making it an interesting therapeutic target18 [17]. Hong Xu et al. [18] found that miRNA-29a (miR-29a) directly targets the CD276 3′-UTR in HeLa cells, and may have potential implications for immune-based therapy of human solid tumors. In addition, the miR-29c/CD276 axis has been identified to play an important role in childhood asthma via regulation of Th2/Th17 cell differentiation [19]. Another study has reported that miR-29 mediates the innate and adaptive immune responses to bacterial infections by targeting IFN-γ [20]. The plasma levels of miR-29 are reduced in both allergic and asthmatic patients compared to healthy subjects [21]. Together, these studies have suggested that miR-29 may be involved in the allergic and immune processes of AR. However, to our knowledge, there has been no prior study examining the in vivo effect of miR-29 on allergy symptoms and AR. Therefore, this study aimed to investigate the role and significance of miR-29 in mice with OVA-induced AR, and to examine whether miR-29 has any effect on the development of AR.
Methods
Tissue samples
This study included tissues samples from 9 AR patients and 9 healthy control patients. The diagnosis and treatment of AR were carried out by their physicians. Out of the 9 AR patients, 5 were males and 4 were females with a mean age of 40.2 years (range: 26–57 years), There were 3 cases with positive mite skin test, 2 cases with family history of asthma and 1 cases with seasonal asthma. There was no history of smoking and no use of hormone drugs in the past 2 weeks. The results of chest X-ray examination were normal.The healthy control group included 7 males and 2 females with a mean age of 38 years (range: 24–58 years). There was no history of rheumatism nor AR. There was no upper respiratory tract infection, no use of hormones and anti allergic drugs in the past 1 month, and the results of chest X-ray examination were normal. The epithelial samples were gently scraped from the surface of the inferior nasal turbinate using a plastic curette. All participants have signed an informed consent form and been informed of all the experiment details in advance.
Animals
A total of 32 8-week-old pathogen-free male BALB/c mice (18–20 g) were purchased from the Shanghai Xinmao Experimental Animal Center (Shanghai, China) and divided randomly into four groups of 8 mice each: control (Con), OVA-induced AR (OVA), OVA-induced AR with miR-29 agomir treatment (OVA + miR-29), and OVA-induced AR with mismatched agomir (MA) treatment (OVA + miR-MA). All mice were housed in the campus animal facility under standard conditions, including 12 h light/dark cycles, average temperature of 18–22 °C, and mild humidity (50–60%), as well as free access to food and water ad libitum. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. At the end of the experiment, mice were anesthetized with 1.25% pentobarbital (40 mg/kg) and sacrificed by CO2 gas asphyxiation for tissue collection.
AR model establishment and treatments
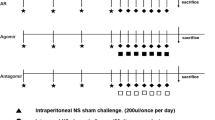
The OVA-induced AR mouse model was generated as previously described [22]. Briefly, mice were injected intraperitoneally with 200 μL saline including 50 μg OVA (Grade V, Sigma, St. Louis, MO, USA) adsorbed to 1 mg aluminum hydroxide (Thermo Scientific, Rockford, MD, USA) on days 1, 8, and 15. From days 22 to 28, the mice received a daily intranasal challenge with 20 μL of 1 mg/mL OVA per nostril. Animal experiments were repeated three times.
Symptom scores
Thirty minutes after the final OVA challenge, we examined the behavior of the AR mice, including the amount of nasal rubbing and sneezing motions in a given time. A video monitoring device recorded all of the symptoms within a 10 min period. The analysis of behavior was then conducted by professional personnel who were blinded to the groups.
Intranasal administration of miR-29 agomir
The miR-29 agomir and the corresponding mismatched agomir (miR-MA) were purchased from Shanghai GeneChem (Shanghai, China). On days 22–28, the miR-29 agomir was diluted to 5 pmol/μL in 20 μL saline and intranasally injected daily into each nostril of the mice in the OVA + miR-29 group 3 h before the OVA challenge. The OVA + miR-MA group was intranasally injected with the same dose of miR-MA. The OVA and Con groups were intranasally treated with saline.
Quantitative real-time qPCR
After euthanasia, nasal mucosal tissues were collected and DNA was extracted using an RNeasy Mini Kit (Qiagen, CA, USA) according to the manufacturer's instructions. The expression of genes of interest was analyzed by fluorescence quantitative polymerase chain reaction (qPCR). Gene expression levels were measured using the 2−ΔΔCt method and normalized to the internal reference gene of β-actin or U6 expression. The primers used for qPCR analyses are listed in Table 1.
Quantitative measurement of cytokines
The nasal lavage fluid (NALF) was collected after irrigation and was centrifuged at 8000×g at 4 °C for 15 min to obtain the supernatant. To evaluate the allergic reaction, cytokines (IL-4, IL-6, IgE, and IFN-γ) were measured using ELISA kits (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions. While the concentrations of Eotaxin and RANTES were measured using the DuoSet Mouse ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Histological analyses
Fresh nasal mucosal tissue was isolated, fixed with formalin, and then embedded in paraffin. Tissue sections (5 μm thick) were then stained with hematoxylin and eosin (H&E) according to the manufacturer’s instructions. The eosinophils in nasal lavage fluid and nasal mucosa were studied by HE staining. We observed the morphology of the nasal mucosal epithelium and the number and distribution of eosinophils in the nasal mucosa. Apoptosis was detected by in situ labeling using a TUNEL kit (Beyotime, Jiangsu, China) according to the manufacturer’s instructions. The TUNEL-positive cells were observed under a fluorescence microscope (Leica DM4000, Wetzlar, Germany). The apoptosis rate was quantified by counting TUNEL-positive cells from five random fields of view.
Cell culture and miRNA transfections
The cell line from the nasal mucosa of mice was purchased from American Type Culture Collection (ATCC) and cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS, 100 mg/mL streptomycin, and 100 units/mL penicillin at 37 °C in an atmosphere of 5% CO2 and 95% relative humidity. For transfection of these cells, miR-29 mimics or their corresponding negative controls (Rio Biotechnology, Guangzhou, China) were diluted in OptiMEM I medium (Invitrogen, Carlsbad, CA, USA) and transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) transfection reagent according to the manufacturer’s instructions.
Luciferase experiments
The luciferase reporter assays were performed to examine the direct binding of miR-29 to the target CD276 mRNA. Wild-type and mutant reporter plasmids of CD276 (Gene ID: 102,657, GenBank) which containing a wild or mutant miR-29 binding sites were synthesized by GenePharma and amplified and cloned into the pMIR-ReportTM vector (RiboBio Co., Guangzhou, China). Nasal mucosal cells from mice were co-transfected with the 3′-UTR of CD276 (with either wild-type or mutant miR-29 binding sites) and either miR-29 mimics or miR-29 negative controls using Lipofectamine 2000 (Invitrogen). After incubation for 48 h, the Dual-Luciferase Reporter Assay (Promega, Madison, WI, USA) was performed to examine whether miR-29 directly binds CD276. Renilla luciferase activity was used as an internal control for normalization.
Western blotting
Total protein was extracted from the nasal mucosal cells of mice using M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific, Shanghai, China). Equal amounts of the protein samples were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. The membrane was blocked by 5% non-fat milk for 2 h at 25 °C and incubated with the primary antibodies overnight at 4 °C. The next day, the membrane was washed 5 times with 1 × PBST and then incubated with the secondary antibody for 1 h at 25 °C. The membrane was developed using enhanced luminol-based chemiluminescence. The results were photographed using a UVP BioSpectrum Imaging System (BioSpectrum, Orangevale, CA, USA).
Statistics
All data are presented as mean ± SEM and compared by one-way ANOVA with Tukey’s post-hoc test. All statistical analyses were performed using the SPSS 16.0 software and graphical representations were generated using GraphPad Prism 5 (San Diego, CA, USA) software. Results were considered significant when they reached a 95% confidence level (P < 0.05).
Results
The levels of miR-29 are suppressed in the nasal mucosa of AR patients and AR mice
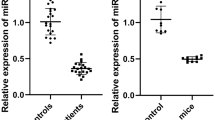
We collected the nasal mucosal tissues of AR patients and matched healthy controls, and performed qPCR to detect the amount of miR-29 in each of the samples. MiR-29 levels were much lower in the AR patients than in the healthy controls (Fig. 1a, t = 7.43, p < 0.001). The levels of miR-29 were also significantly lower in AR mouse tissues than in control tissues (Fig. 1b, t = 6.64, p < 0.001). To further investigate the role of miR-29, we administrated mir-29 in mice AR model (Fig. 1c).
Allergic rhinitis (AR) suppresses the expression of miRNA-29 (miR-29) in the nasal mucosa. a Expression of miR-29 in the nasal mucosa of human AR patients. Nasal mucosa samples were collected from nine pairs of age-matched AR patients and healthy controls. ***, P < 0.001 vs. Con by t-test. b Expression of miR-29 in the nasal mucosa of OVA(ovalbumin)-induce AR mice. The data are presented as mean ± SEM, n = 9 for each group. c Schema showing the design of the animal experiments. ***, P < 0.001 vs. Con
The overexpression of miR-29 alleviates the symptoms of AR
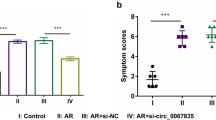
We overexpressed miR-29 in the nasal mucosa of AR mice via intranasal administration of miR-29 agomir. As a control, some mice were intranasally treated with mismatched agomir (miR-MA). Compared to the control group, AR symptoms in the OVA-induced group were more severe, causing a significant increase in nasal friction (Fig. 2a, F = 6.732, p < 0.001, one-way ANOVA) and sneezing (Fig. 2b, F = 8.543, p < 0.001, one-way ANOVA). However, the symptoms of AR were alleviated in the miR-29 overexpression group. After the behavioral test, nasal mucosa tissues were collected to perform RT-qPCR test to verify the effect of miR-29 agomir. The miR-29 agomir treatment did increase the miR-29 level in nasal mucosa (Fig. 2c). These results indicated that miR-29 overexpression is able to reduce AR symptoms.
Restoration of miR-29 reduces the levels of cytokines in the NALF of AR mice
To determine the effect of miR-29 on local inflammation, we collected the serum and NALF from the different mouse groups and measured the levels of inflammatory cytokines. The levels of cytokines were substantially increased in the NALF of AR mice compared to that in the NALF of control mice. However, treatment with miR-29 agomir reduced the levels of these cytokines IL-4 (Fig. 3a, F = 8.554, p < 0.001, one-way ANOVA), IL-6 (Fig. 3b, F = 7.356, p < 0.001, one-way ANOVA), and IFN-γ (Fig. 3c, F = 6.976, p < 0.001, one-way ANOVA), whereas that with miR-MA had no significant effect. Next, we examined the levels of eosinophils and the secreted mediator proteins in the NALF of the mice. Eosinophils (Fig. 3d, F = 7.875, p < 0.001, one-way ANOVA), Eotaxin (Fig. 3e, F = 9.452, p < 0.001, one-way ANOVA), and RANTES (Fig. 3f, F = 5.355, p < 0.001, one-way ANOVA) levels were all elevated in the AR mice compared to the control mice, but significantly reduced in mice treated with miR-29 agomir.
Treatment with miR-29 agomir reduces the levels of inflammatory cytokines in mice. a–c The levels of IL-4 (a), IL-6 (b), and IFN-γ (c) in the NALF of the mice were measured using ELISA. d The amount of eosinophils in the nasal lavage fluid (NALF) of the mice were examined using flow cytometry. e, f The levels of Eotaxin (e) and RANTES (f) in NALF were measured using ELISA. The data are presented as mean ± SEM, n = 8 for each group. ***, P < 0.001 vs. Con; ##, P < 0.01 vs. OVA by ANOVA
Restoration of miR-29 reduces eosinophil infiltration and apoptosis in the nasal mucosa of AR mice
We investigated the histopathological changes in AR mice following overexpression of miR-29. H&E staining demonstrated that there was a significant influx of eosinophils into the nasal mucosa of mice in the AR group compared to the control group. Restoration of miR-29 levels significantly decreased eosinophil infiltration (Fig. 4a, c, F = 8.896, p < 0.001, one-way ANOVA). In addition, TUNEL staining was used to evaluate the level of apoptosis in the nasal mucosa of the mice. OVA administration significantly increased TUNEL-positive cells, indicating increased levels of apoptosis. However, restoration of miR-29 levels efficiently suppressed apoptosis (Fig. 4b, d, F = 7.979, p < 0.001, one-way ANOVA).
Restoration of miRNA-29 expression inhibits infiltration of inflammatory cells and apoptosis in the nasal mucosal tissue of allergic rhinitis (AR) mice. a Hematoxylin and eosin staining of the nasal mucosa sections from the various groups of mice. b TUNEL staining of the nasal mucosa sections from the various groups of mice. c The number of eosinophils present in each group. d The number of TUNEL-positive cells in each group. The data are presented as mean ± SEM, n = 8 for each group. ***, P < 0.001 vs. Con; ##, P < 0.01 vs. OVA by ANOVA
Restoration of miR-29 reduces inflammation-related gene expression in the nasal mucosa of AR mice
To investigate the association between miR-29 and inflammation, the amounts of IL-6 and cleaved caspase-3 were examined by immunostaining in each group. The increased amounts of IL-6 (Fig. 5a, b, F = 13.534, p < 0.001, one-way ANOVA) and Cleaved caspase-3 (Fig. 5c, d, F = 11.164, p < 0.001, one-way ANOVA) following OVA challenge were attenuated by miR-29 overexpression. The mRNA expression of relevant inflammatory factors, including IL-6 (Fig. 5e, F = 10.331, p < 0.001, one-way ANOVA), Cleaved caspase-3 (Fig. 5f, F = 14.434, p < 0.001, one-way ANOVA), IL-4 (Fig. 5g, F = 15.423, p < 0.001, one-way ANOVA), IL-10 (Fig. 5h, F = 14.453, p < 0.001, one-way ANOVA), and IFN-γ (Fig. 5i, F = 7.534, p < 0.001, one-way ANOVA), was assessed and was found to be increased upon administration of OVA and decreased upon treatment with miR-29 agomir. Meanwhile, there was no significant difference between the OVA + miR-MA group and the OVA group.
Restoration of miRNA-29 expression is associated with a reduction in inflammation-related gene expression in the nasal mucosa of allergic rhinitis (AR) mice. a IL-6 staining of the nasal mucosal tissue from each mouse group. b Quantification of the results of IL-6 staining shown in A. c Cleaved caspase-3 staining of the nasal mucosal tissue from each mouse group. d Quantification of the results of cleaved caspase-3 staining shown in C. e–i qPCR results for the expression of IL-6 (e), cleaved caspase-3 (f), IL-4 (g), IL-10 (h), and IFN-γ (i). The data are presented as mean ± SEM, n = 8 for each group. ***, P < 0.001 vs. Con; ##, P < 0.01 vs. OVA by ANOVA
CD276 is the target gene of miR-29
To investigate the possible target genes of miR-29 in regulating inflammation, we used the miRNA target gene prediction website TargetScan (www.targetscan.org). We identified highly conserved miR-29b binding sites in the 3′-UTR of CD276, and partial sequence alignments of miR-29 and CD276 (Fig. 6a, t = 7.24, p < 0.001). Accordingly, a previous study found that miR-29a directly targets the 3′-UTR of CD276 in HeLa cells [18]. To confirm the in silico results, miR-29 mimics or corresponding negative controls were co-transfected with either pCD276-WT or pCD276-MUT (CD276 with a mutated binding site) in murine nasal mucosa cells. A luciferase reporter assay showed that transfection with the miR-29 mimics significantly inhibited the luciferase activity associated with the wild-type, but not the mutated, pCD276 (Fig. 6b, t = 7.12, p < 0.001). These results demonstrated that miR-29 binds to and suppresses CD276 expression in a direct and specific manner. The expression of CD276 was also examined by immunohistochemistry of the nasal mucosa. The results of the staining showed that CD276 levels increased significantly in OVA-treated mice compared to those in control mice. Restoration of miR-29 remarkably decreased the expression of CD276 (Fig. 6c, t = 7.75, p < 0.001). Immunohistochemistry experiments (Fig. 6d) revealed a consistent pattern with immunoblotting results that upregulation of miR-29 caused reduced CD276 protein expression in the nasal mucosa (Fig. 6e, f, F = 6.643, p < 0.001, one-way ANOVA). These data indicate that miR-29 negatively regulates CD276 expression.
MiRNA-29 targets CD276 to regulate inflammation. a Pairing alignments of CD276 and miR-29-3p. b Quantification of the results of relative luciferase activity in each group, n = 6 for each group. c Quantification of the qPCR results for CD276 after miRNA-29 transfection, n = 6 for each group. d CD276 staining of murine nasal mucosa cells, n = 3 for each group. e Representative image of immunoblot results for CD276 expression in each group. f Quantification of the results of the immunoblot shown in E. The data are presented as mean ± SEM, n = 4 for each group. **, P < 0.01 vs. Con, ***, P < 0.001 vs. Con; ##, P < 0.01 vs. OVA by ANOVA
Discussion
AR is triggered after allergen-specific IgE and Th2 cells recognize inhaled allergens in the environment and elicit an inflammatory process that involves many different inflammatory cells and molecules, including eosinophils, cytokines, and other regulatory molecules [23]. MiR-29 consists of three different isoforms: miR-29a, miR-29b, and miR-29c. These mature isoforms of miR-29 are silenced or downregulated in different kinds of cancer [8,9,10]. It has also been reported that suppression of miR-29 may lead to several inflammatory diseases [24, 25]. In addition, miR-29 has been identified as a potential biomarker and therapeutic target for allergies and asthma [26, 27].
The present study demonstrated that miR-29 was downregulated in human patients and mice with AR when compared with control groups. To avoid the possible systemic complications that could be caused by tail vein injection, we intranasally administered miR-29 agomir in AR mice and investigated its direct effects on the nasal mucosa. A previous study had indicated that mice with OVA-induced AR display nasal allergy symptoms similar to humans [28]. Our research showed that the frequencies of nasal rubbing and sneezing were much lower in AR mice overexpressing miR-29 than in the normal AR model mice, indicating that intranasal administration of miR-29 agomir ameliorates AR symptoms.
MiR-29 overexpression also inhibited the expression levels of OVA-specific IgE and proinflammatory cytokines (IL-4, IL-6, IL-10, and IFN-γ) in the NALF of AR mice. Furthermore, we observed that miR-29 markedly inhibited the pathological changes of OVA-induced AR and reduced the number of infiltrating eosinophils. Eosinophil infiltration in the tissues is the main characteristic of allergic inflammation in humans [29]. IL-4 and IL-10 are Th2 cytokines produced by mast cells, T cells, and macrophages. They play an important role in regulating IgE isotype switching in B cells and the differentiation of T cells into Th2 cells [30]. IFN-γ, a Th1-related cytokine, has been found to regulate IgE-mediated allergies and asthma [31, 32]. The above results indicate that the decrease in Th1 and Th2 cytokines may underlie the anti-inflammatory effect of miR-29 in an AR model.
In addition to inflammation, aberrant apoptosis of nasal mucosa cells can contribute to AR symptoms [33]. Through the use of TUNEL assays, immunohistochemistry, and qPCR of the apoptosis-related gene caspase-3, we determined that miR-29 was able to prevent apoptosis in cells of the nasal mucosa. These results suggest that miR-29 alleviates OVA-induced AR in mice, at least partially, through an anti-apoptotic mechanism.
CD276 (also known as B7-H3) belongs to a family of immune modulators that are expressed in various immune cells, such as dendritic cells (DCs), monocytes, and activated T cells [34]. An anti-CD276 monoclonal antibody has previously been used as an effective treatment to alleviate asthmatic syndromes [35]. Therefore, inhibition of CD276 signals may provide a novel therapeutic approach for the treatment of allergic asthma. In this study, the luciferase reporter gene assay in murine nasal mucosa cells showed that CD276 is a target gene of miR-29, which is consistent with a previous study by Hong Xu et al. [18]. Thus, the mechanism by which miR-29 overexpression can inhibit the expression of inflammatory cytokines and prevent the development of AR-related histological changes likely involves the ability of miR-29 to reduce expression of CD276. Although there have been no previous studies linking miR-29 to AR, miR-29c was previously reported to be involved in allergic asthma inflammatory diseases [19]. The current study, together with the miR-29c study by Zhang et al. (38), suggests that the miR-29/CD276 regulatory axis may be a general mechanism underlying inflammatory diseases, including allergic airway inflammation diseases. The role of this regulatory axis in a potentially wide array of inflammatory diseases warrants further investigation.
In conclusion, the results of our study demonstrate that miRNA-29 alleviates the symptoms and allergic responses of OVA-induced AR, potentially by regulating the expression of CD276 and other inflammation-related cytokines.
Availability of data and materials
The datasets used or/and analyzed during the current study are available from the corresponding author on reasonable request.
Change history
14 June 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s13223-023-00807-1
Abbreviations
- mir-29:
-
Microrna-29
- AR:
-
Allergic rhinitis
- OVA:
-
Ovalbumin
- ige:
-
Immunoglobulin E
- NALF:
-
Nasal lavage fluid
- IFN:
-
Interferon
- UTR:
-
Untranslated region
- MA:
-
Mismatched agomir
- ATCC:
-
American Type Culture Collection
- dcs:
-
Dendritic cells
References
Teng Y, Zhang R, Liu C, et al. miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem Biophys Res Commun. 2015;457(1):58–64.
Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352.
Alhamwe Bilal A, Sarah M, von Strandmann Elke P, et al. Epigenetic regulation of airway epithelium immune functions in asthma. Front Immunol. 2020;11:1747.
Watts Annabelle M, Cripps Allan W, West Nicholas P, et al. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front Pharmacol. 2019;10:294.
Small P, Keith PK, Kim H. Allergic rhinitis. allergy asthma. Clin Immunol. 2018;14(Suppl 2):51.
Campo P, Eguiluz-Gracia I, Bogas G, et al. Local allergic rhinitis: implications for management. Clin Exp Allergy. 2019;49(1):6–16.
Watts AM, Cripps AW, West NP, Cox AJ. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front Pharmacol. 2019;10:294.
Zhang N, Li H, Jia J, He M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell Immunol. 2015;298(1–2):88–95.
Amin K, Janson C, Bystrom J. Role of Eosinophil Granulocytes in Allergic Airway Inflammation Endotypes. Scand J Immunol. 2016;84(2):75–85.
Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5.
Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–6.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Long H, Wang X, Chen Y, Wang L, Zhao M, Lu Q. Dysregulation of microRNAs in autoimmune diseases: pathogenesis, biomarkers and potential therapeutic targets. Cancer Lett. 2018;428:90–103.
Shan H, Geping W, Xiaodan G, et al. Effect of biospray dressings on eosinophil infiltration in the nasal mucosa and serum IgE levels after nasal provocation in experimental allergic rhinitis. Allergy Rhinol. 2020;11:2152656720902142.
Li L, Zhang S, Jiang X, Liu Y, Liu K, Yang C. MicroRNA-let-7e regulates the progression and development of allergic rhinitis by targeting suppressor of cytokine signaling 4 and activating Janus kinase 1/signal transducer and activator of transcription 3 pathway. Exp Ther Med. 2018;15(4):3523–9.
Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7–H3. Immunol Rev. 2017;276(1):26–39.
Castellanos JR, Purvis IJ, Labak CM, et al. B7–H3 role in the immune landscape of cancer. Am J Clin Exp Immunol. 2017;6(4):66–75.
Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7–H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–81.
Zhang X, Zhao X, Sun H, et al. The role of miR-29c/B7-H3 axis in children with allergic asthma. J Transl Med. 2018;16(1):218.
Ma F, Xu S, Liu X, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12(9):861–9.
Specjalski K, Jassem E. MicroRNAs: potential biomarkers and targets of therapy in allergic diseases? Arch Immunol Ther Exp (Warsz). 2019;67(4):213–23.
Bui TT, Piao CH, Hyeon E, et al. The protective role of Piper nigrum fruit extract in an ovalbumin-induced allergic rhinitis by targeting of NFκBp65 and STAT3 signalings. Biomed Pharmacother. 2019;109:1915–23.
Licari A, Castagnoli R, Brambilla I, et al. Biomarkers of immunotherapy response in patients with allergic rhinitis. Expert Rev Clin Immunol. 2018;14(8):657–63.
Tang K, Zhao J, Xie J, Wang J. Decreased miR-29b expression is associated with airway inflammation in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2019;316(4):L621-l629.
Patel SA, Gooderham NJ. IL6 Mediates immune and colorectal cancer cell cross-talk via miR-21 and miR-29b. Mol Cancer Res. 2015;13(11):1502–8.
Sheu CC, Tsai MJ, Chen FW, et al. Identification of novel genetic regulations associated with airway epithelial homeostasis using next-generation sequencing data and bioinformatics approaches. Oncotarget. 2017;8(47):82674–88.
Émile B, Anne-Marie M, Anne-Marie B-L, et al. Eosinophil microRNAs play a regulatory role in allergic diseases included in the atopic march. Int J Mol Sci. 2020;21(23):9011.
Wang H, Zhang J, Gao C, Zhu Y, Wang C, Zheng W. Topical levamisole hydrochloride therapy attenuates experimental murine allergic rhinitis. Eur J Pharmacol. 2007;577(1–3):162–9.
Kim KA, Jung JH, Choi YS, Kang G, Kim ST. Anti-inflammatory effect of wogonin on allergic responses in ovalbumin-induced allergic rhinitis in the mouse. Allergy Rhinol. 2018;9:2152656718764145.
Zhang Y, Feng J, Sun J, et al. H2-Eb1 expression is upregulated in the nasal mucosa of allergic rhinitis. Asian Pac J Allergy Immunol. 2014;32:308–15.
Takada S, Kambe N, Kawasaki Y, et al. Pluripotent stem cell models of Blau syndrome reveal an IFN-γ-dependent inflammatory response in macrophages. J Allergy Clin Immunol. 2018;141(1):339-349.e311.
Leavy O. Asthma and allergy: An IFNγ bias in severe asthma. Nat Rev Immunol. 2015;15(8):466–7.
Wang T, Chen D, Wang P, Xu Z, Li Y. miR-375 prevents nasal mucosa cells from apoptosis and ameliorates allergic rhinitis via inhibiting JAK2/STAT3 pathway. Biomed Pharmacother. 2018;103:621–7.
Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7–H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–46.
Chen ZR, Zhang GB, Wang YQ, et al. Therapeutic effects of anti-B7-H3 antibody in an ovalbumin-induced mouse asthma model. Ann Allergy Asthma Immunol. 2013;111(4):276–81.
Acknowledgements
All experimental protocols were approved by the Ethics Committee of the Beijing Shijitan Hospital of Capital Medical University (sjtkyll-lx-2020(9)).
Funding
The study was funded by departmental resources.
Author information
Authors and Affiliations
Contributions
Y.J-S. conceptualized and designed the research. W.J. and L.A-Z. performed experiments, acquired and analyzed the data, and wrote the paper. P.H. prepared the data figures. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Ethics Committee of the Beijing Shijitan Hospital of Capital Medical University (sjtkyll-lx-2020(9)). All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s13223-023-00807-1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Yin, J., Peng, H. et al. RETRACTED ARTICLE: MicroRNA-29 mediates anti-inflammatory effects and alleviation of allergic responses and symptoms in mice with allergic rhinitis. Allergy Asthma Clin Immunol 17, 24 (2021). https://doi.org/10.1186/s13223-021-00527-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-021-00527-4