Abstract
Purpose
The present research work focuses on the extraction of chitosanase enzyme from soil fungi. Chitosan hydrolysis by chitosanase is one of the most effective methods to produce chitosan oligosaccharides which are new biomaterials that have many biological activities such as antitumour, antioxidant, antidiabetic and antimicrobial.
Method
A strain producing chitosanase was screened and identified as Fusarium oxysporum D18 with an accession number OL343607. Various physiological parameters (incubation type, carbon source, additive nitrogen source, statistical evaluation, solid state fermentation) were assessed to increase chitosanase production.
Results
Fusarium oxysporum D18 produced a considerable value of chitosanase (1.220 U/ml). After 7 days of incubation, the best carbon source was lactose, and the best nitrogen source was ammonium chloride. Statistical evaluation was carried out by using Plackett–Burman and Box-Behnken designs. The highest chitosanase production (1.994 U/ml) was induced by the medium composition g/l: KH2PO4 (1.5), MgSO4 (0.269), lactose (18), NH4Cl (1.26), pH (6.68), using a 5-day-old inoculum and chitosanase activity was 1.63 folds that of the original medium. The production of chitosanase by Fusarium oxysporum D18 in solid state cultures using different solid substrates was studied and the best solid substrate for higher chitosanase activity (2.246 U/ml) was raw shrimp heads and shells and chitosanase activity was 1.13 folds that of the optimized liquid cultures. An extracellular chitosanase was isolated and partially purified by using 75% saturation of ammonium sulphate. The highest chitosanase activity (3.667 U/ml) with a specific activity of 0.390 U/mg protein was obtained at enzyme protein concentration of 9.391 mg/ml, substrate concentration of 1.2 % (w/v), Vmax of the enzyme of approximately 0.430 U/mg protein, and KM of 0.26 % (w/v), at pH 5.6 and reaction temperature of 50 °C. The activity of the purified and characterized chitosanase increased by 3 times than that the original isolate activity. The enzyme was thermostable and retained about 55% of its original activity after heating at 70 °C for 15 min. The enzyme preparations were activated by Ca2+ ions and inactivated by Zn+2, Cu+2 ions, and EDTA.
Conclusion
An antitumour activity of chitooligosaccharides produced by the chitosanase was applied to the MCF-7 (breast carcinoma cells) and they had a cytotoxicity inhibitory effect against them about IC50 = 448 μg/ml.
Highlights
(1) We screened and identified a chitosanase producing strain from the soil as Fusarium oxysporum D18 with an accession number OL343607.
(2) The enzyme activity of chitosanase was greatly improved by optimizing the fermentation medium of the fungus.
(3) The activity of chitosanase was optimized by statistical evaluation that was carried out by using Plackett-Burman and Box-Behnken designs.
(4) The production of chitosanase by Fusarium oxysporum D18 in solid state cultures using different solid substrates was studied for increasing activity.
(5) Partial purification of the enzyme was performed by using 75 % saturation of ammonium sulphate.
(6) Physical and chemical properties of the enzyme were performed leading to a highly thermostable chitosanase.
(7) An antitumour chitosanase activity was applied to the MCF-7.
Similar content being viewed by others
Background
Chitin is the second most abundant biopolymer on the planet after cellulose. Chitin, known as 2-acetamide-2-deoxy-β-d-glucan, has molecular weights ranging from hundreds of thousands to millions (Abo Elsoud and El Kady 2019). Its sources are very wide, common in insects and invertebrate exoskeletons and crustaceans. Chitosan, a D-glucosamine polymer, whose scientific name is 2-amino-2-deoxy-β-d-glucan, has a relative molecular weight ranging from hundreds of thousands to millions (Beier and Bertillsson 2013). It is present in the cell walls of a limited group of fungi in nature (Muzzarelli et al. 2012). It is usually prepared from chitin by the artificial deacetylation in the presence of alkali. Chitosan and its derivatives showed functional properties making them useful in many fields including food, cosmetics, medicine and pharmaceutical chitooligosaccharides (COS); are composed of 2 to 10 units of D-glucosamines; are easily absorbed in the intestine and quickly get into the blood flow (Chang et al. 2007; Aktuganov et al. 2019).
Conversion of chitosan into COS can be done either by acid or enzyme hydrolysis. Enzymatic hydrolysis has some advantages in producing COS (Cruz et al. 2004). It requires milder reaction conditions (relatively low temperatures and slightly acidic pH) and does not generate harmful by-products or wastes, which makes it safer and more environmentally friendly approach (Suresh 2019).
Chitosanase (EC 3.2.1.132) is an enzyme that catalyses the endo-type cleavage of β-1,4-linkages between D-glucosamine (GlcN) residues in chitosan, producing chitosan oligosaccharides and glucosamine (Weikert et al. 2017; Miguez et al. 2021). COS are new biomaterials that have been reported to have many biological activities such as antitumour, anti-HIV-1, antioxidant, antidiabetic and antimicrobial. They also had a number of potential applications in food, pharmaceutical and agricultural industries (Zhai et al. 2019). Chitosanases have many industrial and biotechnological applications since chitosanase-producing microorganisms have an application in the bioconversion of marine crustacean biomaterials to bioactive molecules such as antitumours and antioxidants (Wang et al. 2010). They can be also used to improve the resistance of plants against different phytopathogenic fungi (Gao et al. 2008). These various applications attract the research focus to improve the COS productivity to meet the industrial requirements.
Chitosanases have been found from many microorganisms, including fungi; although chitosanase has been studied for about 50 years (Choi et al. 2004), only certain microorganisms can produce it. The fungi producing chitosanase include Aspergillus, Penicillium, Fusarium, Gongronella and Mucor. Recent studies on chitosanases have been dominated by bacterial species belonging to the genera Bacillus (Durkin et al. 2009) and Streptomyces, and they have been purified and characterized. Few chitosanases have been reported to be produced by some plants (Thadathil and Velappan 2014).
The aim of the present work was the production of chitosanase from the most promising and potent fungus showing high chitosanase activity, identification by 18S-rRNA, statistical optimization, solid state fermentation, purification, characterization and application of chitosanase as an antitumour agent.
Materials and methods
Isolation of chitosanase-producing microorganisms
Seven soil samples were collected from different Egyptian sites for the isolation of chitosanase-producing microorganisms, 0.1 ml of soil suspension was transferred to the petri plates containing the chitosanase-detection agar medium (CDA) composed of (g/l): chitosan (10.0), glucose (10.0), yeast extract (2.0), (NH4)2SO4 (2.0), KH2PO4 (2.0), MgSO4 (0.24) and agar (15.0). The plates were incubated at 30°C and examined after 7 days of incubation for the appearance of a clear zone around colonies (Wangtueai et al. 2006; Myat et al. 2019).
Identification of the chitosanase-producing microorganism
The promising fungal isolate was selected for identification by 18S-rRNA sequence analysis amplified by polymerase chain reaction (PCR) (Hashem et al. 2018) at Sigma Scientific Services Company, Lebon building- La Cite mall - El Hossary - 6 of October, Cairo-Egypt.
Preparation of the fermentation liquid medium
Fermentation was performed in 250-ml Erlenmeyer flasks containing 50 ml medium (g/l): chitosan (10.00), yeast extract (2.0), KH2PO4 (2.0 g) and MgSO4 (0.5). After sterilization, each flask was inoculated with 1 ml of fungal spore suspension and incubated for 7 days at 30°C in the static and shaker incubators. Afterwards, the contents of each flask were taken for analysis.
Assay for chitosanase activity
Chitosanase activity was assayed by measuring the reducing sugars liberated during the hydrolysis of chitosan with a DDA (degree of deacetylation) of 83%. The reaction mixture contained 1 ml of soluble chitosan (1 % (w/v)) and 1 ml of diluted enzyme solution at pH 5.6 using acetate buffer. Then the mixture was incubated at 37°C for 30 min (Chen et al. 2008; Abdel-Aziz et al. 2014).
One enzyme unit was defined as the amount of enzyme required to produce 1 µmol of reducing sugar per min. The resulting reducing sugar in the filtrate were measured using the modified dinitrosalicylic acid (DNS) method and were measured spectrophotometrically at 540 nm. The protein assay was determined by the method of Lowry et al. (1951).
Effect of different carbon sources on the production of chitosanase from Fusarium oxysporum D18
Chitosan was substituted by equal carbon amounts (10.0 g/l) of different carbon sources, one at a time (fructose, sucrose, dextrose, lactose, glucose and starch) and keeping other medium constituents at their basal level (Zhang and Zhang 2013; Liaqat et al. 2018).
Effect of different nitrogen sources on the production of chitosanase from Fusarium oxysporum D18
Yeast extract was substituted by potassium nitrate, ammonium sulphate, ammonium chloride and sodium nitrite as inorganic nitrogen sources in an equivalent nitrogen amount to that used in the fermentation medium (2.0 g/l), one at a time, and keeping other medium constituents at their basal level (Liaqat et al. 2018).
Statistical optimization
Statistical optimization with a two-level experimental design was carried out as follows, Plackett–Burman design followed by Box-Behnken design to optimize the variables of the highest effect.
Plackett-Burman design
The variables that significantly influence chitosanase production were screened using a fractional factorial Plackett–Burman (PB) design (Plackett and Burman 1946). In this experiment, eight factors were screened in twelve combinations organized according to the Placket-Burman Design matrix as shown in Table 1. Using Microsoft Excel, statistical t-vales for equal unpaired samples were calculated for the determination of variable significance.
Box-Behnken design
It was used to obtain the optimum levels of the key factors determined by the Plackett-Burman design. Each factor was examined at three levels, high (+1), low (−1) and basal (0) (Box and Behnken 1960). This design was carried out according to the matrix represented in Table 2 (Liaqat et al. 2018). For predicting the optimum point, a 2nd-order polynomial model was fitted to correlate a relationship between the independent factors and the response (chitosanase production). For the three factors, the used equation is:
where y is the predicted response (chitosanase production); b0 is the model constant; x1, x2, x3 are the independent factors; b1, b2, b3 are the linear coefficients; b12, b13, b23 are the cross-product coefficients; b11, b22, b33 are the quadratic coefficients.
The predicted optimum concentrations of the three significant factors were calculated using Microsoft Excel. The optimum value of chitosanase activity was determined by the solver function of Microsoft Excel. Three-dimensional surface plots were established by Statistica software.
Production of chitosanase by solid state fermentation (SSF)
Five grams of raw shrimp heads and shells, wheat bran and sugarcane bagasse was transferred to 250-ml Erlenmeyer flasks and moistened with 5 ml of media, then sterilized at 121°C for 15 min. After sterilization, each flask was inoculated with 1 ml of fungal spore suspension for enzyme production and then incubated at 25°C for 7 days.
At the end of the incubation period, the fermentation mass was extracted by the simple method of extraction using distilled water as extracting agent (Shehata and Abd El Aty 2015). The filtrate was used for the estimation of chitosanase activity and protein content. Different types and volumes of moistening agents and different incubation periods were studied.
Fractional precipitation of chitosanase by salting out with ammonium sulphate
The crude chitosanase solution was kept in an ice bath. This was followed by fractional precipitation of the enzyme produced from SSF by salting out with ammonium sulphate (Chasanah et al. 2011), the protein content and chitosanase activity of each fraction were measured.
Physical, chemical and kinetic characterization of chitosanase
Effect of enzyme concentration
Different enzyme concentrations from 3.13 to 12.52 mg/ml were used, then the chitosanase activity was measured (EL-Sayed et al. 2011). Each reaction mixture was incubated at 37°C for 30 min.
Effect of substrate concentration
Different substrate concentrations from 0.4 to 1.6 % (w/v) were used, and then the chitosanase activity was measured (Liang et al. 2016). KM and Vmax of chitosanase were estimated by linear regression technique utilizing Lineweaver–Burk plot according to the equation: (Gooch 2011)
where V is the enzyme velocity (activity), KM is the Michaelis–Menten constant, Vmax is the maximum enzyme activity, and S is the substrate concentration.
Effect of the incubation pH
The pH ranges from 5 to 6 of the chitosanase activity were determined by using two buffers (0.2 M of acetate buffer and of phosphate buffer) (Prakash and Gopal 2017).
Effect of the incubation temperature
Enzyme reactions were carried out at different incubation temperatures (25 to 60°C) for 30 min (Kassem et al. 2013).
Thermal stability
The enzyme solutions were pre-incubated for different time intervals (15, 30 and 60 min) at different temperatures (from 40 to 80°C) in the absence of substrate, and then the residual activity was measured (Kassem et al. 2013).
Effect of some activators and inhibitors on chitosanase activity
They were added to each reaction mixture at different concentrations (0.1, 0.01, 0.001 M), and the residual activity was measured and compared to the control. The tested substances were ZnSO4, CuSO4, CaCl2 and EDTA (Prakash and Gopal 2017).
Evaluation of cytotoxic effect of chitosanase on mammalian cell lines: MCF-7 cells (human breast cancer cell line)
According to the procedure described by Mohan et al. (2021), the chitooligosaccharides produced by the partially purified chitosanase were used in this experiment which was performed at the Institute of Graduate Studies and Research, Alexandria, Egypt. Mammalian cell lines were obtained from VACSERA Tissue Culture Unit. The cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated foetal bovine serum, 1% L-glutamine, HEPES buffer and 50 μg/ml gentamycin. All cells were maintained at 37°C in a humidified atmosphere with 5% CO2 and were sub-cultured two times a week. Cytotoxicity was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay by seeding the cells in a 96-well plate at a cell concentration of 1×104 cells per well in 100 μl of growth medium. A fresh medium containing different concentrations of the test sample was added after 24 h of seeding. Serial twofold dilutions of the tested chemical compound were added to confluent cell monolayers. The microtiter plates were incubated at 37°C in a humidified incubator with 5% CO2 for a period of 48 h. Three wells were used for each concentration of the test sample. Control cells were incubated without the test sample and with or without DMSO. After incubation of the cells at 37 °C, various concentrations of the sample were added, and the incubation was continued for 24 h, and viable cell yield was determined by a colorimetric method. In brief, after the end of the incubation period, the media were aspirated and the crystal violet solution (1% (w/v)) was added to each well for at least 30 min. The stain was removed and the plates were rinsed using tap water until all the excess stain was removed. Then, glacial acetic acid (30% (v/v)) was added to all wells and mixed thoroughly, and the absorbance of the plates was measured after gently shaking on a microplate reader (TECAN, Inc.), using a test wavelength of 490 °nm. All results were corrected for background absorbance detected in wells without added stain. Treated samples were compared with the cell control in the absence of the tested compounds. All experiments were carried out in triplicate. The cell cytotoxic effect of each tested compound was calculated. The optical density was measured with the microplate reader (SunRise, TECAN, Inc. USA) to determine the number of viable cells and the percentage of viability was calculated as [1 − (ODt / ODc) × 100 % where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells. The relationship between surviving cells and drug concentration is plotted to get the survival curve of each tumour cell line after treatment with the specified compound. The 50% inhibitory concentration (IC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots of the dose-response curve for each conc. using Graphpad Prism software (San Diego, CA. USA).
Results
Isolation and screening of chitosanase-producing fungi
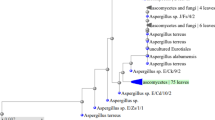
Seven chitosanase-producing fungi were isolated. The most potent chitosan-degrading fungus which formed the largest clear zone on a CDA plate was selected and identified as Fusarium oxysporum D18 and the fungal strain nucleotide sequences were deposited in GenBank as Fusarium oxysporum D18 with accession number OL343607. Construction of the phylogenetic tree was done using the maximum likelihood method as shown in Fig. 1.
Studying the effect of some physiological factors on the chitosanase produced by Fusarium oxysporum D18
Under static incubation condition, the highest chitosanase activity (1.165U/ml) was obtained from Fusarium oxysporum D18 as compared to that obtained under shaken conditions after 7 days of incubation and the activity decreased gradually reaching the lowest activity after 11 days as shown in Table 3. Each treatment was carried out in triplicate, and the results obtained throughout the work were the arithmetic mean of at least 2 experiments.
As shown in Table 3, the enzyme activity of the culture filtrate was differentially affected by the nature of the carbon source. The most preferable carbon source for Fusarium oxysporum D18 was lactose, producing high chitosanase activity (1.191 U/ml) with maximum protein content (2.428 mg/ml), followed by chitosan.
Also, by testing of different nitrogen sources on the chitosanase production, it was found that ammonium chloride was the most preferable nitrogen source, yielding maximum chitosanase activity (1.220 U/ml) with protein content (2.574 mg/ml) followed by ammonium sulphate yielding chitosanase activity (1.133 U/ml) as shown also in Table 3.
Statistical optimization of the physicochemical parameters influencing chitosanase production by Fusarium oxysporum D18
Plackett-Burman design
All experiments were performed in duplicates and the average results of chitosanase activity were measured. The main effect of each variable on enzyme production was estimated and presented graphically in Fig. 2.
According to these suggestions, it can be predicted that the optimum medium for producing an extracellular chitosanase from the culture of Fusarium oxysporum D18 with a relatively high activity was (g/l): KH2PO4 (1.5), MgSO4 (0.25), lactose (13), NH4Cl (1.26), pH (7), using a 5-day-old inoculum, inoculum size (60 ml/l) and medium volume (35 ml).
On the basis of statistical analysis, the three variables evidencing the most significant effects on chitosanase production were MgSO4 (t-value= 1.733), pH (t-value =1.678) and lactose (t-value =1.454).
Box-Behnken design
In this experiment, the three significant factors, MgSO4, lactose and pH, were examined at three levels, which were high (+1), low (−1) and basal (0) included 15 trials. In all of these trials, non-significant factors were used at their near optimum levels, obtained from the Plackett-Burman design. The results were illustrated in surface plots in Fig. 3 to clarify the interaction between the three significant factors and chitosanase activity (response). The optimum levels of the three tested factors that were predicted to give the highest chitosanase activity were as follows (g/l): MgSO4 (0.269), lactose (18) and pH (6.68).
Production of chitosanase by solid state fermentation (SSF)
The effect of different substrates on chitosanase production is shown in Fig. 4. The highest chitosanase activity (1.723 U/ml) was obtained when raw shrimp heads and shells were used, but wheat bran gave the lowest activity (0.283 U/ml).
By optimizing the (SSF) process for production of the enzyme, the highest chitosanase activity (2.246 U/ml) was obtained when raw shrimp heads and shells were moistened with 8 ml of the optimized medium after 5 days of incubation with (1.13 folds) that of the optimized liquid cultures as illustrated in Table 4.
Purification of chitosanase produced by Fusarium oxysporum D18 by salting out with ammonium sulphate
An extracellular chitosanase was isolated and purified from SSF medium of Fusarium oxysporum D18 by ammonium sulphate saturation. The results in Table 5 indicate that fractional precipitation with ammonium sulphate brought about 15.13% protein recoveries.
The recovered protein showed its peak value at the fraction precipitated with 75% ammonium sulphate saturation. This fraction has the highest protein content (7.83 mg/ml) represented about 28.57 % of the total recovered protein. The chitosanase activity of the fraction precipitated with different ammonium sulphate saturations increased gradually up to the 75% fraction, where the maximum activity (3.44 U/ml) was obtained. The activity of this fraction was 26.72 % of the total recovered activity. Higher or lower ammonium sulphate saturation showed lower chitosanase activity. The total recovered activity obtained by ammonium sulphate fractions was 9.69 % of the activity present in the crude enzyme solution.
Physical, chemical and kinetic characterization of chitosanase
Influence of enzyme protein concentration on chitosanase was assayed toward a parallel relationship that occurred between the enzyme concentration and chitosanase; the maximum specific activity of the chitosanase (0.374 U/mg protein) was obtained at an enzyme protein concentration of 9.391 mg/ml. Lower protein concentrations showed a decrease in enzyme activity.
Substrate (chitosan) concentration dependence of the enzyme activity was also characterized with a parallel relationship as shown in Fig. 5 which represents the Michaelis-Menten non-linear regression curve. The maximum activity was obtained at a substrate concentration of 1.2 % (w/v) and the chitosanase activity at that point reached 3.498 U/ml with a specific activity of 0.372 U/mg protein. Further increases in substrate concentration yielded a slightly lower enzyme activity. The maximum value of enzymatic activity (Vmax) was approximately 0.430 U/mg protein as extrapolated by the Lineweaver–Burk plot. The value of KM is the substrate concentration required to attain half of the maximum enzyme velocity. The value of KM thus obtained was 0.26 % (w/v). Smaller KM value is a representative of powerful affinity towards substrate. If an enzyme has a small value of KM, it achieves its maximum catalytic efficiency at low substrate concentrations. Hence, the smaller the value of KM, the more efficient is the catalyst.
Influence of pH on the chitosanase stability was assayed toward soluble chitosan under limitedly narrow range of values (pH 5–6). As expected, the optimum pH value of the reaction was 5.6 at which the maximal enzyme activity was recorded (0.366 U/mg protein) with relative chitosanase activity (100%). Above or below this pH value, the activity of the partially purified enzyme was gradually decreased.
Temperature dependence of the enzyme activity was characterized with pronounced optimum activity (0.390 U/mg protein) at 50°C after salting out with ammonium sulphate. A further increase in temperature resulted in a gradual decrease in activity.
The thermostability of the partially purified chitosanase was studied; the results indicated that the chitosanase was stable over a wide range of temperatures. The stability of it in the absence of chitosan depends on the temperature of heating and the time of exposure. The enzyme became more activated when exposed to 50°C for 15 and 30 min, and this activity increased by 39.27 and 20.49%, respectively, more than that of the control. The enzyme was nearly not affected by increasing the temperature of heat treatment to 60°C for 15 min, but it retained about 27% of its original activity after 60 min. The enzyme was thermostable and retained about 55 and 30% of its original activity after heating at 70°C for 15 and 30 min of exposure, respectively.
The partially purified chitosanase was assayed in presence of some metal ions (activators and inhibitors). The results indicated that the enzyme was activated in the presence of Ca+2. While the results showed that Zn+2, Cu+2 ions and EDTA inhibited chitosanase activity. 0.001 M of CaCl2 slightly increased the activity by about 8%, and by increasing the concentration to 0.01 and 0.1 M, it activated the enzyme by 20.50 and 28.70%, respectively. 0.001 M of CuSO4 brought about 5% increase in the enzyme activity, and increasing the concentration to 0.01 and 0.1 M resulted in a decrease in the activity, reaching 5.74 and 17.06%, respectively.
On the other hand, 0.01 M of ZnSO4 reduced the relative activity by about 35.85%, and 0.01 M of EDTA brought about 80% inhibition of the enzyme activity, while 0.1 M of EDTA totally inhibited the enzyme activity.
Evaluation of the cytotoxic effects of the partially purified chitosanase on mammalian cell lines: MCF-7 cells (human breast cancer cell line)
In this experiment, the chitooligosaccharides, which are produced by the partially purified chitosanase, were used to estimate an antitumour activity against mammalian cell lines and MCF-7 cells (human breast cancer cell line) and they had the highest cytotoxicity inhibitory effect against them at concentration of about IC50 = 448 μg/ml. Where IC50 is the concentration required to cause toxic effects in 50% of intact mammalian cells, it was estimated from graphic plots of the dose-response curve for each conc. At this concentration, the cells had the lowest viability (30.21%).
Discussion
On studying the effect of some physiological factors on the chitosanase produced by Fusarium oxysporum D18, the highest activity of chitosanase produced by Fusarium oxysporum D18 (1.165U/ml) was obtained after 7 days of static incubation. This result was in agreement with Shehata and Abd El Aty (2015), who reported that the optimum incubation period for the chitosanase production by Chaetomium globosum KM651986, Aspergillus fumigatus and Aspergillus terreus were 7 days at 28°C.
In contrast, it was demonstrated by Singh and Vidyasaga (2017) that colloidal chitosan was the best inducer for chitosanase production by Aspergillus fumigatus. However, Abdel-Aziz et al. (2014) showed that molasses is the best C-source for chitosanase activity by Mucor rouxii and biomass yield followed by chitosan.
The majority of microorganisms produced chitosanase by induction (Pagnoncelli et al. 2010; Hosny et al. 2016), but some microorganisms constitutively produced chitosanase without chitosan. Our strain used in this work also produced the enzyme in the absence of chitosan as an inducer and this characteristic has advantages at the fermentation stage because chitosan increases the viscosity of the culture medium and makes production costs high (Choi et al. 2004).
These results were different from those obtained by Singh and Vidyasaga (2017) who reported that yeast extract was the optimal nitrogen source for Aspergillus fumigatus.
Statistical evaluation of the physicochemical parameters influencing chitosanase production by Fusarium oxysporum D18 was carried out by using Plackett-Burman and Box-Behnken designs.
By applying Plackett-Burman design, the most significant factors affecting chitosanase production were MgSO4, pH and lactose. It was conducted that the highest chitosanase production (1.994 U/ml) was induced by the medium composition g/L: KH2PO4 (1.5), MgSO4 (0.269), lactose (18), NH4Cl (1.26), pH (6.68), using a 5-day-old inoculum. These results were in accordance with those obtained by Zhang and Zhang (2013), who found that chitosanase production by Aspergillus fumigatus YT-1 was significantly affected by MgSO4. Zhang et al. (2012) reported that pH factor had a significant effect on chitosanase production by Aspergillus sp. QD-2.
On production of chitosanase by (SSF), the results obtained in this manuscript were in agreement with those results previously noted by Nidheesh et al. (2015), who reported that Puroureocillium lilacinum CFRNT12 produced chitosanase with an activity of 2.34 U/g IDS (initial dry substrate) under SSF by the use of solid shrimp by-product as a substrate. On the other hand, wheat bran was chosen by Xu et al. (2021) as a supplementary carbon source for high chitosanase production by Streptomyces.
Purification of chitosanase produced by Fusarium oxysporum D18 was performed by salting out with ammonium sulphate; the crude extract of chitosanase produced from SSF medium of Fusarium oxysporum D18 was precipitated with finely powdered 75% ammonium sulphate saturation.
El-Sayed et al. (2012) reported that using acetone for precipitation of chitosanase revealed unsuitability as precipitating agent due to the poor yield obtained relative to the crude enzyme. Hence, the crude enzyme was precipitated by using ammonium sulphate with different saturations. Also, chitosanase produced by Gongronella butleri was partially purified by ammonium sulphate precipitation (Seki et al. 2019). Pagnoncelli et al. (2010) found that the crude chitosanases hydrolyzed soluble chitosan into biofunctional oligomers (COS) and the use of crude enzymes from Paenibacillus ehimensis B-23118 instead purified ones were of industrial interest because enzyme purification steps were expensive.
Upon studying of some physical, chemical and kinetic characterization of chitosanase, it was found that the highest activity of chitosanase produced by Fusarium oxysporum D18 was observed at a substrate concentration of (1.2 % (w/v)). The same result was obtained by Sarni and Dali (2016), who reported that the optimum concentration of the substrate required for maximum chitosanase activity produced by Klebsiella sp. was 1.0 % (w/v). Cheng and Li (2000); Aktuganov et al. (2003); Chen et al. (2005) reported that the optimum concentration of the substrate required for maximum chitosanase activity produced by Aspergillus sp., Bacillus sp. 739 and Aspergillus sp. CJ22-326, respectively, was from 0.5 to 1.0 % (v/v). KM (0.26% (w/v)) and Vmax (4.04 U/ml) of chitosanase were estimated by linear regression technique utilizing Lineweaver–Burk method (Gooch 2011). Cao et al. (2022a, b) found that KM and Vmax of the recombinant chitosanase (PoCSN75A) were 0.27 mg/ml and 4.36 U/ml respectively. The KM and Vmax of the chitosanase (Csn-SH) were 0.50 mg/mL and 140.05 μmol mg–1 min–1, respectively (Cui et al. 2021).
In this manuscript, chitosanase showed the highest activity at pH 5.6. This result showed an agreement with Jiang et al. (2021) who reported that the recombinant chitosanase (PbCsn8) was most active at pH 5.5. Also, Hosny et al. (2016) reported that the optimum pH of the chitosanase from Dothideomycetes sp. css035 and Aspergillus fumigatus KB-1 using acetate buffer was pH 5.5. Also, Cao et al. (2022a, b) found that pH 5.5 was optimum for chitosanase produced by Penicillium oxalicum M2. In contrast, Guo et al. (2022) reported that the optimum pH that produced the highest activity of the recombinant chitosanase (SaCsn46A) was 6.2.
The influence of temperature on chitosanase was also examined and the result obtained in this study was in agreement with the study carried out by El-Sherbiny (2011) who reported that the activity of the partially purified chitosanase from Chaetomium globosum KM651986 and Streptomyces cyaneogriseus was found optimal at 50°C and at lower or higher temperatures, the activities were reduced. Chiang et al. (2003) found that the optimum activity for the purified chitosanase produced from Bacillus sp. was at 45 °C. Kitasatospora setae KM-6054 chitosanase which was cloned in E. coli had the highest activity at 60 °C (Xu et al. 2023). Also, recombinant chitosanase (CsnS) showed its highest activity at 60°C (Zheng et al. 2021).
Thermostability of chitosanase produced by Fusarium oxysporum D18 was examined. The enzyme was thermostable and retained about 55% of its original activity after heating at 70°C for 15 min.
These results indicated that chitosanase had a thermal stability and agree with the results obtained by El-Sherbiny (2011) who reported that the purified chitosanase produced by Streptomyces cyaneogriseus was stable at 40 and 50°C when it was incubated without substrate for 30–90 min, and gradually inactivated at 60 and 70°C and significantly lost activity above 70°C. Han et al. (2018) reported that the engineered chitosanase (CsnA) from Renibacterium sp. QD1 retained about 40% activity after being kept at 60°C for 60 min. The recombinant chitosanase (CsnQ) could retain 77.72 and 71.79% of the original activity after 60 min of incubation at 20 and 30 °C, respectively. The residual activity of purified CsnQ reduced dramatically after 60 min of incubation at temperatures above 30 °C (Ma et al. 2020).
The activity of the partially purified chitosanase was tested in presence of some metal ions. The enzyme preparations were activated by Ca2+ ions and inactivated by Zn+2, Cu+2 ions and EDTA.
The results of this study showed an agreement with those obtained by Chasanah et al. (2011) who reported that the chitosanase produced by Aeromonas media KLU 11.16 was activated by the presence of Ca+2 and was inhibited by Zn2 +, Cu2+ and EDTA (concentration of 1mM of EDTA decreased significantly (100%) the chitosanase activity). In contrast, Aktuganov et al. (2022) found that chitosanase produced by Bacillus Thuringiensis B-387 was slightly suppressed by Ca+2. Cheng and Li (2000) reported that the purified chitosanase of Aspergillus sp. was greatly enhanced by Cu+ ions at low concentrations.
This result differed from that obtained by Liang et al. (2016) who reported that the activity of chitosanase produced by Bacillus mycoides was nearly unaffected by Ca2+, but agreed with them when they reported that the enzyme was inhibited by Cu+.
An antitumour activity of chitooligosaccharides produced by chitosanase was applied to the MCF-7 cells (human breast cancer cell line), and they had a cytotoxicity inhibitory effect against them at concentration about IC50 = 448 μg/ml, where IC50 is the concentration required to cause toxic effects in 50% of intact mammalian cells. At this concentration, the cells had the lowest viability (30.21%).
Other results were obtained by Hashem et al. (2018) who reported that the chitooligosaccharides produced by chitosan hydrolysis using chitosanase obtained from Dothideomycetes sp. NRC-SSW had a stronger cytotoxic activity against human liver cancer cells (Hep-G2) in comparison to breast cancer cell line (MCF7) as the IC50 of Hep-G2 was 12 μg/ml while the IC50 of MCF-7 was 85.5 μg/ml. Also, Chokradjaroen et al. (2018) reported that chitooligosaccharides have a cytotoxic effect against human uterine cervix cancer cell line (HeLa cells), human breast adenocarcinoma cell line (MCF-7 cells) and human lung cancer cell line (H460 cells). The IC50 of chitooligosaccharides against cancer cells HeLa, MCF-7 and H460 were approximately 2.3, 2.0 and 4.1 mg/ml, respectively. Chitooligosaccharides can easily permeate the cancer cells. Chitooligosaccharides derivatives greatly limit the formation of new blood vessels in tumours, thereby blocking tumour progression and metastasis. It has the ability to decrease free radicals in normal cells, which are implicated in cellular damage (Pavan et al. 2022).
Conclusion
In this study, a chitosanase-producing fungus, Fusarium oxysporum D18, was isolated from soil, and it showed high chitosanase activity after optimization by statistical evaluation using Plackett-Burman and Box-Behnken designs of different physical and chemical factors. Physical and chemical properties of the partially purified enzyme were performed leading to a highly thermostable antitumour chitosanase with high cytotoxicity against mammalian cell lines: MCF-7 cells.
Availability of data and materials
Available from the Botany and Microbiology Department.
References
Abdel-Aziz SM, Kahil T, Keera AA (2014) Kinetic behavior of free and in situ immobilized chitosanases produced by the Fungus Mucor rouxii. World Appl Sci J 30(1):01–09
Abo Elsoud MM, El Kady EM (2019) Current trends in fungal biosynthesis of chitin and chitosan. Bull Natl Res Cent 43:59
Aktuganov G, Shirokov A, Melent` A, (2003) Isolation and characterization of chitosanase from the strain Bacillus sp. 739. Appl Biochem Microbiol 39:469–474
Aktuganov GE, Galimzianova NF, Gilvanova EA, Pudova EA, Kuzmina LYu, Melentiev AI, Safina VR (2019) Purification and characterization of exo-β-1,4-glucosaminidase produced by chitosan degrading fungus, Penicillium sp. IB-37- A. World J Microbiol Biotechnol 35:18
Aktuganov G, Safina V, Galimzianova N, Gilvanova E, Kuzmina L, Melentiev A, Baymiev A, Lopatin S (2022) Constitutive chitosanase from Bacillus Thuringiensis B-387 and its potential for preparation of antimicrobial chitooligomers. World J Microbiol Biotechnol 38(10):167
Beier S, Bertillsson S (2013) Bacterial chitin degradation – mechanisms and ecophysiological strategies. Review Front Microbiol 4:149
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technomet 2:455–475
Cao S, Gao P, Xia W, Liu S, Liu X (2022a) A) Cloning and characterization of a novel GH75 family chitosanase from Penicillium oxalicum M2. Process Biochem 120:41–52
Cao S, Gao P, Xia W, Liu S, Wang B (2022b) B) A novel chitosanase from Penicillium oxalicum M2 for chitooligosaccharide production: purification, identification and characterization. Mol Biotechnol 64:947–957
Chang WT, Chen YC, Jao CL (2007) Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour Technol 98:1224–1230
Chasanah E, Patantis G, Zilda DS, Ali M, Risjani Y (2011) Purification and characterization of Aeromonas media KLU 11.16 chitosanase isolated from shrimp waste. J Coastal Develop 15:104–111
Chen X, Xia W, Yu X (2005) Purification and characterization of two types of chitosanase from Aspergillus sp. CJ22-326. Food Res Int 38:315–322
Chen X, Fang X, Xia W (2008) Strain improvement and optimization of the medium composition of chitosanase-producing fungus Aspergillus sp. CJ 22–326. Afr J Biotech 7(14):2501–2508
Cheng C, Li C (2000) An Aspergillus chitosanase with potential for large-scale preparation of chitosan oligosaccharides. Biotech Appl Biochem 32:197–203
Chiang CL, Chang CT, Sung HY (2003) Purification and properties of chitosanase from a mutant of Bacillus subtilis IMR-NK1. Enzyme Microb Technol 32:260–267
Choi YJ, Kim EJ, Piao Z, Yun YC, Shin YC (2004) Purification and characterization of chitosanase from Bacillus sp. strain KCTC 0377BP and its application for the production of chitosan oligosaccharides. Appl Environ Microbiol 70(8):4522–4531
Chokradjaroen C, Theeramunkong S, Yui H, Saito N, Rujiravanit R (2018) Cytotoxicity against cancer cells of chitosan oligosaccharides prepared from chitosan powder degraded by electrical discharge plasma. Carbohydr Polym 201:20–30
Cruz CR, Sanchez PO, Rojas ANG, Gymez RM, Rojas ALI (2004) Chitosanase activity in Bacillus thuringiensis. Folia Microbiol 49:94–96
Cui D, Yang J, Lu B, Shen H (2021) Efficient preparation of chitooligosaccharide with a potential chitosanase Csn-SH and its application for fungi disease protection. Front Microbiol 12:682829
Durkin CA, Mock T, Armbrust EV (2009) Chitin in diatoms and its association with the cell wall. Eukaryot Cell 8:1038–1050
EL-Sayed M, El-Sayed S, Shousha W, Shehata A, Omar N (2011) Isolation and characterization of chitosanase enzyme from different parts of some higher plants. J Am Sci 7(3):713
El-Sayed S, El-Sayed E, Shousha W, Shehata A, Omar N (2012) Production of novel antitumor chitooligosaccharides by using purified chitosanases from Capsicum annuum leaves. J Basic and Appl Sci 6(4):1–15
El-Sherbiny E (2011) Purification and characterization of chitosanase enzyme from Streptomyces cyaneogriseus. Asian J Biol Sci 4(1):15–24
Gao XA, Ju WT, Jung WJ, Park RD (2008) Purification and characterization of chitosanase from Bacillus cereus D-11. Carbohydr Polym 72:513–520
Gooch JW (2011) Lineweaver-Burk Plot. In: Gooch JW (eds) Encyclopedic Dictionary of Polymers Han Y, Gao P, Yu W and Lu X (2018) N-Terminal seven-amino-acid extension simultaneously improves the pH stability, optimal temperature, thermostability and catalytic efficiency of chitosanase CsnA. Biotechnol Lett 40(1):75–82
Guo J, Wang Y, Gao W, Wang X, Gao X, Man Z, Cai Z, Qing Q (2022) Gene cloning, functional expression, and characterization of a novel GH46 chitosanase from Streptomyces avermitilis (SaCsn46A). Appl Biochem Biotechnol 194:813–826
Han Y, Gao P, Yu W, Lu X (2018) N-Terminal seven-amino-acid extension simultaneously improves the pH stability, optimal temperature, thermostability and catalytic efficiency of chitosanase CsnA. Biotechnol Lett 40(1):75–82
Hashem AM, Ismail SA, Hosny AMS, Awad G, Ismail SA (2018) Optimization of Dothideomycetes sp. NRC-SSW chitosanase productivity and activity using response surface methodology. Egypt J Chem 61:973–987
Hosny AMS, Ismail SAI, Abdel-Naby MA, Hashem AM (2016) Evaluation of culture conditions for chitosanase production by Dothideomycetes sp. css035. Inventi Impact: Pharm Biotech Microbiol 4:180–189
Jiang Z, Ma S, Guan L, Yan Q, Yang S (2021) Biochemical characterization of a novel bifunctional chitosanase from Paenibacillus barengoltzii for chitooligosaccharide production. World J Microbiol Biotechnol 37:83
Kassem M, El-Toukhy N, Abdelnabi R, Fanaki N, Abou-Shleib H (2013) An environmental chitosanase-producing isolate from Alexandria governorate, Egypt: its molecular characterization, biofilm formation and enzyme purification and characterization. New Egyptian J Microbiol 36:177–197
Liang T, Chen W, Lin Z, Kuo Y, Nguyen AD, Pan P, Wang S (2016) An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int J Mol Sci 17:2–14
Liaqat F, Bahadir PS, Elibol M, Eltem R (2018) Optimization of chitosanase production by Bacillus mojavensis EGE-B-5.2i, J. Basic Microbiol 58(10):836–847
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ma C, Li X, Yang K, Li S (2020) Characterization of a new chitosanase from a marine Bacillus sp. and the antioxidant activity of its hydrolysate. Mar Drugs 18:126
Miguez N, Kidibule PE, Santos-Moriano P, Ballesteros AO, Lobato MF, Plou FJ (2021) Enzymatic synthesis and characterization of different families of chitooligosaccharides and their bioactive properties. Appl Sci 11:3212
Mohan KM, Vasudevarao P, Bramanandam M, Appa RP (2021) Chitooligosaccharides induce apoptosis in human breast cancer cell. Carbo Poly Tech and Appl 2:100–77
Muzzarelli RA, Boudrant J, Meyer D, Manno N, Marchis De, Paoletti MG (2012) Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: atribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr Polym 87:995–1012
Myat AA, Lae KZW, Ngwe DH (2019) Extraction and characterization of chitosanase enzyme from Bacillus megaterium under liquid state fermentation. J Myanmar Acad Arts Sci. XVII(1A):573
Nidheesh T, Kumar PG, Suresh PV (2015) Enzymatic degradation of chitosan and production of D-glucosamine by solid substrate fermentation of exo-β-D glucosaminidase (exo-chitosanase) by Penicillium decumbens CFRNT15. Int Biodeterior Biodegrad 97:97–106
Pagnoncelli MGB, de Araújo NK, da Silva NMP, de Assis CF, Rodrigues S, de Macedo GR (2010) Chitosanase production by Paenibacillus ehimensis and its application for chitosan hydrolysis. Braz Arch Biol Technol 53(6):1461–1468
Pavan SR, Venkatesan J, Kim SK, Prabhu A (2022) Anticancer effects of chitooligosaccharides. In: Kim SK (ed) Chitooligosaccharides. Springer, Cham. https://doi.org/10.1007/978-3-030-92806-3_8
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Prakash N, Gopal S (2017) Characterization of an extracellular chitosanase from the soil bacterium Bacillus cereus CH12. IIOAB J 8(1):1–6
Sarni N, Dali S (2016) Production and characterization chitosanase of sponge symbiont Bacteria Klepsiella sp. To Hydrolyze chitosan be chitooligosaccarides. Int J Marina Chimica Acta 17:1441–2132
Seki K, Nishiyama Y, Mitsutomi M (2019) Characterization of a novel exo-chitosanase, an exo-chitobiohydrolase, from Gongronella butleri. J Biosci Bioeng 127(4):425–429
Shehata AN, Abd El Aty AA (2015) Improved production and partial characterization of chitosanase from a newly isolated Chaetomium globosum KM651986 and its application for chitosan oligosaccharides. J Chem Pharm Res 7(1):727–740
Singh PS, Vidyasagar GM (2017) Isolation, purification and optimization of chitosanase production from a common mahabubnagar agricultural field fungi Aspergillus fumigatus of telangana state. World J Pharm Pharm Sci 6(9):886–898
Suresh PV (2019) Enzymatic technologies of chitin and chitosan. In: Trincone A (ed) Enzymatic technologies for marine polysaccharides. CRC Press, Taylor and Francis Group, Boca Raton, pp 449–491
Thadathil N, Velappan S (2014) Recent developments in chitosanase research and its Biotechnological applications: a review. Food Chem 150:392–399
Wang SL, Chang TJ, Liang TW (2010) Conversion and degradation of shellfish wastes by Serratia sp. TKU016 fermentation for the production of enzymes and bioactive materials. Biodegradation 21:321–333
Wangtueai S, Worawattanamateeku W, Sangjindavong M, Naranong N, Sirisansaneeyakul S (2006) Isolation and screening of chitosanase producing microorganisms, Kasetsart J. Nat Sci 40:944–948
Weikert T, Niehues A, Cord-Landwehr S, Hellmann MJ, Moerschbacher BM (2017) Reassessment of chitosanase substrate specificities and classification. Nat Commun 8(1):1698
Xu X, Song Z, Yin Y, Zhong F, Song J, Huang J, Ye W, Wang P (2021) Solid state fermentation production of chitosanase by streptomyces with waste mycelia of Aspergillus niger. Ad in Enz Res 9:10–18
Xu Y, Wang H, Zhu B, Yao Z (2023) Biochemical characterization and elucidation action mode of a new endolytic chitosanase for efficient preparation of chitosan oligosaccharides. Biomass Conv Bioref
Zhai X, Yuan S, Yang X, Zou P, Li L, Li G, Shao Y, Abd El-Aty AM, Haclmüftüo A, Wang J (2019) Chitosan oligosaccharides induce apoptosis in human renal carcinoma via reactive oxygen-species-dependent endoplasmic reticulum stress. J Agric Food Chem 67(6):1691–1701
Zhang H, Zhang W (2013) Induction and optimization of chitosanase production by Aspergillus fumigatus YT-1 using response surface methodology. Chem Biochem Eng Q 27(3):335
Zhang H, Sang Q, Zhang W (2012) Statistical optimization of chitosanase production by Aspergillus sp. QD-2 in submerged fermentation. Ann Microbiol 62:193–201
Zheng Q, Meng X, Cheng M, Li Y, Liu Y, Chen X (2021) Cloning and characterization of a new chitosanase from a deep-sea bacterium Serratia sp. QD07. Front Microbiol 12:619731
Acknowledgements
The financial support received from the Botany and Microbiology Department, Faculty of Science, Alexandria University is gratefully acknowledged.
Human and animal rights
No human subjects or livestock were included in this research.
Informed consent
The scientists certify that this study adhered to ethical and professional standards.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Prof. Dr. RM.A.A: participate in the development of the research plan, statistical experiments, statistical tables, figures, preparation, writing and revision of the manuscript. Dr. AE.AE-S: supervising the student in preparing and conducting laboratory and statistical experiments, statistical tables, figures, preparation and revision of the manuscript. DR.M.AE: conducting laboratory and statistical experiments, writing and revision of the thesis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abedin, R.M.A., Abd Elwaly, D.R.M. & Abd El-Salam, A.E. Production, statistical evaluation and characterization of chitosanase from Fusarium oxysporum D18. Ann Microbiol 73, 27 (2023). https://doi.org/10.1186/s13213-023-01731-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-023-01731-w