Abstract
Purpose
In recent years, considerable research has been conducted on the use of plant growth regulators (PGRs) to improve crop yields. Large amounts of PGRs are applied to regulate crop growth. However, few studies have assessed the impact of PGRs, which leave soil residues, on soil microorganisms, especially rhizobia.
Methods
In this study, the influence of inoculation of soybean roots with Sinorhizobium fredii and Bradyrhizobium japonicum exposed to gibberellin A3 (GA3) and mepiquat chloride (MC) on nodule number, nitrogenase activity, and a symbiotic nitrogen fixation gene (fixA) expression was studied. The nitrate content, free amino acids, and nitrate reductase activity of the roots, and seed yield and quality of soybean were measured.
Result
Nodulation was promoted to some extent, whereas nitrogenase activity and fixA expression were inhibited to some extent by treatment with GA3; however, these effects were reversed by treatment with MC. In a pot experiment to study phenotypic characteristics, rhizobia treatment in combination with GA3 or MC significantly improved soybean yield and seed nitrogen content, and increased the root nitrate content, free amino acid content, and nitrate reductase activity.
Conclusion
The results indicated that PGRs, which leave soil residues, have significant positive effects on the growth and activity of soybean and rhizobia.
Similar content being viewed by others
Significance and impact of study
Although plant growth regulators (PGRs) are important factors in regulating legume growth and development
Limited information is available on their effects on legume symbioses with rhizobia. in this study
The impact of the PGRs gibberellin A3 (GA3) and mepiquat chloride (MC) on rhizobia-induced effects in soybean was examined. an understanding of the effects of PGR residues on the soybean–rhizobia symbiosis will be helpful in the evaluation of soil ecological security and rational application of these regulators
Introduction
The legume–rhizobia symbiosis is a specialized plant–microbial symbiotic system in the rhizosphere that helps to supply nitrogen nutrition to the host plant. Legumes can provide a stable environment and energy for microbes. It has been reported that 50–60% of nitrogen nutrition in soybean stems from its symbiosis with rhizobia (Rellán-Alvarez et al. 2010). Nitrogenases, a group of metalloenzymes, play a crucial role in the rhizobia–legume symbiosis (Dixon and Kahn 2004). Normally, rhizobia nitrogenase consists of two components: the homodimeric Fe protein and the tetrameric Mo–Fe protein, which contains the Mo–Fe cofactor. Many studies have reported that the nitrogenase activity of rhizobia is significantly influenced by external factors. A high concentration of NO3−, Fe2+-free (LeVier et al. 1996), or Cu2+-free (Maas et al. 1979) can suppress the nitrogenase activity of nodules in different manners. It has been found that indole acetic acid (IAA), which is secreted by rhizosphere microbes, can improve the nitrogen-fixation ability, thereby increasing the dry weight and yield of sorghum (Ashraf et al. 2011). Therefore, it is important to consider how to improve the nitrogenase activity to increase soybean yield. As early as the 1980s, some researchers found that host plants could secrete flavonoids when infected by rhizobia, which can induce the synthesis and release of nod factors (NFs) in the rhizobia. Many studies have shown that plant growth regulators (PGRs) regulate plant nodulation and nitrogen metabolism. For example, ethylene (ET), abscisic acid (ABA), jasmonic acid (JA), salicylic acid (SA), and brassinolide (BR) negatively affect nodule formation (Tirichine et al. 2007). The root and shoot dry weights, nodule number, nodule weight, nodule nitrogen content, and nitrogenase activity of soybean were improved significantly after foliar spray application of BR and SHK-6 (a type of novel plant growth regulator, the main component is diethyl aminoethyl hexanoate mepiquat). It was also found that the nitrogenase activity of soybean roots was increased by cytokinin (CTK)/gibberellic acid (GA) and CTK/IAA treatments, while the effect of IAA/ABA and GA/ABA treatments was the opposite. It has been shown that pea mutants deficient in GA3 biosynthesis could not form nodules with rhizobia, but the addition of exogenous GA3 in moderation can restore nodule formation (Ferguson et al. 2005). Moreover, it has been suggested that in aluminium (Al)-treated roots, nitrate reductase (NR) activity is increased but the IAA concentration is maintained at the same level as in the pretreatment (Tomioka et al. 2012). However, indole-3-acetyl-L-aspartic acid (IA-Asp), which is a metabolic intermediate of IAA degradation, is not detected in the roots. In calcium-treated roots, NR activity and IA-Asp concentration increases, but the IAA concentration decreases. It has been found that the activity of NR, nitrite reductase, glutamine synthetase, and glutamate synthase decreases significantly (Gangwar et al., 2011); however, the ammonium content and activity of glutamate dehydrogenase are increased by chromium and 100 μM IAA treatments in pea seedlings. The effects of PGRs on rhizobia cells in free-living cultures have also been confirmed. It has been reported that 0.1% D1 (a synthetic phytohormone analogue) suppresses bacterial growth (Kosenko et al. 2001; 2003); however, bactozole (a plant growth stimulant of bacterial origin) at different concentrations (0.001%, 0.01%, and 0.1%) exerted similar effects on the growth of bacteria when grown under a low nitrate concentration (6 mM). There is also evidence for the role of conventional PGRs in nodule structure (Brewin 1993). PGRs play an important role in regulating the grain-filling process and protein accumulation. Yang et al. (2013) demonstrated that the grain-filling rate is positively and significantly dependent on the contents of GA, IAA, and ABA and that the content of free amino acids and activity of glutamine synthetase is dependent on the contents of ABA and GA. In addition, the content of soluble proteins is significantly affected by the contents of ABA and IAA (Yang et al. 2013). Thus, it could be concluded that exogenous hormones can affect the grain-filling process and nitrogen metabolism characteristics by changing the contents of endogenous hormones.
Several genes and proteins have been identified as essential for symbiotic nitrogen fixation by the bacterium Sinorhizobium meliloti and are termed symbiotic nitrogen fixation (fix) genes. Fix genes were first identified in S. meliloti (Mueller and Gonzalez 2010) and later in Bradyrhizobium japonicum, Azorhizobium caulinodans (Wu 2012), Rhizobium leguminosarum bv. viciae (Elsayed et al. 2013), R. leguminosarum bv. trifolii (Miller et al. 2007), and R. leguminosarum bv. phaseoli (Dombrecht et al. 2002). Mutations in any one of the fixA/B/C/X genes of R. meliloti, B. japonicum, or A. caulinodans completely abolish nitrogen fixation. It has been proposed that fixA/B/C/X gene products may be involved in electron transport to nitrogenase (Dai et al. 2014). In a study using mutant plants, it has been suggested that, in addition to the electron transfer flavoprotein, the fixA locus is required for symbiotic efficiency (Delmotte et al. 2014). It has been shown that application of IAA and 2,4-dichlorophenoxyacetic acid in a symbiotic environment can significantly increase the mRNA transcript levels of fix genes in S. meliloti (Bianco, 2010).
In our previous work, we confirmed that PGRs could change the growth rate and structure of rhizobia cells in free-living cultures. However, the effect of PGRs on the influence of rhizobia on nitrogen metabolism, yield, and quality of soybean remains unclear. In this study, we investigated the effect of soybean root inoculation with S. fredii and B. japonicum exposed to GA3 and MC on nodule number, nitrogenase activity, fixA expression, nitrate content, free amino acid content, NR activity, and seed yield and quality.
Materials and methods
Rhizobial strains and PGRs
Two strains of rhizobia, Bradyrhizobium japonicam (strain number GIM 1.94) and Sinorhizobium fredii (strain number GIM1.227), were obtained from the China Agricultural Culture Collection Center (ACCC). GA3 and MC were obtained from our laboratory. Uniform soybean “Suinong 28” seeds were obtained from YiBin University.
Culture media
Yeast mannitol agar (YMA) liquid medium was prepared. The nitrogen-free nutrient solution contained 0.15 g Na2HPO4·2H2O, 0.1 g CaCl2·2H2O, 0.12 g MgSO4·7H2O, 0.1 g K2HPO4, 5 mg ferric citrate, and 1 mL minor elements and diluted with distilled water to 1000 mL, sterilized at 121 °C for 15 min. The stock of minor elements was prepared using 2.86 g H3BO3, 2.03 g MnSO4·4H2O, 0.22 g ZnSO4·7H2O, 0.13 g Na2MoO4·2H2O, and 0.08 g CuSO4·5H2O and diluted with distilled water to 1000 mL. The GA3 mother solution comprised 10.0 g GA3 diluted with absolute ethyl alcohol (100 mL) and was stored at 4 °C until use. The MC mother solution contained 10.0 g MC diluted with distilled water to 100 mL and was stored at 4 °C until use.
Soybean pot experiments
Plant growth regulator GA3 and MC mother solutions at 10−3 and 10−2 (v/v) concentrations, respectively, were added to the YMA liquid medium, then S. fredii and B. japonicum were inoculated and cultured at 28 °C with shaking at 170 rpm for 3 days. The strains in each treatment were collected after centrifugation of the 1 mL bacterial solution at 8000 × g for 10 min, rinsed with sterile water to remove PGRs in the YMA liquid medium, and then suspended in 1 mL sterile water. Soybean seeds were surface-sterilized with 70% ethanol (v/v) for 5 min followed by extensive rinsing with sterile water. The surface-sterilized seeds were inoculated individually with 1 mL bacterial suspension, then planted in an autoclaved mixture (1:1) of perlite and vermiculite in pots (25 cm diameter × 30 cm height). Each pot contained three plants and was supplied daily with 500 mL nitrogen-free nutrient solution. Each treatment was replicated five times. Plants were grown in an artificial climate with sunlight and 60% relative humidity at 28 °C.
Root nodule number and nitrogenase activity in acetylene reduction assay
Plants were carefully uprooted from 08:00 to 09:00 during the soybean pod stage, and the root systems were rinsed thoroughly with sterile water. Nodules of similar number and size in each treatment were counted and detached carefully, rinsed with sterile water, and pooled. Ten nodules, selected at random from the pooled sample, were placed in serum bottles. After removal of 10% of the volume of air from the flask and injection with an equal volume of C2H2 30 min after removing the reaction gases, a gas chromatograph (Agilent 7890A) was used to determine the amount of C2H4 (Kurz, et al., 1975). Each treatment was replicated five times.
Real-time PCR assay for fixA gene expression
Total RNA of rhizobia in the collected nodules (as described in the preceding section) was extracted using the QIAGEN RNeasy Mini Kit. Reverse transcription was performed using the TransScript II First-Strand cDNA Synthesis Super Mix in accordance with the manufacturer’s instructions. All reagents were treated with DEPC on ice to ensure that the entire process was performed on a clean bench. The cDNA was reverse-transcribed and diluted 30 times as a template for the real-time PCR experiment. The PCR mixture was prepared to a total volume of 12.5 μL and included 6.25 μL Eco GreenI fluorescent dye MIX, 0.25 μL forward primer, 0.25 μL reverse primer, 2 μL cDNA first reaction product, and 3.5 μL ddH2O. The fixA gene primers used were forward 5′-CTATGATCTGTTCGCGCTTG-3′ and reverse 5′-AGACGATATCGGGCGTACC-3′. The reference gene primers were forward 5′-CCTACGGGAGGCAGCAG-3′ and reverse 5′-ATTACCGCGGCTGCTGG-3′. Amplifications used the following program: 95 °C for 5 min, 1 cycle; 95 °C for 15 s, 55 °C for 30 s, 5 cycles. After the amplification, melting curve analysis was performed.
Nitrogen metabolism-related indicators
Root samples were collected and frozen in liquid nitrogen for 1 h and then stored at − 40 °C. The frozen samples were used for determination of physiological indicators. Free amino acids were determined using the ninhydrin method (Gerhard et al. 2002), nitrate-nitrogen was determined using the salicylic acid method (Ratushnyak et al. 2011), and NR was determined using the reference method (Yin et al. 2015).
Soybean yield and quality assessment
The yield and quality of soybean were assessed by measuring plant height, pod number per plant, seed number per plant, and 100-seed weight. The nitrogen content of the seeds was determined using the Kjeldahl method.
Data analysis
Data processing and mapping were performed using Microsoft Excel 2003, fixA relative gene expression levels were analysed using SDS 2.3 software, and the SPSS statistical analysis system was used for analysis of variance and significance tests.
Results and discussion
Effects of PGRs and rhizobia on nodule number and nitrogenase activity
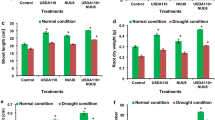
Leguminous plants can form a symbiotic relationship with rhizobium and capture nitrogen in the atmosphere to provide to host plants by forming nodules in the roots. Plant hormones play a key role in this process. GA3 is a kind of plant hormone known to be involved in a variety of biological processes (such as cell growth and germination). Previous studies have also shown that gibberellin is involved in the formation and maturation of root nodules in legumes (Liu H et al., 2018). To study the effects of PGRs on nodulation and nitrogen fixation of soybean infected by rhizobia, GA3 and MC were incorporated separately in combination with S. fredii and B. japonicum in the culture medium. Inoculation with GA3–S. fredii and GA3–B. japonicum increased the nodule number of soybean roots by 12.9% and 10.5% compared with the control, respectively, but the increase was not significant. Many studies have found that plant hormones such as ethylene, jasmonic acid, abscisic acid, and gibberellin can negatively regulate the formation of infection lines and the development of root nodules (Li et al., 2022; Velandia et al., 2022; Lin et al., 2020). Exogenous application of GA3 can restore the phenotypic changes of these plants, suggesting that the formation of root nodules in leguminous plants depends on the effect of GA3. However, in vitro studies have shown that high concentrations of GA3 above the threshold level will in turn inhibit nodulation (Ferguson et al. 2005). These data suggest that the dependence of nodulation on GA3 is regulated by its concentration. It was also reported that exogenous GA3 treatment inhibited ammonium nitrate-induced root hair deformation, infection line formation and nodule development, and the application of GA3 biosynthesis inhibitors saved this phenotypic change. The results showed that GA3 played a negative role in the formation of infection lines and nodule development (Maekawa et al., 2009; Fonouni-Farde et al., 2016; Hung et al., 2016). In contrast, inoculation with MC–S. fredii and MC–B. japonicum decreased the nodule number of soybean roots by 15.8% and 5.6% compared with the control, respectively (Fig. 1A).
Effects of plant growth regulators (PGRs) and rhizobia on nodule number and nitrogenase activity. A Influence of PGRs (gibberellin A3 [GA3] and mepiquat chloride [MC]) and inoculation with rhizobia (Sinorhizobium fredii and Bradyrhizobium japonicum) on the nodule number of soybean roots. B Influence of rhizobial inoculation combined with PGRs (GA3 and MC) on the nitrogenase activity of soybean roots. Each bar is the mean of eight measurements of nodule number and nitrogenase activity. Student’s t test was used to evaluate the difference between the control and the treatments
Although the differences were statistically non-significant, the results were consistent. This study represented a preliminary exploration and the sample size was relatively small. Therefore, a larger sample size in a future study will provide a better understanding of the effect of PGRs on nitrogen fixation by rhizobia and infected legumes.
In contrast to the nodule number, the nitrogenase activity of soybean roots inoculated with GA3–S. fredii, GA3–B. japonicum, MC–S. fredii, or MC–B. japonicum were decreased by 11.1% and 5.9% and increased by 18.1% and 6.2%, respectively, compared with the control. However, the changes in nitrogenase activity were not significant (Fig. 1B). These findings were consistent with previous studies. There are many potential reasons for the differences being not statistically significant. The main reason may be that the sample size was small and that variation among individuals may have impacted on the statistical results. Previous studies have shown that the growth rate and cell structure of free-living rhizobia cultures may be regulated by GA3 and MC, and the nodule number and nitrogenase activity of soybean roots were affected after inoculation with the rhizobia. It has been observed that R. japonicum and R. phaseoli differ in their responses to PGRs in a manner depending on the type and concentration (Stearn et al. 1980). Furthermore, it has been demonstrated that auxin, which is produced by growth-promoting bacteria, significantly increases nitrogenase activity (Egamberdiyeva et al., 2004). In contrast to previous studies, to avoid the interference of plant secretions and exogenous sources that were produced by the applied rhizobia, in the present study rhizobia were applied simultaneously with PGRs. The present study showed that nodulation of soybean roots was promoted by inoculation with GA3–rhizobia to varying degrees, but nitrogenase activity in the soybean roots was inhibited. The opposite results were observed for MC. This might be because the expression of certain regulatory signals of rhizobia is stimulated or inhibited by PGRs. It has been demonstrated previously that expression of the receptor for activated protein C kinase 1 mRNA, which is responsible for nodule meristem initiation and rhizobia infection, is induced by PGRs (namely, auxins, ABA, CTK, and GA) (Islas-Flores et al., 2012). In a previous study, S. meliloti wild-type cells were treated with 0.5 mM IAA or a derivative S. meliloti strain that overproduced IAA (RD64) was used. Medicago truncatula plants inoculated with RD64 (Mt-RD64) showed increases in acetylene reduction activity and root dry weight (Imperlini et al. 2009).
Effect of PGRs–rhizobia on fixA gene expression
The expression levels of fixA from S. fredii were significantly upregulated by GA3 and MC treatment compared with that of the S. fredii control (Fig. 2). However, fixA was significantly downregulated by MC treatment in combination with B. japonicum. It is evident that PGRs have different effects on the transcription level of fixA among different rhizobia, and the present experiments showed that this effect is significant. In previous studies, we have preliminarily showed that GA3 and MC have the same effect on the level of nodulation nitrogen fixation among rhizobia, although not with statistical support. The difference in fixA transcription level shows the specificity of PGRs. If studies with a large sample size support this conclusion, the mechanism of PGR-specific regulation of the fixA transcription level should be investigated in future studies. Previous research has demonstrated that the operon formed by the fixA, fixB, fixC, and fixX genes (Earl et al. 1987) is essential for nitrogen fixation by S. meliloti and that homologous genes are present in Sinorhizobium and Bradyrhizobium. The present results showed that expression of fixA of S. fredii was stimulated by GA3, but significantly inhibited by MC in B. japonicum. Wheatley et al. (2020) also found not only 27 genes were annotated as nif and fix in Rhizobium leguminosarum, but also 603 genetic regions (593 genes, 5 transfer RNAs, and 5 RNA features) related to nitrogenase activity and symbiotic nitrogen fixation using mariner-based transposon insertion sequencing. This demonstrated that the nitrogenase activity of soybean roots is regulated by PGRs by regulation of the expression of symbiotic nitrogen-fixation genes in the rhizobia. However, differences between the nitrogenase activity and the expression of fixA were observed.
Effect of PGR–rhizobia on nitrogen metabolism-related indicators
As the main form of nitrogen absorbed by plants, the content of nitrate-nitrogen in soybean roots was increased compared with that of the control in all PGR–rhizobia treatments (Fig. 3A). Inoculation with GA3–S. fredii and MC–S. fredii significantly (P < 0.05) increased the nitrate–nitrogen content of soybean roots by 26.0% and 18.1%, respectively. Inoculation with MC–B. japonicum significantly increased the nitrate-nitrogen content in soybean roots by 12.3%, whereas inoculation with GA3–B. japonicum increased the nitrate-nitrogen content by 9.3% but this was not statistically significant.
Effect of plant growth regulators and rhizobia on nitrogen metabolism-related indicators. A Content of nitrate nitrogen. B Content of free amino acids. C Activity of nitrate reductase. Each bar is the mean of three independent experiments. Student’s t test was used to evaluate the difference between the control and the treatments
Free amino acid content is an important indicator of the nitrogen content and nutrient metabolism in plants. Inoculation with GA3–S. fredii and MC–S. fredii significantly (P < 0.05) increased the free amino acid content in soybean roots by 175.3% and 215.4%, respectively. In addition, the inoculation with GA3–B. japonicum and MC–B. japonicum significantly (P < 0.05) increased the content of free amino acids in soybean roots by 49.0% and 60.2%, respectively (Fig. 3B).
Nitrate reductase is a rate-limiting enzyme involved in the nitrate assimilation process in plants. Inoculation with GA3–S. fredii and MC–S. fredii increased the NR activity by 17.4% and 15.5%, respectively. However, inoculation with GA3–B. japonicum and MC–B. japonicum significantly (P < 0.05) increased NR activity, respectively (Fig. 3C).
Nitrate and free amino acids are the main substances involved in physiological metabolism in the roots of plants and play an important role in the regulation of plant metabolism. The nitrate metabolic rate was determined based on the activity of NR in plants. Salem et al. (2017) analyzed the phenotype of the mutant of raptor1b, a protein related to TOR1B regulation. The results showed that the mutant of raptor1b led to delayed seed germination and poor resistance to exogenous stress. At the same time, studies at the molecular level showed that these phenotypic changes were accompanied by an increase in the level of free amino acids and a decrease in protective secondary metabolites and storage proteins. In addition, the levels of plant hormones also changed significantly, and the levels of abscisic acid, auxin, and jasmonic acid increased significantly. The supply of exogenous gibberellin could restore the above phenotypic and molecular changes. Wang et al. (2023) found that nitrogen application can promote nitrogen absorption and utilization by increasing the expression of genes related to the absorption and transport of NH4+ and NO3− and increasing the activities of nitrate reductase and glutamine synthetase in rice. At the same time, it was also found that nitrogen could affect the levels of GA3 and ABA by regulating the synthesis and metabolism of GA3 and ABA. Evensen et al. (1981) showed that the nodule strains lacking nitrate reductase were inoculated with Lima bean. Compared with the control group, they all responded to exogenous GA3. After 3–5 weeks of inoculation, the response of the control group to GA3 was weakened, while that of the NR deficient strain did not change. After the application of GA3 biosynthesis inhibitor, the height of Lima bean inoculated with NR deficient strain decreased by 20%, while that of the control group was affected. In addition, it was also found that Lima bean inoculated with NR-deficient strain would form nodules, and the content of extractable gibberellic acid analogues was 4–50 times higher than that of the control group. Previous studies have shown that the root activity and NR of soybean are increased by 2-N,N-diethyl aminoethyl hexanoate (DTA-6) and (DTA-6)–GA3 treatment (He et al., 2014; Hassan et al., 2021). However, in the present study, the accumulation and output of free amino acids were increased by GA3, whereas the nitrate content and NR activity were reduced. It has been shown that the nitrogen fixation ability of soybean roots is well maintained by inoculation with different rhizobia under salt, drought, acid, alkaline, nutrition, fertilizer, pesticide, and heavy-metal stresses (Zahran 1999). The contents of seed protein and amino acids and NR activity of soybean are increased significantly in response to treatment with rhizobia with nitrogen and phosphorus fertilizers (Sital et al. 2011). The NR activity in rhizobia is largely suppressed by cadmium (Bianucci et al. 2013). In the present study, we speculated that the efficiency of root uptake and accumulation of nitrogen were improved with increase in the number of root nodules increased or nitrogenase activity of rhizobia.
Effect of PGRs–rhizobia on seed yield and quality of soybean
Inoculation with GA3–rhizobia or MC–rhizobia significantly increased the plant height, number of pods per plant, seed number per plant, and total nitrogen content of soybean (Table 1). Inoculation with GA3–S. fredii treatment increased the plant height and number of seeds per plant by 22.9% and 47.1% compared with the S. fredii control, respectively. Inoculation with MC–S. fredii increased the number of pods per plant by 41.1% and 19.7% compared with the S. fredii control, respectively. The total quality of soybean seeds was increased by inoculation with GA3–rhizobia or MC–rhizobia. Inoculation with GA3–B. japonicum or MC–B. japonicum significantly increased the total nitrogen content of the soybean seeds by 5.1 or 6.0% compared with the B. japonicum control, respectively.
As a type of protein-rich food, the yield and quality of soybean seeds are intimately linked with nitrogen metabolism and thus is affected by many external factors. We found that the yield components and quality of soybean were regulated by inoculation with PGRs–rhizobia. These results indicated that nitrogen metabolism in soybean roots was regulated by inoculation with rhizobia under treatment with GA3 and MC, thereby affecting the yield and quality of soybean; however, the mechanism was not clear. Roy Choudhury et al. (2019) have shown that plant hormones such as abscisic acid and jasmonic acid have an overall inhibitory effect on nodulation in leguminous plants, while plant hormones such as gibberellin and brassine steroids play a positive role in promoting nodulation. Tatsukami et al. (2016) showed that the hosts inoculated with M. loti mutants with insufficient GA3 synthesis formed more nodules 4 weeks after inoculation than those inoculated with wild type, which indicated that GA3 from added rhizobium could prevent the formation of new nodules. These studies partly explain some of the conclusions of this study that the regulatory mechanism between legume growth and nodulation induced by plant hormones needs to be further studied. In the follow-up work, we will focus on the role of plant hormones as a bridge between plant growth and nodulation.
Conclusion
The present study examined the effects of PGRs on the growth and physiological activity of soybeans and rhizobia by simulating PGR soil residues. It was indicated that a suitable concentration of PGRs played an active role in soybean production. Interestingly, the regulation differed when soybeans were inoculated with different genera of rhizobia in combination with PGRs. This difference might be due to physiological diversity among rhizobium genera, which requires further tests.
Availability of data and materials
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ashraf MA, Rasool M, Mirza MS (2011) Nitrogen fixation and indole acetic acid production potential of bacteria isolated from rhizosphere of sugarcane (Saccharum officinarum L.). Adv Biol Res 5(6): 348–355.
Bianco C, Defez R (2010) Auxins upregulate nif and fix genes. Plant Signal Behav 5(10):1290–1294
Bianucci E, Fullana C, Furlan A, Castro S (2013) Antioxidant defense system responses and role of nitrate reductase in the redox balance maintenance in Bradyrhizobium japonicum strains exposed to cadmium. Enzyme Microb Technol 53(5):345–350
Brewin NJ (1993) The Rhizobium-legume symbiosis: plant morphogenesis in a nodule. Semin Cell Biol 4(2):149–156
Dai Z, Guo X, Yin H, Liang Y, Cong J, Liu X (2014) Identification of nitrogen-fixing genes and gene clusters from metagenomic library of acid mine drainage. PLoS ONE 9(2):e87976
Delmotte N, Mondy S, Alunni B, Fardoux J, Chaintreuil C, Vorholt JA, Giraud E, Gourion B (2014) A proteomic approach of Bradyrhizobium/Aeschynomene root and stem symbioses reveals the importance of the fixA locus for symbiosis. Int J Mol Sci 15(3):3660–3670
Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2(8):621–631
Dombrecht B, Tesfay M, Verreth C, Heusdens C, Nápoles M, Vanderleyden J, Michiels J (2002) The Rhizobium etli gene iscN is highly expressed in bacteroids and required for nitrogen fixation. Mol Genet Genomics 267(6):820–828
Earl CD, Ronson CW, Ausubel FM (1987) Genetic and structural analysis of the Rhizobium meliloti fixA, fixB, fixC, and fixX genes. J Bacteriol 169(3):1127–1136
Egamberdiyeva D, Hoeflich G (2004) Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J Arid Environ 56(2):293–301
Elsayed BB, Hassan MM, Ramady EHR (2013) Phylogenetic and characterization of salt-tolerant rhizobial strain nodulating faba bean plants. African Journal of Biotechnology 12(7).
Evensen KB, Blevins DG (1981) Differences in Endogenous Levels of Gibberellin-Like Substances in Nodules of Phaseolus lunatus L. Plants Inoculated with Two Rhizobium Strains. Plant Physiol 68(1):195–198
Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS (2005) Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 437(7056):209–214
Fonouni-Farde C, Diet A, Frugier F (2016) Root Development and Endosymbioses: DELLAs Lead the Orchestra. Trends Plant Sci 21(11):898–900
Gangwar S, Singh VP (2011) Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: Implication of oxidative stress. Scientia Horticulturae 129(2): 321–328.
Gerhard H, Peter Z, Gert B (2002) Plant species affect the concentration of free sugars and free amino acids in different types of soil. J Plant Nutr Soil Sci 165(5):557–565
Hassan M, Israr M, Mansoor S, et al (2021) Acclimation of cadmium-induced genotoxicity and oxidative stress in mung bean seedlings by priming effect of phytohormones and proline. PLoS One 16(9):e0257924
He S, Wu Q, He Z (2014) Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 117:132–138
Hung CY, Qiu J, Sun YH, et al (2016) Gibberellin deficiency is responsible for shy-flowering nature of Epipremnum aureum. Sci Rep 6:28598
Imperlini E, Bianco C, Lonardo E, Camerini S, Cermola M, Moschetti G, Defez R (2009) Effects of indole-3-acetic acid on Sinorhizobium meliloti survival and on symbiotic nitrogen fixation and stem dry weight production. Appl Microbiol Biotechnol 83(4):727–738
Islas-Flores T, Guillén G, Sánchez F, Villanueva MA (2012) Changes in RACK1 expression induce defects in nodulation and development in Phaseolus vulgaris. Plant Signal Behav 7(1):132–134
Kosenko LV, Krugova ED, Mandrovskaia NM, Okhrimenko SM (2001) Effect of plant growth stimulators on Rhizobium leguminosarum BV. VICIAE 263b and efficiency of symbiotic nitrogen-fixation in peas. Mikrobiolohichny Zhurnal 63(5): 59–66.
Kosenko LV, Mandrovskaya NM, Krugova ED, Varbanets LD (2003) The effect of the plant growth stimulant bactozole on Rhizobium leguminosarum bv. viciae 250a and its nitrogen-tolerant mutant M-71 under different nitrogen supply conditions. Microbiology 72(1), 30–36.
Kurz WGW, LaRue TA (1975) Nitrogenase activity in rhizobia in absence of plant host. Nature 256(5516):407–409
LeVier K, Day DA, Guerinot ML (1996) Iron uptake by symbiosomes from soybean root nodules. Plant Physiol 111(3):893–900
Li Y, Pei Y, Shen Y, et al (2022) Progress in the Self-Regulation System in Legume Nodule Development-AON (Autoregulation of Nodulation). Int J Mol Sci 23(12):6676
Lin J, Frank M, Reid D (2020) No Home without Hormones: How Plant Hormones Control Legume Nodule Organogenesis. Plant Commun 1(5):100104
Liu H, Zhang C, Yang J, Yu N, Wang E (2018) Hormone modulation of legume-rhizobial symbiosis. J Integr Plant Biol 60(8):632–648
Maas MR, Post FJ, Salunkhe DK (1979) Effect of inorganic nutrients on production of steroid glycoalkaloids by Phytophthora infestans race 1.2.4 In Vitro. J Food Prot 42(1): 27–30.
Maekawa T, Maekawa-Yoshikawa M, Takeda N, Imaizumi-Anraku H, Murooka Y, Hayashi M (2009) Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J 58(2):183–194
Miller SH, Elliot RM, Sullivan JT, Ronson CW (2007) Host-specific regulation of symbiotic nitrogen fixation in Rhizobium leguminosarum biovar trifolii. Microbiology 153(9):3184–3195
Mueller K, Gonzalez JE (2010) Complex regulation of symbiotic functions is coordinated by MucR and quorum sensing in Sinorhizobium meliloti. J Bacteriol 193(2):485–496
Ratushnyak AP, Vi A, Ki A, Trushin MV (2011) The effect of nitrate nitrogen and salicylic acid on aerenchyma formation in Typha angustifolia grown in mesocosms. Plant Root 5:25–30
Rellán-Alvarez R, Andaluz S, Rodríguez-Celma J, Wohlgemuth G, Zocchi G, Alvarez-Fernández A, Fiehn O, López-Millán AF, Abadía J (2010) Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol 10(1):1–15
Roy Choudhury S, Johns SM, Pandey S (2019) A convenient, soil-free method for the production of root nodules in soybean to study the effects of exogenous additives. Plant Direct 3(4):e00135
Salem MA, Li Y, Wiszniewski A, Giavalisco P (2017) Regulatory-associated protein of TOR (RAPTOR) alters the hormonal and metabolic composition of Arabidopsis seeds, controlling seed morphology, viability and germination potential. Plant J 92(4):525–545
Sital JS, Kaur K, Sharma S, Sandhu JS, Singh S (2011) Effect of Rhizobium inoculation, phosphorus and nitrogen supplements on protein quality in developing lentil (Lens culinaris) seeds. Indian J Agricult Biochem 24:17–22
Stearn WC, Miller RH, Cakmakci L (1980) Possible effects of PGRs on competitiveness of Rhizobium spp.
Tatsukami Y, Ueda M (2016) Rhizobial gibberellin negatively regulates host nodule number. Sci Rep 6:27998
Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J (2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315(5808):104–107
Tomioka R, Takenaka C, Maeshima M, Tezuka T, Kojima M, Sakakibara H (2012) Stimulation of root growth induced by aluminum in Quercus serrata Thunb. Is related to activity of nitrate reductase and maintenance of IAA concentration in roots. American J Plant ences 3(11): 1619.
Velandia K, Reid JB, Foo E (2022) Right time, right place: The dynamic role of hormones in rhizobial infection and nodulation of legumes. Plant Commun 3(5):100327
Wang H, Zhong L, Fu X, et al (2023) Physiological analysis reveals the mechanism of accelerated growth recovery for rice seedlings by nitrogen application after low temperature stress. Front Plant Sci 14:1133592
Wheatley RM, Ford BL, Li L, Aroney ST, Knights HE, Ledermann R, Easta AK, Ramachandrana VK, Poole PS (2020) Lifestyle adaptations of Rhizobium from rhizosphere to symbiosis. Proc Natl Acad Sci 117(38):23823–23834
Wu L (2012) Mutants of the hybrid sensor kinase CstA affect cyst cell development in Azospirillum brasilense Sp7. Afr J Microbiol Res 6(2):348–354
Yang W, Cai T, Li Y, Guo J, Peng D, Yang D, Yin Y, Wang Z (2013) Effects of exogenous abscisic acid and gibberellic acidonfilling process and nitrogen metabolism characteristics in wheat grains. Aust J Crop Sci 7(1):58–65
Yin X, Liang X, Zhang R, Yu L, Xu G, Zhou Q, Zhan X (2015) Impact of phenanthrene exposure on activities of nitrate reductase, phosphoenolpyruvate carboxylase, vacuolar H+-pyrophosphatase and plasma membrane H+-ATPase in roots of soybean, wheat and carrot. Environ Exp Bot 113:59–66
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63(4):968–989
Acknowledgements
Thanks to all the authors for their efforts.
Funding
This research was funded by the Sichuan Science and Technology Program (No. 2021YJ0294), Scientific Research Project of Yibin University (No. 2017RC03), Key Lab of Aromatic Plant Resources Exploitation and Utilization in Sichuan Higher Education (No. 2018XLZ00301), Research Initiation Project of Yibin University (grant number: 2020YY04), and Solid-state Fermentation Resource Utilization Key Laboratory of Sichuan Province (grant number: 2018GTJ014).
Sichuan Science and Technology Program,2021YJ0294,Wenhao Chen,Scientific Research Project of Yibin University,2017RC03,Wenhao Chen,Key Lab of Aromatic Plant Resources Exploitation and Utilization in Sichuan Higher Education,2018XLZ00301,Wenhao Chen,Research Initiation Project of Yibin University,2020YY04,Wenhao Chen,Solid-state Fermentation Resource Utilization Key Laboratory of Sichuan Province,2018GTJ014,Wenhao Chen
Author information
Authors and Affiliations
Contributions
Conceptualization, Wenhao Chen; methodology, Huawei Yuan; software, Juan Li; validation, Sipei Jiang and Xiaohong Zhao; formal analysis, Juan Li; investigation, Ling You; resources, Qin Wei; data curation, Ruizhang Feng; writing—original draft preparation, Wenhao Chen. The authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, W., Li, J., Yuan, H. et al. Plant growth regulators improve nitrogen metabolism, yield, and quality of soybean–rhizobia symbiosis. Ann Microbiol 73, 15 (2023). https://doi.org/10.1186/s13213-023-01721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-023-01721-y