Abstract
Purpose
This study aims to screen bacterial isolates from the Addis Ababa municipal solid waste dumping site (Koshe) for the biodegradation of low-density polyethylene bags and analyzes their efficiency in degrading plastic bags.
Methods
In this study, low-density polyethylene bag-degrading bacteria were isolated from the Koshe municipal solid waste disposal area in Addis Ababa, Ethiopia. Screening of isolates for low-density polyethylene bag biodegradation was carried out using a clear zone method. Additionally, the efficiency of the isolates for low-density polyethylene biodegradation was evaluated using the weight loss method, scanning electron microscopy analysis, and Fourier transform infrared analysis. Finally, molecular identification of potential low-density polyethylene degrader bacterial isolates was done by 16S rDNA sequencing.
Results
Isolates KS35, KS14, and KS119 resulted in significant weight loss of low-density polyethylene film (42.87 ± 1.91%, 37.2 ± 3.06%, and 23.87 ± 0.11% weight loss, respectively). These isolates were selected for further biodegradation study using scanning electron microscopy and Fourier transform infrared analysis. Scanning electron microscopy analysis shows the formation of pores, pits, and distortion of the plastic surface. Fourier transform infrared analysis indicates the appearance of new peaks at the surface of low-density polyethylene films. Phylogenetic analysis of the three potential bacterial isolates was also carried out, and the result indicates that the sequence of isolate KS35 had 99% similarity with sequences of Methylobacterium radiotolerans MN525302. Isolate KS119 had 100% similarity with Methylobacterium fujisawaense KT720189, and the sequence of isolate KS14 had 99% similarity with species of Lysinibacillus fusiformis.
Conclusions
Weight loss, scanning electron microscopy analysis, and Fourier transform infrared analysis results show that isolates KS35, KS14, and KS119 have high potential in degrading low-density polyethylene bags.
Similar content being viewed by others
Background
Polyethylene is one of the most abundant commercially produced synthetic plastic materials. It is a polymer of ethylene, CH2-CH2, having the formula (−CH2-CH2-)n, where “n” is the number of carbon atoms (Sandhu and Shakya 2019). Among the polyethylene family, LDPE (low-density polyethylene) accounts for 60% of the total production of plastic bags, and it is the major component of municipal solid waste (Gajendiran et al. 2017).
Due to its high molecular weight, long carbon chain backbone, three-dimensional structures, hydrophobic nature, and lack of functional groups recognizable by microbial enzyme systems, LDPE is very resistant to biodegradation (Chiellini et al. 2003). Under normal conditions, the mineralization of LDPE takes more than ten decades (Otake et al. 1995). The extensive usage of LDPE is a severe environmental threat to terrestrial and marine ecosystems. Plastic waste disposed of into the environment entangles the animal or bird’s body, thereby causing the mortality of the organism (Kumar and Raut 2015). It also blocks the sewage system and creates a breeding ground for mosquitoes. Improper disposal of plastic waste can result in lost revenue from tourism by deteriorating the natural beauty of the environment (Muthukumar and Veerappapillai 2015). Plastic materials dumped into the earth prevent the production and mobilization of nutrients in the soil (Gharahi and Zamani-Ahmadmahmoodi 2022). Some plastic products cause human health problems by causing immune and enzyme disorders, hormonal disruption, and even infertility and are carcinogenic (Koteswararao et al. 2014).
Different countries have adopted a range of approaches to discourage the use of plastic bags. In Ethiopia, Environmental Protection Authority has cited a proclamation prohibiting granting permits for the company manufacturing or importing non-biodegradable plastic bags with a thickness of less than 0.03 mm (Gazeta 2007). However, due to poor awareness of society, and the lack of a strong regulation system, different types of plastic bags are produced in large quantities and improperly disposed of.
Different methods are practiced for the management of plastic waste. These include recycling, incineration, biodegradation, and dumping in a landfill. Moreover, biodegradation is an environmentally sound full and cost-effective method of plastic waste management (Kumar and Raut 2015). Recent reports on discovering certain fungi and bacteria that degrade synthetic plastics have received scientific attention. Bacterial species associated with the degradation of LDPE bags include Bacillus cereus (Raut et al. 2015), Pseudomonas knackmussii, Pseudomonas aeruginosa (Hou et al. 2022), and Streptococcus species (Das and Kumar 2015). Polyethylene bags could be degraded by some fungal species such as Aspergillus niger, Aspergillus flavus (Deepika and Jaya 2015), and Aspergillus versicolor (Gajendiran et al. 2016). Biodegradation of LDPE film using Bacillus amyloliquefaciens has been reported by Das and Kumar (2015). They incubated LDPE film with two strains of Bacillus amyloliquefaciens for 60 days and obtained a 16% weight loss of film. The incubation of polyethylene film with Pseudomonas species, A. niger, A. flavus, and Streptomyces species for 6 months revealed the reduction in molecular weight of LDPE film by 24.22 ± 0.01%, 26.17 ± 0.05%, 16.45 ± 0.01%, and 46.7 ± 0.01%, respectively (Lee et al. 1991). Gajendiran et al. (2016) identified Aspergillus clavatus as polyethylene-degrading fungi with 35% weight loss of films after 90 days of incubation.
Even though many researchers have reported bacterial and fungal degradation of LDPE, significant degradation of LDPE wastes for environmental applications has not yet been achieved (Montazer et al. 2020). This study aimed to screen indigenous bacterial isolates that have the potential to degrade LDPE from plastic harboring municipal solid waste soil samples.
Materials and methods
Sample collection and substrate preparation
Soil samples were collected from Koshe solid waste disposal area in Addis Ababa, Ethiopia. The site is located in the Kolfe Keraniyo sub-city southeastern part of Addis Ababa (Fig. 1). This area serves as a solid waste disposal site for over 50 years. A total of 15 samples were collected from different sites randomly using closed sterile containers and transported to the laboratory in the ice box. The samples were homogenized and stored at 4 °C until used. LDPE granules were collected from Ethiopia Plastic Factory and used for enriching LDPE-degrader bacterial isolates after being prepared in powder form. The powder was prepared by immersing LDPE granules in xylene and boiling them for 15 min (Bhatia et al. 2014). The powder was washed with 95% ethanol, dried overnight in a hot air oven at 50 °C, and stored at room temperature for further use. Low-density polyethylene films required for the biodegradation study were purchased from a local market and prepared by cutting into 1.5 cm × 1.5 cm size pieces.
Culture enrichment and isolation of LDPE-degrading bacteria
Culture enrichment was performed to isolate bacteria that use LDPE as the sole source of carbon. The culture was enriched by suspending 1 g of the soil sample in 50 ml of sterile saline water and incubating it in a rotary shaker at 120 rpm for 4 h. Then, 5 ml of soil suspension was transferred into a 250 ml Erlenmeyer flask containing 100 ml of sterile mineral salt broth (1g/L K2HPO4, 0.2 g/L KH2PO4, 1 g/L (NH4)2SO4, 0.5 g/L MgSO4.7H2O, 1 g/L NaCl, 0.01 g/L FeSO4.7H2O, 0.002 g/L CaCl2.2H2O, 0.001 g/L MnSO4.7H2O, 0.001 g/L CuSO4.5H2O, 0.001 g/L ZnSO4.7H2O and pH 7.0) and 0.2% (w/v) LDPE powder. All Erlenmeyer flasks were incubated in a shaker incubator at 35 °C and 120 rpm. After 1 week of growth, 5 ml of the enriched culture was transferred into 100 ml of freshly prepared mineral salt medium supplemented with 0.2% (w/v) LDPE powder. The third and fourth transfers were done successively under similar conditions. After four cycles of enrichment, 0.1 ml of serially diluted sample was spread on nutrient agar plates. Isolated pure bacterial colonies were transferred into the nutrient broth and used for further study.
Screening of isolates for biodegradation of LDPE
Screening of bacterial isolates for LDPE degradation was carried out by the clear zone method. The synthetic medium required to determine clear zone formation around the colony was prepared by mixing polyethylene glycol (the soluble form of polyethylene) with a mineral salt medium at a concentration of 0.2% (w/v) and 15% (w/v) agar (Rosario and Baburaj 2017). The media was autoclaved at 121 °C, 15 lbs pressure for 15 min, and allowed to cool to 45 °C, and poured into sterile Petri plates. Once solidified, the isolated colonies grown on nutrient agar were inoculated using an inoculation loop and then incubated at 30 °C for 2 weeks. After 2 weeks of incubation, plates were stained with 0.1% Coomassie Brilliant Blue solution and destained to visualize a clear zone around the colony. Coomassie Brilliant Blue solution was prepared by dissolving 0.1% (w/v) of Coomassie Blue into 40% (v/v) methanol and 10% (v/v) acetic acid. The destaining solution was prepared by adding 40% (v/v) methanol into 10% (v/v) acetic acid. Agar plates were flooded with 0.1% solution of Coomassie Blue R-250 for 20 min. The solution of Coomassie Blue was poured off, and the plates were flooded with a destaining solution for 20 min. The bacteria producing a clear zone in a blue background are considered polyethylene degraders (Gupta et al. 2016).
Biodegradation studies
In the present study, untreated LDPE was used to analyze the biodegradation efficiency of bacterial isolates, while most early studies of microbial degradation of LDPE used pretreated films. A biodegradation test was performed using 0.2% (w/v) of LDPE films (1.5 × 1.5 cm) that had been dried overnight at 60 °C, weighed, disinfected (30 min in 70% ethanol), and air-dried for 15 min in laminar air flow chamber. The films (0.2 g) were aseptically added to Erlenmeyer flasks containing 100 ml of sterile mineral salt medium supplemented with 0.01% (w/v) of yeast extract. Each flask was inoculated with 1 ml of 24-h old culture grown in a nutrient broth medium. Then, cultures were incubated on a rotary shaker at 35 °C and 120 rpm for 60 days. Flask without inoculation served as a sterile control. The extent of biodegradation of LDPE film was determined after 60 days of incubation using the weight loss method, scanning electron microscope, and Fourier transforms infrared (FT-IR) analysis.
Determination of dry weight of the residual polymer
The percentage of weight loss was determined after 60 days of incubation on a rotary shaker (120 rpm) at 35 °C. To determine weight loss, residual LDPE films were collected and mixed with 2% (w/v) of aqueous sodium dodecyl sulfate (SDS). The mixture was incubated in a shaker incubator (120 rpm) for 4 h and then rinsed with distilled water to remove microbial film and residual medium. Finally, residual LDPE samples were collected on filter paper and dried overnight at 60 °C before being weighed. The weight loss was calculated and compared based on the following formula (Montazer et al. 2019):
Analysis of surface topography
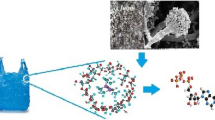
The surface morphology of the LDPE film was analyzed after 60 days of incubation at 35 °C and 120 rpm through scanning electron microscopy (SEM) to check for any structural changes made by the activities of bacteria on LDPE film. The film was subjected to SEM analysis after washing with 2% (w/v) of aqueous sodium dodecyl sulfate and distilled water repeatedly through mild shaking for a few minutes. Additionally, the film was flushed with 70% ethanol to get a maximum surface to be exposed for visualization. A piece of air-dried film was placed on the sample holder, coated using a master coater, and then analyzed under a high-resolution scanning electron microscope (Gajendiran et al. 2016).
Fourier transform infrared (FT-IR) analysis
The structural change in the LDPE surface was investigated using the Fouriiier transform infrared (FT-IR) spectrometer. FT-IR analysis detects any change in the functional groups. Spectrum was recorded at 450–4000 wave numbers cm−1 for all LDPE samples (Gajendiran et al. 2016).
Sequencing and phylogenetic analysis
Total genomic DNA was isolated from the bacterial cultures grown for 24 h using the Bacterial Genomic DNA Purification Kit (GeneMark) following the manufacturer’s instructions. The 16S rRNA gene was amplified using universal primers (forward primer (27F 5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer (1492R 5′-GGTTACCTTGTTACGACTT-3′)) (Olukunle 2019). The PCR was performed on a Prime thermal cycler, UK, using Taq DNA polymerase. A total of 30 cycles of amplification were performed with template DNA. PCR reaction was performed as follows: denaturation at 94 °C for 4min, primer annealing at 56 °C for 1min, primer extension at 72 °C for 1 min, and final extension at 72 °C for 8 min. Finally, the PCR product was visualized through electrophoresis on a 1% agarose gel and sequenced using the Sanger sequencing technology (Applied Biosystems, India). Sequence analysis was done using the NCBI blast tool, and the best-matched organisms having valid names were recovered. The phylogenetic tree was constructed using the neighbor-joining method (MEGA version 11) after multiple sequence alignments with a bootstrap value of 1000 replicates. The 16S rRNA gene partial sequences are deposited in the NCBI database under accession numbers OK336096, OL315394, and OK465137.
Statistical analysis
Data were subjected to one-way ANOVA to observe the variation in weight loss among the bacterial isolates after 60-day incubation. Post hoc (Duncan) test (p < 0.05) was performed to determine the significance of the difference between bacterial isolates in reducing LDPE film weight after 60 days of incubation. Statistical analysis was done using the software SPSS version 16 (Awasthi et al. 2017).
Results
Isolation and screening of LDPE-degrading bacteria
Isolation of bacteria for biodegradation of LDPE was made after successive enrichment of culture using mineral salt broth supplemented with 0.2% of LDPE powder as a sole carbon source. Isolation of the bacteria was carried out on a nutrient agar medium, and a total of sixty bacterial isolates were obtained. Screening of bacterial isolates for LDPE degradation was carried out by the clear zone method. Out of 60 bacterial isolates obtained, fourteen isolates formed detectable clear zone around their colony (Fig. 2). Bacterial isolates were selected based on the diameter of the clear zone around their colony.
Clear zone formed by isolates after 2 weeks incubation in mineral salt medium supplemented with 0.2% polyethylene glycol (“A” growth of bacteria on medium before staining with 0.1% Coomassie Brilliant Blue solution. “B” and “C” Clear zone around bacterial colony after staining with 0.1% Coomassie Brilliant Blue solution)
Biodegradation studies
Determination of dry weight of residual LDPE
We calculated the weight loss of the polythene strips. Out of fourteen bacterial isolates that form a clear zone around their colony, ten resulted in weight loss of LDPE films (Table 1). In our study, some isolates which form a clear zone around their colony did not show a significant change in the final weight of LDPE film. The maximum degradation was achieved by isolate KS35, followed by KS14 and KS119 (42.87 ± 1.91%, 37.12 ± 3.06%, and 23.87 ± 0.11%, respectively) after 60 days of incubation. Furthermore, strain KS35 and KS14 showed significant (p < 0.005) weight loss of LDPE film compared to other isolates. Three of them resulted in maximum weight loss (KS14, KS119, and KS35) were selected as potential LDPE degraders for further SEM and FT-IR analysis.
Scanning electron microscopic (SEM) analysis
Scanning electron microscopic analysis was carried out for three potential bacterial isolates (KS14, KS119, and KS35), which showed better activity during the biodegradation study using the weight loss method. Scanning electron micrograph showed various holes, cracks, pits, and irregularities on the LDPE film. The control film appeared with a smooth surface (Fig. 3).
Fourier transform infrared spectroscopy analysis
Oxidation or hydrolysis of LDPE by bacterial enzymes creates functional groups that improve the polymer hydrophilicity and degradability by microorganisms. In this study, the LDPE film biodegradation potential of the isolates was confirmed by FT-IR analysis. FT-IR analysis was used to detect the change in concentration of existing functional groups or the formation of new functional groups. FT-IR spectra of LDPE films after 60 days of incubation with selected bacterial isolates are shown in Fig. 4. The result shows that incubation of LDPE film with bacterial isolates has resulted in the generation of new functional groups, changes in the concentrations of existing functional groups, and disappearance of a few functional groups at the surface of LDPE film either because of their consumption or production.
FT-IR spectra of polyethylene sheet treated with bacterial inoculum and the control incubated at 35 °C for 60 days. Peak at 1643.78 cm−1 which represent C–C=C stretch of alkenes disappeared in all bacterial-treated LDPE film except KS 119-treated film. New peaks at 1250.9 cm−1 and 1246.44 cm−1 attribute to C–O stretch of ester’s group
The FTIR spectrum of LDPE after treatment with KS14 and KS119 showed a decrease in wavelength number for O–H stretching of alcohol, and the band shifted from 3391.53 to 3401.04 cm−1 (O–H stretching of alcohol). The peak at 3391.53 cm−1, 1473 cm−1 (C–H bend stretching vibration of alkanes), and 1643.40 (C=C stretching of alkenes) in KS35-treated LDPE film disappeared. Additionally, the peak at 1643.40 cm−1 (C=C stretching of alkenes) in KS14-treated film and peak at 1473 cm−1 in KS119-treated LDPE film disappeared due to the effective degradation of polyethylene film by bacterial isolates. A new peak appeared in KS35- and KS14-treated LDPE film at 1250.33 cm−1 and 1246.44 cm−1 wavelength numbers, respectively. Both peaks correspond to the C–O stretching of esters groups. The most prominent structural change was observed in the LDPE film treated with KS35 bacterial isolate.
Sequencing and phylogenetic analysis
PCR amplification of 16S rRNA gene was carried out, and the PCR products were sequenced using Sanger sequencing technology (Fig. 5). Sequences of the three bacterial isolates were compared against the sequences available in the NCBI, nr database using BLASTn. The sequence of isolate KS35 had 99% similarity with Methylobacterium radiotolerans MN525302. The sequences of isolate KS119 had 100% similarity with Methylobacterium fujisawaense KT720189, and isolate KS14 had 99% similarity with species of Lysinibacillus fusiformis (Fig. 6).
Discussion
Low-density polyethylene could degrade by different fungal and bacterial species isolated from various sources. Several researchers explored microbial populations inhabiting landfills (Muhonja et al. 2018), rhizosphere soil of mangroves (Sangale 2012), and marine water (Ambika 2014) for their polyethylene-degrading potential. Similarly, in this study, the Koshe solid waste disposal area in Addis Ababa, Ethiopia, was selected for isolation and screening of LDPE-degrading bacteria. In many LDPE biodegradation studies, the weight loss method was used to determine microbial consumption of polymers (Das and Kumar (2015), Jamil et al. (2017), and Gyung Yoon et al. (2012)).
In our study, the percentage of LDPE weight loss was calculated, and the highest value was recorded by isolates KS35 and KS14 (42.87 ± 1.91% and 37.2 ± 3.06%, respectively) (Table 1). This shows better degrading ability than the previously reported work by Kalia and Dhanya (2022), in which they have documented 4.38% (untreated) and 12.09% (xylene treated) LDPE films weight loss after 30 days of incubation with Lysinibacillus fusiformis. Maroof et al. (2021) also reported a comparatively low percentage of thermo-oxidized and UV-treated LDPE films weight loss (8.46 ± 0.3%) after 90 days of incubation with B. siamensis. Montazer et al. (2019) observed a percent decrease in LDPE (untreated and without additives) mass by 33.7% ± 1.2% for C. necator H16, which is comparable with our result. Gajendiran et al. (2017) also reported a 35% weight loss of LDPE films after 90 days of incubation with the fungi Aspergillus clavatus. A maximum decrease in weight loss (48.40%) of pre-treated LDPE films after 90 days of incubation with C. lunata SG1 in T-80 added medium was reported by Raut et al. (2015).
While weight loss provides solid evidence of polymer degradation, SEM analysis confirms the biodegradation ability of isolates by elucidating the change of the surface of LDPE films. Scanning electron microscopy analysis showed the deformation of the LDPE film and the formation of pits and holes after incubation of the film for 60 days with selected bacterial isolates (Fig. 3). In agreement with the present study, Das and Kumar (2015) reported that several cracks developed on the surface of LDPE film treated with the bacterial isolate Bacillus amyloliquefaciens BSM-1 after 60 days of incubation, whereas the control sample had an appearance of a smooth surface. Esmaeili et al. (2013) have also noticed surface erosion and the formation of pits and cavities on the surface of the LDPE samples after bacterial treatment. In a study by Yoon et al. (2012), SEM evidence confirmed that the smooth surface of the LDPE sheet became eroded as a result of the biodegradation of the polymer by Pseudomonas sp. E4. Incubation of LDPE films with Aspergillus clavatus strain JASK1 for 90 days also resulted in surface erosion, cracks, folding, and fungal colonization (Gajendiran et al. 2016).
In our present study, Fourier transform infrared spectral analysis was carried out to check the chemical degradation of polyethylene. The spectrum of LDPE films, incubated with the selected bacterial isolates, showed the appearance of new bands and the disappearance of existing bands due to bacterial activity. Analysis of the polyethylene spectral figures indicates the formation of new peaks at 1250.33 cm−1 and 1246.44 cm−1, which corresponds to the C–O stretching of esters groups in KS35 and KS14, treated LDPE film, respectively. Functional groups such as an ester group, a carbonyl group, or an ether group are formed when a hydrogen atom on a long carbon-carbon bond is replaced by an oxygen atom (Ren et al. 2019). Alkane hydroxylases are the key enzymes mediating aerobic alkane degradation by hydroxylation of carbon–carbon bonds and the formation of primary or secondary alcohols (Montazer et al. 2020). In our study, the peak intensity of the band 1045.72 cm−1, which corresponds to C–O of the ether group, increased. Ether is formed during the biodegradation of LDPE as a result of the epoxidation of alkenes by microbial alkene monooxygenase (Hou et al. 1979). A new peak was also observed at 888.07 (=C–H stretch of alkenes) in KS14-treated film (Fig. 4). A similar pattern of LDPE film spectra was reported by Gajendiran et al. (2017), where new peaks were observed at 1263.37, 1078.21 cm−1 (C–O stretch of ethers), and 987.55 cm−1 (=CH2 stretch of alkenes) after 90-day incubation of film with fungi Aspergillus. Muhonja et al. (2018) also analyzed the biodegradability of untreated LDPE using FT-IR and observed the formation of new peaks at 1700–1650 cm−1 and 1000–1100 cm−1. The shifting, addition, and deletion of peaks indicate structural changes made by microbial activity (Bhatia et al. 2014).
Phylogenetic analysis of the three potential LDPE degrader bacterial isolates was performed using MEGA 11 software, and the result showed that they are closely related to Methylobacterium radiotolerans (KS35), Lysinibacillus fusiformis (KS14), and Methylobacterium fujisawaense (KS119). Previously, these bacterial species were reported to involve in the biodegradation of LDPE and other hydrocarbons. Montazer et al. (2021) have reported biodegradation of low-density polyethylene by Lysinibacillus fusiformis species isolated from larvae of the greater wax moth, Galleria mellonella. Lysinibacillus species isolated from dumpsites were also identified as effective polyethylene degraders (Muhonja et al. 2018). Kalia and Dhanya (2022) reported that Lysinibacillus fusiformis had xylenes treated and untreated LDPE degradation potential. Photolo et al. (2021) isolated M. radiotolerans that can detoxify heavy metals and promote plant growth from municipal solid waste. Nzila et al. (2016) also reported that M. radiotolerans isolated from soil contaminated with petroleum products was able to use naphthalene as the sole source of carbon, and this bacterial strain grows efficiently in the presence of ethanol. Degradation of LDPE by microorganisms had known for several years, and there is no report on the biodegradation of LDPE by Methylobacterium radiotolerans and Methylobacterium fujisawaense so far. This is the first experimental report on LDPE utilization as a carbon source under laboratory conditions by showing the effective ability of Methylobacterium radiotolerans and Methylobacterium fujisawaense.
The present work indicates that soil bacteria from solid waste dump sites show great efficacy in degrading virgin polyethylene. Previously, many researchers evaluated the biodegradability of LDPE after abiotic pretreatment. Abiotic pretreatment such as UV irradiation, chemical oxidation, and thermal treatment was employed to facilitate the microbial degradation of the polymer (Yoon et al. 2012). In the present study, untreated LDPE film was used for the biodegradation study, and promising results were obtained. A further effort to improve this degrading capacity through the assessment of optimum conditions for microbial activity is necessary. Pre-treatment of polyethylene with environmentally friendly substances could also be adopted as a means to enhance polyethylene biodegradation so that this concept can be applied commercially and on a larger scale.
Conclusions
The present study aimed to screen and analyze the LDPE bag-degrading ability of bacteria isolated from the Koshe waste disposal area. The result from biodegradation studies (weight loss percentage, surface analysis using SEM, and FTIR analysis) reveals that isolates KS35, KS14, and KS119 which are closely related to Methylobacterium radiotolerans, Lysinibacillus fusiformis, and Methylobacterium fujisawaense, respectively, have high ability to degrade LDPE bags.
Availability of data and materials
16S rRNA gene sequences supporting the results of this article are available in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers OK336096, OL315394, and OK465137. All other data generated or analyzed during this study are included in this published article.
Abbreviations
- LDPE:
-
Lower density polyethylene
- FTIR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning electron microscopic
References
Ambika K (2014) Isolation of polyethylene degrading bacteria from marine waters of Vishkhapatnam, India. Curr Microbiol 3:269–283
Awasthi S, Srivastava P, Singh P, Tiwary D, Mishra PK (2017) Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. Biotech. 7:332
Bhatia M, Girdhar A, Tiwari A, Nayarisseri A (2014) Implications of a novel pseudomonas species on low density polyethylene biodegradation: an in vitro to in silico approach. SpringerPlus 3:1–10
Chiellini E, Corti A, Swift G (2003) Biodegradation of thermally-oxidized, fragmented low-density polyethylenes. Polym Degrad Stab 81:341–351. https://doi.org/10.1016/s0141-3910(03)00105-8
Das MP, Kumar S (2015) An approach to low-density polyethylene biodegradation by bacillus amyloliquefaciens. 3 Biotech 5:81–86. https://doi.org/10.1007/s13205-014-0205-1
Deepika S, Jaya M (2015) Biodegradation of low density polyethylene by microorganisms from garbage soil. J Exp Biol Agric Sci 3:1–5
Esmaeili A, Pourbabaee AA, Alikhani HA, Shabani F, Esmaeili E (2013) Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus Niger in soil. PLoS One 8:e71720. https://doi.org/10.1371/journal.pone.0071720
Gajendiran A, Krishnamoorthy S, Abraham J (2016) Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech 6:52. https://doi.org/10.1007/s13205-016-0394-x
Gajendiran A, Subramani S, Abraham J (2017) Effect of Aspergillus versicolor strain JASS1 on low density polyethylene degradation. IOP Confer Ser: Mater Sci Eng 263:022038. https://doi.org/10.1088/1757-899x/263/2/022038
Gazeta FN (2007) Solid waste management proclamation. No. 513/2007
Gharahi N, Zamani-Ahmadmahmoodi R (2022) Effect of plastic pollution in soil properties and growth of grass species in semi-arid regions: a laboratory experiment. Environ Sci Pollut Res Int 29(39):59118–59126. https://doi.org/10.1007/s11356-022-19373-x. Epub 2022 Apr 5
Gohel HR, Contractor CN, Ghosh SK, Braganza VJ (2014) A comparative study of various staining techniques for determination of extra cellular cellulase activity on carboxy methyl cellulose (CMC) agar plates. Int J Curr Microbiol App Sci 3:261–266
Gupta KK, Devi D, Rana D (2016) Isolation and screening of low density polyethylene (Ldpe) degrading bacterial strains from waste disposal sites. World J Pharmaceut Res 5:1633–1643
Hou H, Patel R, Laskin A, Barnabe N (1979) Microbial oxidation of gaseous hydrocarbons: epoxidation of C2 to C4 n-alkenes by methylotrophic bacteria. Appl Environ Microbiol 38:1
Hou L, Xi J, Liu J, Wang P, Xu T, Liu T, Qu W, Lin YB (2022) Biodegradability of polyethylene mulching film by two pseudomonas bacteria and their potential degradation mechanism. Chemosphere 286:131758
Jamil SUU, Zada S, Khan I, Sajjad W, Rafiq M, Shah AA, Hasan F (2017) Biodegradation of polyethylene by bacterial strains isolated from Kashmir cave, Buner, Pakistan. J Cave Karst Stud 79:73–80. https://doi.org/10.4311/2015mb0133
Kalia A, Dhanya MS (2022) Evaluation of biodegradation efficiency of xylene pretreated polyethylene wastes by isolated Lysinibacillus fusiformis. Nat Environ Pollut Technol 21:3. https://doi.org/10.46488/NEPT.2022.v21i03.045
Kim MN, Yoon MG (2010) Isolation of strains degrading poly (vinyl alcohol) at high temperatures and their biodegradation ability. Polym Degrad Stab 95(1):89–93
Koteswararao PR, Tulasi S, Pavani Y (2014) Impact of solvents on environmental pollution. National seminar on impact of toxic metals, minerals and solvents leading to environmental lution. J Chem Pharm Sci 3:132–135
Kumar SS, Raut S (2015) Microbial degradation of low density polyethylene (LDPE): a review. J Environ Chem Eng 3:462–473. https://doi.org/10.1016/j.jece.2015.01.003
Lee B, Pometto AL III, Fratzke A, Bailey TB Jr (1991) Biodegradation of degradable plastic polyethylene by Phanerochaete and Streptomyces species. Appl Environ Microbiol 57:678–685
Maroof L, Khan I, Yoo HS, Kim S, Park H, Ahmad B, Azam S (2021) Identification and characterization of low density polyethylene degrading bacteria isolated from soils of waste disposal sites. Environ Eng Res 26(3):200167
Montazer Z, Habibi Najafi MB, Levin DB (2019) Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can J Microbiol 65:224–234. https://doi.org/10.1139/cjm-2018-0335
Montazer Z, Najafi MBH, Levin DB (2021) In vitro degradation of low-density polyethylene by new bacteria from larvae of the greater wax moth, galleria mellonella. Can J Microbiol 67:249–258. https://doi.org/10.1139/cjm-2020-0208%M33306436
Montazer Z, Habibi Najafi MB, Levin DB (2020) Challenges with verifying microbial degradation of polyethylene. Polymers (Basel) 12. https://doi.org/10.3390/polym12010123
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS One 13:e0198446. https://doi.org/10.1371/journal.pone.0198446
Muthukumar A, Veerappapillai S (2015) Biodegradation of plastics–a brief review. Int J Pharmaceut Sci Rev Res 31:204–209
Nzila, A, Sankaran S, Al-Momani M, Musa MM (2018) Isolation and characterization of bacteria degrading polycyclic aromatic hydrocarbons : phenanthrene and anthracene. Archives of Environmental Protection 44(2):43–54
Olukunle OF (2019) Molecular identification of crude oil-degrading bacteria and screening for catechol 2, 3 dioxygenase (C23O) gene. Biotechnol J Int 23(4):1–14
Otake Y, Kobayashi T, Asabe H, Murakami N, Ono K (1995) Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J Appl Polym Sci 56:1789–1796
Photolo MM, Sitole L, Mavumengwana V, Tlou MG (2021) Genomic and physiological investigation of heavy metal resistance from plant endophytic Methylobacterium radiotolerans MAMP 4754, isolated from Combretum erythrophyllum. Int J Environ Res Public Health 18. https://doi.org/10.3390/ijerph18030997
Raut S, Raut S, Sharma M, Srivastav C, Adhikari B, Sen SK (2015) Enhancing degradation of low density polyethylene films by curvularia lunata sg1 using particle swarm optimization strategy. Indian J Microbiol 55:258–268. https://doi.org/10.1007/s12088-015-0522-z
Ren L, Men L, Zhang Z, Guan F, Tian J, Wang B, Wang J, Zhang Y, Zhang W (2019) Biodegradation of polyethylene by Enterobacter sp. D1 from the guts of wax moth galleria mellonella. Int J Environ Res Publ Health 16:1941. https://doi.org/10.3390/ijerph16111941
Rosario LD, Baburaj S (2017) Isolation and screening of plastic degrading bacteria from polythene dumped garbage soil. Int J Res Appl Sci Eng Technol 887(Xii):2321–9653 [Online]. Available: www.ijraset.com1028
Sandhu R, Shakya M (2019) Comparative study of synthetic plastics and biodegradable plastics. Glob J Bio-Sci Biotechnol 8:107–112
Sangale MK (2012) A review on biodegradation of polythene: the microbial approach. J Bioremed Biodegrad 03. https://doi.org/10.4172/2155-6199.1000164
Tareen A, Saeed S, Iqbal A, Batool R, Jamil N (2022) Biodeterioration of microplastics: a promising step towards plastics waste management. Polymers 14:2275 Note: MDPI stays neutral with regard to jurisdictional claims in published
Yoon M, Jeong JH, Nam KM (2012) Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J Bioremed Biodegrad 03. https://doi.org/10.4172/2155-6199.1000145
Acknowledgements
The authors would like to acknowledge Addis Ababa Science and Technology University for providing funds to accomplish this research. We extend our sincere gratitude to the Biotechnology Department for providing all laboratory facilities to perform our research work. We also thanks the Biology Department of Adama Science and Technology University, Ethiopia) for their cooperation in scanning electron microscopy, Kadila Pharmaceutical Industry for providing us FT-IR analysis service, and Ethiopian Plastic Factory for providing us polymer granules required for the research work.
Funding
This research was financially supported by Addis Ababa Science and Technology University which utilized for procurement of laboratory chemicals and reagents, sample collection, and some laboratory works.
Author information
Authors and Affiliations
Contributions
ZM is the main investigator and performed the laboratory bacterial isolation, screening, and analysis of lower-density polyethylene biodegradation and was a major contributor to writing the manuscript. MT conceived the idea of the study, led the project, and edited the manuscript. NT performed interpretation of Fourier transform infrared spectroscopy analysis results and managed the research activities of the study. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nademo, Z.M., Shibeshi, N.T. & Gemeda, M.T. Isolation and screening of low-density polyethylene (LDPE) bags degrading bacteria from Addis Ababa municipal solid waste disposal site “Koshe”. Ann Microbiol 73, 6 (2023). https://doi.org/10.1186/s13213-023-01711-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-023-01711-0