Abstract
Purpose
To explore the methane production potential and microbial community changes of combined anaerobic digestion of chicken manure and corn straw. Increase methane production, reduce the environmental pollution caused by the burning of livestock manure and straw, and provide some theoretical references for the construction and operation of actual biogas projects.
Methods
Different proportions (3%, 5%, 10%) of corn straw were added to the anaerobic digestion systems of chicken manure in order to improve the C/N ratio and to evaluate the feasibility and potential synergistic effect on the co-digestion. The key point was to use 16S rDNA sequencing to analyze the relationship between the microbial diversity and the hydrolase activity during the anaerobic digestion.
Result
The results showed that the volumetric gas production of methane in the 3% straw addition group was 227.66 ml/gVS, which was 18% higher than the cumulative methane production in the pure chicken manure experimental group. However, with the increase of straw concentration, methane production and the utilization rate of the raw materials continued to decrease. The change in activity of each hydrolase was in agreement with changes in hydrolytic acidifying bacteria, and the activity of the main hydrolase also increased with the addition of straw; the correlation coefficient was 0.9943. Sequencing results showed that the dominant strains of methanogenic archaea were Methanosarcina, Methanosaeta, Methanobacterium, and Methanospirillum. Mainly for hydrogen-eating, acetic acid-eating methanogens, its role is to use H2, methanol and acetic acid, and other substances to metabolize methane, and convert it into CH4 and CO2.
Conclusion
The addition of a small amount of straw enhanced the production capacity of hydrogen-nutritive methane to some extent, and the species richness and evenness were also improved, reducing the pollution caused by livestock manure to the environment while controlling the pollution caused by straw burning.
Graphical Abstract

Similar content being viewed by others

Introduction
The growing population worldwide has led to the increasing scale of livestock farming and the large amount of solid organic waste generated. According to statistics in 2018, China produced about 3803 million tons of livestock manure and 1155 million tons of straw (Kung and Mu 2019; Yu et al. 2019), which will cause a series of environmental problems (including greenhouse gas emissions and water eutrophication) if these agricultural wastes are not treated in a timely manner. Anaerobic digestion is a clean and renewable energy technology that can effectively treat wastes such as livestock manure and agricultural straw (Ebner et al. 2016).
Single feedstock anaerobic fermentation limits methane production to a certain extent due to its low biodegradability. Mixed anaerobic fermentation can effectively dilute the concentration of harmful substances in the reactor and avoid their toxic effects on the microbial community. Secondly, the nutrients and trace elements in mixed anaerobic fermentation are more abundant, and the system stability is stronger. Fang et al. (2019) used the combined anaerobic fermentation of mushroom residues and sewage sludge to increase the yield of volatile fatty acids. Their results showed that the best C/N ratio promoted hydrolysis and acidification, although it did not increase VFAs/SCOD. The fermentation system displayed a strong buffer capacity, and the modified logistics model was used to obtain the fitting of the system. Böjti et al. (2017) increased methane yield by 24% by extracting water from chicken manure to improve C/N and then co-fermenting it with corn silage and other feeds, and verified the sustainability of co-fermentation. Microbial community structure is an important factor to maintain the stable operation of anaerobic fermentation. By analyzing the changes of microbial community and the changes of hydrolytic enzyme activity during the mixed fermentation process, we can more accurately grasp the reaction mechanism during the mixed fermentation process and further understand the synergistic effects between microorganisms within each stage of the reaction. Li found that the mixed anaerobic fermentation of cow manure with kitchen waste was rich in microbial diversity, high in biodegradability, and rich in functional microbial taxa with strong volatile fatty acids conversion and methanogenic capacity, which could improve the methanogenic efficiency (Li et al. 2015). Yan found powder activated carbon also stimulates hydrolysis in anaerobic digestion of thermal hydrolysis pretreated sludge and enhances methanogenic activity and volatile solid removal rate (Yan et al. 2020).
During anaerobic fermentation with a single feedstock, the anaerobic fermentation process is highly susceptible to ammonia inhibition problems due to the relatively low C/N of livestock manure (Yusof et al. 2019). Therefore, by mixing livestock manure with corn stover with high C/N ratio for fermentation, the volatile fatty acid produced from corn stover degradation can balance the ammonia concentration in the reactor and help to improve the methane production during anaerobic fermentation. The focus of this study is to explore the optimal ratio of chicken manure and straw co-fermentation to improve the methane production efficiency, and to analyze the mechanism of microbial diversity change and its synergistic effect by using the dynamic change process of molecular biology and the change of related hydrolytic enzyme activity, to reduce the pollution caused by livestock manure to the environment while controlling the pollution caused by straw burning, to increase the utilization rate of agricultural waste, and to provide some theoretical reference for the construction and operation of actual biogas projects.
Materials and methods
Experimental materials
The chicken manure used in this experiment was obtained from a meat chicken farm located in Chaoyang, Liaoning Province, while the straw was obtained from a peasant household located in Shenbei, New District of Shenyang City. Before the experiment, the straw was pretreated, ground, and passed through a 60-mesh screen. This was done in order to increase the contact area and improve the efficiency of the anaerobic fermentation. The inoculation sludge was obtained from the municipal sewage treatment plant located in the north of Shenyang. The experimental raw materials and the properties of the inoculation sludge are shown in Table 1.
Experimental design
Figure 1 displays the self-designed anaerobic reactor that was used for the sequencing batch anaerobic digestion experiments. The whole device mainly includes drainage collection equipment, gas collection equipment, anaerobic digestion reaction equipment, and constant temperature water bath with temperature control and connecting equipment. The original details are shown in Fig. 1. Each device was connected to form a through-type airtight equipment, where the effective volume of the reaction equipment, the gas collection equipment, and the drainage collection device was 1L. The air entrances were controlled to ensure an anaerobic environment.
In the present investigation, we used fresh chicken manure and crushed straw. The experimental design consisted of four groups M1, M2, M3, and M4, in which M1 was the control group (only chicken manure was added). The other three groups of M2–M4 were supplemented with corn stover which accounted for 3%, 5%, and 10% of total chicken manure mass, respectively. The amounts of each material used in these experiments are shown in Table 2. Experiments were performed in duplicate and the results were averaged. Materials were added to the sequencing batch reactor and volumes were adjusted to 1L using deionized water. The reactor was placed in a water bath and temperature was maintained at 37±0.2°C. Experiments were run for 50 days.
Analytical method
Biogas production was measured by drainage method, methane production was measured by methane gas analyzer GT 901 (Beijing Tiandi Shouhe Technology Development Co. LTD., China), pH was measured by Remagnet PHS-2F pH meter (Shanghai Yidian Scientific Instruments Co., Ltd., China), initial total solid and volatile solid of samples were measured by gravimetric method (GB/T28731-2012), chemical oxygen demand was measured by potassium dichromate oxidation method (GB/T32208-2015), total ammonia nitrogen was measured by Nessler reagent spectrophotometry (HJ535-2009), and total volatile fatty acids were measured using a liquid chromatograph SPD-10A (Shimadzu, Japan). The enzyme activity was prepared by using ELISA detection kit (Shanghai Enzyme Link Biotechnology Co., Ltd., China) to prepare standard samples and samples to be tested, and then a microplate reader RaytoRT-6100 (Shenzhen Redu Life Science Co., Ltd., China) was used to measure the absorbance and calculate the enzyme activity (Wei et al. 2019).
16S rDNA technology was used for the abundance and diversity of microbial flora. First, the DNA in the samples was extracted by CTAB method, and the purity and concentration of DNA were detected by agarose electrophoresis. Then, using the DNA of the genome to be tested as a template, the 16SV4 region primer 515F and the specific primer 806R were selected for PCR bacterial amplification, and the 16SV4+V5 region primer Arch519F and the specific primer Arch915R were selected for PCR archaeal amplification; the PCR products were detected and purified by electrophoresis using 2% agarose gel; and then, the library was constructed and sequenced by Ion S5TMXL. The high-throughput sequencing platform was Miseq PE300 (Illumina, USA).
Results and discussion
Daily gas production, pH, COD, and ammonia nitrogen
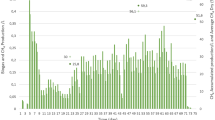
The performance of the anaerobic digestion system can be characterized by methane production, chemical oxygen demand, and ammonia nitrogen (Fig. 2). The significant advantage of anaerobic digestion is waste reduction and energy generation, so methane production can be used to evaluate the efficiency of anaerobic digestion, and other process parameters are used to evaluate the stability of anaerobic digestion (Zhao et al. 2018). According to the results displayed in Fig. 2, the highest daily methane production occurred during the first day of the experiments. Later, the whole reaction process displayed a long delay period. Methane production was recovered until day 16, when the hydrolysis stage of the anaerobic fermentation started. This may have occurred because the activity of the inoculated sludge was poor. It is likely that the 24-h acclimation was not enough to restore its activity. With progress reaction, all the experiments reached the methane production peak on day 25. Therefore, according to the results of the whole experiment, the fermentation process was divided into three stages. The first stage corresponds to the lag and adaptation period (1-20 days); the second stage corresponds to the growth and stable period (20–40 days); and the third stage the decay period (40–51 days). In the first stage, there was a lag period of about 10 days. The reason may be that the initial pH of the inoculated sludge was low, and the acclimation time of the microorganisms in the sludge was short, resulting in a certain acidification phenomenon. When the microorganisms entered the new environmental system, the product inhibition became more and more serious. By adjusting the pH in time, the anaerobic digestion system gradually returned to normal, and the methane production gradually increased. By comparing the daily gas production of each group, it was determined that the maximum daily methane production for the samples containing 3% straw was 16.03 mL/gVS, followed by that containing pure chicken manure and 5% straw, which daily methane production was 13.75 mL/gVS and 11.06 mL/gVS, respectively. Also, the samples containing 10% straw displayed a highest daily methane production of 9.46 mL/gVS. Therefore, it can be concluded that the addition of a small amount of straw was able to adjust the Cmax N of the reaction system and promote the anaerobic fermentation of chicken manure to a certain extent (Zahan et al. 2018a, b). Since large effective straw volumes make difficult the cellulose and lignin degradation, the continuous addition of straw results in a reduction of the reaction rate of the whole anaerobic fermentation system.
Figure 2 also shows that a 3% straw resulted in the highest cumulative methane production. The volumetric gas production was of 227.66 mL/gVS, followed by 185.69 mL/gVS and 174.04 mL/gVS, which corresponded to straw concentrations of 0% and 5%, respectively. The lowest cumulative methane production was observed when straw content was 10%. In this case, the volumetric gas production was only 128.58 mL/gVS. The concentration of ammonia nitrogen was low at the beginning of the experiment and increased with time. The reason for this was that the hydrolysis rate required to produce ammonia nitrogen was higher than the consumption rate of ammonia nitrogen, which was stabilized around day ten. The concentration range of ammonia inhibition was 3000–6000mg/L; the change trend of ammonia nitrogen content in each group was similar and maintained at a low level, indicating that different proportions of raw materials had little effect on ammonia nitrogen production. Thus, no inhibition was observed. The chemical oxygen demand of each group showed an increasing trend in the first stage (lag period and adaptation period). As fermentation progressed, the gas production continuously increased. At this point, the degradation rate of small molecules in the organic matter was higher than that of macromolecules. Thus, the chemical oxygen demand decreased during the growth period and the stable stage. When the reaction entered the third stage (decay period), the results indicated that the gas production potential and fermentation rate were the fastest when straw concentration was 3%. At this point, the largest consumption of organic matter occurred. Given the large amount of cellulose, lignin, and other substances in the group containing 10% straw, the hydrolysis rate was slow. This occurred because cellulose and lignin are difficult to degrade. Thus, chemical oxygen demand still showed a high level at the end of the experiment (Wang et al. 2017). In summary, a 3% straw concentration promoted the anaerobic fermentation of chicken manure. Also, the addition of a small amount of straw can not only optimize the C/N rate in the reaction system, but also increase the porosity and reaction area. These results provide theoretical guidance and technical support for practical biogas engineering processes.
Determination of the correlation between VFAs and pH
Figure 3 presents the changes of volatile fatty acids and pH during anaerobic fermentation with different straw concentrations. According to our data, the change of volatile fatty acids was related to the hydrolysis and acidification rate during anaerobic fermentation, as well as the degradation efficiency of the selected methane-producing bacteria (Mahdy et al. 2020). It was also determined that during the first stage of the fermentation, pH showed low values. This probably occurred because the macromolecules in the organic matter were mainly hydrolyzed and acidified during the lag and adaptation periods. When the macromolecular organic matter in the fermentation system is transformed into fatty acids, a large amount of free H+ is produced. This results in the decrease of pH. In addition, the content of volatile fatty acids continuously increases reaching a peak value. When the microorganisms entered the growth and stable period, the utilization efficiency of volatile fatty acids and H+ by methanogenic bacteria was greater than that of organic matter. Methanogenic bacteria gradually adapted to the environment and used the free H+ present in the system to synthesize methane, CO2, and volatile fatty acids. The content of methane, CO2, and volatile fatty acids decreased and the pH value increased. These results were similar to those reported by Yang et al. (2017). Comparing the four different experiments, it was observed that at straw concentrations of 0%, 3%, 5%, and 10%, the highest volatile fatty acid concentrations occurred on days 16, 13, 16, and 28, respectively. Peak volatile fatty acid concentrations were 4.61, 5.68, 7.34, and 5.23 mg/mL, which decreased to reach values of 2.29, 2.32, 1.91, and 3.21 mg/mL, respectively. It was also determined that, in the sample containing 10% straw, which is a high straw content, the hydrolytic acidification stage lasted a significant amount of time. In addition, in the middle and late stage of the experiment, the value of the hydrolysis rate was almost the same as that of the utilization efficiency of fatty acids and H+ by methanogenic bacteria. In this case, the volatile fatty acid concentration was stable at about 3.21 mg/mL. Data showed that addition of the straw improved the utilization rate of the raw materials and improved the efficiency of the anaerobic digestion.
Changes in hydrolytic bacteria and hydrolase activity
Figure 4 displays changes of the main enzymes (amylase, protease, cellulase) and the genus level of the main hydrolytic acidification bacteria in a given hydrolysis stage. In this experiment, OTU clustering and species clustering analyses were carried out based on effective data. Four groups of experiments determined that 1717, 1778, 1754, and 1751 bacterial OTU, identified by OTU were further allocated to 30 different phyla, among which the main taxa were Firmicutes, Proteobacteria, Bacteroidetes, Spirochaete, and Cloacimonetes. According to the data displayed in Fig. 4a, a positive correlation between amylase and flora abundance occurred. At the genus level, the main flora was unidentified_Spirochaetaceae, unidentified_Prevotellaceae, unidentified_Lentimicrobiaceae, Lactobacillus, and Bacteroides. Unidentified_Spirochaetaceae belongs to Spirulina, a group of Gram-negative bacteria with the effect of decomposing lignocellulosic substances. The increasing abundance of bacteria in the early stage of the experiment indicates that Spirochaetaceae bacteria accelerated the degradation of corn straw (Song et al. 2014). In addition, Unidentified_Prevotellaceae and Bacteroides belong to Bacteroides and are relatively rich members of the community in anaerobic environments. These microorganisms are able to decompose glucose and protein and play an important role in acid and acetic acid production. The changes in Bacteroides were associated with changes in short-chain fatty acids, and in the fourth group of experiments, the abundance of bacteria in the phylum Bacteroides increased significantly because the addition of a certain amount of corn stover promoted the hydrolysis and acidification of the substrate (Krause et al. 2008). Lactobacillus belongs to thick-walled bacteria, which participates in the hydrolytic fermentation of organic compounds and uses various sugars to produce VFAs as the final fermentation products (Peng et al. 2018).
Figure 4b displays the relative microflora abundance and protease activity. Data indicated that the change of enzyme activity and relative abundance of microflora increased at the beginning of the process and later decreased. The active bacteria were Candidatus_Cloacimonas, Ruminiclostridium, Bacteroides, and Acidaminococcus. Figure 4c shows the change in cellulase activity and the main active bacteria. Cellulase, which is widely present in natural organisms, displayed a higher activity as compared to that of amylase and protease. The cellulose present in the system was decomposed into oligosaccharides and monosaccharides. Also, at the beginning of the process, the abundance of microflora increased later decrease. In this case, the main secretory flora was unidentified_Spirochaetaceae, Candidatus_Cloacimonas, Megasphaera, Bacteroides, and unidentified_Clostridiales. Candidatus_Cloacimonas is the main bacterial species in anaerobic digestion. These bacteria produce cellulase, lipase, protease, and other extracellular enzymes, which participates in hydrolysis and acid production. It has been reported that Clostridium is also involved in acetic acid production and synaptic oxidation (Niu et al. 2015; Theuerl et al. 2015). Ruminiclostridium and Bacteroides belong to Bacteroides, which are able to decompose sugars, amino acids, and other organic matter, and produce acid and gas (Jang et al. 2014). Acidaminococcus mainly metabolizes amino acids, especially glutamic acid. The products of this process are acetic acid, butyric acid, and CO2 (Peng et al. 2018). Megasphaera is able to ferment fructose and lactic acid, which are widely found in the rumen of cattle and sheep. Unidentified_Clostridiales belongs to Clostridium, which usually decomposes sugars and proteins to produce mixed organic acids and alcohols (Nelson et al. 2011). By observing the changes of hydrolase activity and microflora abundance in the four groups of experiments, it was determined that hydrolase increased at first and later decreased. This may have occurred because in the sequencing batch experiment, the content of starch, protein, and other organic matter was quantitative and constantly consumed. The change of each hydrolase accords with the change law of microflora; according to the data presented in Fig. 4, the peak values of amylase activity in the four groups were 277.00, 318.73, 335.47, and 309.28 IU/L. Peak values of protease activity were 106.81, 101.03, 133.82, and 160.03 IU/L, respectively. The activity of cellulase is relatively higher than other enzymes because the reaction system contains a large amount of straw and cellulose is difficult to degrade. Peaks of cellulase activity were 189.44, 238.70, 237.18, and 175.89 U/mL. Comparing the relative microbial abundance and enzyme activity of the samples containing pure chicken manure with those containing small amounts of straw, it was determined that the presence of straw resulted in increased microbial abundance and hydrolase activity. The straw also increased the C/N ratio, which improved the ability of the microorganisms to use the raw materials and in turn improved the efficiency of the anaerobic digestion.
Diversity of archaeal flora
In these experiments, 32 samples were sequenced using the 16SrDNA technology. The sequences were clustered into OTU with 97% sequence similarity. The experiments were based on valid data for OTUs clustering and species clustering analysis. The four groups of experiments obtained 436 and 459. The OTUs of 456 and 450 archaebacteria were all identified as belonging to the Euryarchaeota phylum. Among them, the dominant bacteria were Methanosarcina, Methanosaeta, Methanobacterium, Methanospirillum, Methanosphaera, Methanoculleus, Methanoregula, and Methanobrevibacter. Methanosarcina and Methanosaeta belong to Methanosarcinales. As shown in Fig. 5, Methanosarcina was mainly used as the dominant strain at the end of the experiment, with a greater relative abundance at the end of the experiment, and Methanosaeta was used as the dominant strain at the beginning of the experiment, with a gradual decrease in relative abundance over time, both of which were the main contributors to methane production. Methanosarcina is the only genus of methanogenic microorganisms that can utilize all methanogenic pathways. The genus is tolerant and can adapt to higher volatile fatty acids (Luo and Angelidaki 2014). Methanosaeta is more sensitive to environmental changes compared to Methanosarcina and its number decreases with increasing acetic acid concentration. Therefore, the relative abundance of Methanosaeta decreased significantly in the early stage and was consistent with the trend of pH and volatile fatty acids (Song et al. 2014).
Relative abundance of archaeal flora. a Changes of relative abundance of archaeal flora in pure chicken feces. b Changes of relative abundance of archaeal flora in 3% straw addition group. c Changes of relative abundance of archaeal flora in 5% straw addition group. d Changes of relative abundance of archaeal flora in 10% straw addition group
Table 3 shows the alpha diversity for each group of bacteria and archaea present during the anaerobic digestion. In terms of indicators such as OTU, abundance, uniformity, and diversity, the values of the parameters obtained for the bacterial community were higher than those obtained for the archaeal community. These results were consistent with those previously reported by Ros et al. (2017). The Chao1 index can be used to estimate the index of the number of OTU contained in a given sample. The higher the index, the greater the diversity of the species (Ros et al. 2017). Also, the Shannon index evaluates the richness and consistency of species in a sample. In this case, a high value indicates an abundance of species in the environment. It also indicates that the species are evenly distributed (Karakashev et al. 2006). Additionally, the Simpson index refers to the probability that two OTU, randomly selected from a sample data belong to different species. Here, the greater the probability, the higher the species diversity of the sample, and vice versa (Ziganshina et al. 2015). This index can also evaluate the status and the role of the dominant species present in the community. Data presented in Table 3 shows that the number of OTU of Archaea was much lower than that of bacterial. With respect to Archaea, the results obtained for the Shannon index, the Simpson index, and the Chao1 index were lower than those for bacteria. This indicates that the richness and evenness of bacterial species in the samples were higher than those of Archaea. Comparing the four groups of experiments, the data indicated that M2 (samples containing 3% straw) displayed a higher richness and evenness of the species. This result may indicate that the addition of a small amount of straw promotes the anaerobic digestion and improve the utilization rate of the raw materials.
Conclusion
Compared with the pure chicken manure group, the 3% straw addition group increased methane production by 18%. In the hydrolysis stage, the hydrolase activity was directly proportional to the change of the main acting bacterial groups, and the hydrolase activity and flora abundance in samples containing straw concentrations of 3% and 5% were higher than those containing either pure chicken manure or 10% straw. The dominant bacterial groups of methanogenic archaea are mainly Methanosarcina, Methanosaeta, Methanobacterium, and Methanospirillum. The addition of a small amount of straw enhanced the production capacity of hydrogen-nutritive methane to some extent, and the species richness and evenness were also improved.
References
Böjti T, Kovács KL, Kakuk B, Wirth R, Rákhely G, Bagi Z (2017) Pretreatment of poultry manure for efficient biogas production as monosubstrate or co-fermentation with maize silage and corn stover. Anaerobe 46:138–145
Ebner JH, Labatut RA, Lodge JS, Williamson AA, Trabold TA (2016) Anaerobic co-digestion of commercial food waste and dairy manure: characterizing biochemical parameters and synergistic effects. Waste Manag 52:286–294
Fang W, Zhang P, Zhang T, Requeson DC, Poser M (2019) Upgrading volatile fatty acids production through anaerobic co-fermentation of mushroom residue and sewage sludge: performance evaluation and kinetic analysis. J Environ Manag 241:612–618
Jang HM, Kim JH, Ha JH, Park JM (2014) Bacterial and methanogenic archaeal communities during the single-stage anaerobic digestion of high-strength food wastewater. Bioresour Technol 165:174–182
Karakashev D, Batstone DJ, Trably E, Angelidaki I (2006) Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl Environ Microbiol 72(7):5138–5141
Krause L, Diaz NN, Edwards RA, Gartemann KH, Kromeke H, Neuweger H, Puhler A, Runte KJ, Schluter A, Stoye J, Szczepanowski R, Tauch A, Goesmann A (2008) Taxonomic composition and gene content of a methane-producing microbial community isolated from a biogas reactor. J Biotechnol 136(1-2):91–101
Kung CC, Mu JE (2019) Prospect of China’s renewable energy development from pyrolysis and biochar applications under climate change. Renew Sustain Energy Rev 114:109343
Li D, Liu S, Mi L, Li Z, Yuan Y, Yan Z, Liu X (2015) Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and pig manure. Bioresour Technol 187:120–127
Luo G, Angelidaki I (2014) Analysis of bacterial communities and bacterial pathogens in a biogas plant by the combination of ethidium monoazide, PCR and Ion Torrent sequencing. Water Res 60:156–163
Mahdy A, Bi S, Song Y, Qiao W, Dong R (2020) Overcome inhibition of anaerobic digestion of chicken manure under ammonia-stressed condition by lowering the organic loading rate. Bioresour Technol Rep 9:100359
Nelson MC, Morrison M, Yu ZT (2011) A meta-analysis of the microbial diversity observed in anaerobic digesters. Bioresour Technol 102(4):3730–3739
Niu QG, Kubota K, Qiao W, Jing ZQ, Zhang YL, Yu-You L (2015) Effect of ammonia inhibition on microbial community dynamic and process functional resilience in mesophilic methane fermentation of chicken manure. J Chem Technol Biotechnol 90(12):2161–2169
Peng XY, Zhang SY, Li L, Zhao XF, Ma Y, Shi DZ (2018) Long-term high-solids anaerobic digestion of food waste: effects of ammonia on process performance and microbial community. Bioresour Technol 262:148–158
Ros M, Oliveira JD, Murcia MDP, Bustamante MA, Moral R, Coll MD, Santisima-Trinidad ABL, Pascual JA (2017) Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: dissection of the microbial community structure. J Clean Prod 156:757–765
Song CH, Li MX, Jia X, Wei ZM, Zhao Y, Xi BD, Zhu CW, Liu DM (2014) Comparison of bacterial community structure and dynamics during the thermophilic composting of different types of solid wastes: anaerobic digestion residue, pig manure and chicken manure. Microb Biotechnol 7(5):424–433
Theuerl S, Kohrs F, Benndorf D, Maus I, Wibberg D, Schluter A, Kausmann R, Heiermann M, Rapp E, Reichl U, Puhler A, Klocke M (2015) Community shifts in a well-operating agricultural biogas plant: how process variations are handled by the microbiome. Appl Microbiol Biotechnol 99(18):7791–7803
Wang YZ, Ren GX, Zhang T, Zou SZ, Mao CL, Wang XJ (2017) Effect of magnetite powder on anaerobic co-digestion of pig manure and wheat straw. Waste Manag 66:46–52
Wei Y, Yuan H, Wachemo AC, Li X (2019) Impacts of modification of corn stover on the synergistic effect and microbial community structure of co-digestion with chicken manure. Energy Fuel 34(1):401–411
Yan W, Zhang L, Wijaya SM, Zhou Y (2020) Unveiling the role of activated carbon on hydrolysis process in anaerobic digestion. Bioresour Technol 296:122366
Yang ZY, Wang W, Zhang SY, Ma ZH, Anwar N, Liu GQ, Zhang RH (2017) Comparison of the methane production potential and biodegradability of kitchen waste from different sources under mesophilic and thermophilic conditions. Water Sci Technol 75(7):1607–1616
Yu WS, Li TX, Zhu J (2019) What determines China’s grain imports and self-sufficiency? The role of rising domestic costs and varying world market prices. China World Econ 27(5):1–24
Yusof TRT, Rahman NA, Ariff AB, Man HC (2019) Evaluation of hydrogen and methane production from co-digestion of chicken manure and food waste. Pol J Environ Stud 28(4):3003–3013
Zahan Z, Georgiou S, Muster TH, Othman MZ (2018a) Semi-continuous anaerobic co-digestion of chicken litter with agricultural and food wastes: a case study on the effect of carbon/nitrogen ratio, substrates mixing ratio and organic loading. Bioresour Technol 270:245–254
Zahan Z, Othman MZ, Muster TH (2018b) Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: a comparative batch study for C/N optimisation. Waste Manag 71:663–674
Zhao YB, Sun FR, Yu JD, Cai YF, Luo XS, Cui ZJ, Hu YG, Wang XF (2018) Co-digestion of oat straw and cow manure during anaerobic digestion: stimulative and inhibitory effects on fermentation. Bioresour Technol 269:143–152
Ziganshina EE, Belostotskiy DE, Ilinskaya ON, Boulygina EA, Grigoryeva TV, Ziganshin AM (2015) Effect of the organic loading rate increase and the presence of zeolite on microbial community composition and process stability during anaerobic digestion of chicken wastes. Microb Ecol 70(4):948–960
Acknowledgements
Thanks to all the authors for their efforts, and thanks to the Scientific Research Fund of Liaoning Provincial Education Department for its financial support.
Funding
This study was financially supported by Scientific Research Fund of Liaoning Provincial Education Department (JYT2020146).
Author information
Authors and Affiliations
Contributions
XY Lin and L Feng conceived and designed research. XY Lin conducted experiments. L Feng contributed new reagents or analytical tools. XY Lin and X Li analyzed the data. XY Lin wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, L., Lin, X. & Li, X. Combined anaerobic digestion of chicken manure and corn straw: study on methanogenic potential and microbial diversity. Ann Microbiol 72, 44 (2022). https://doi.org/10.1186/s13213-022-01704-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-022-01704-5