Abstract
Purpose
The application of bio-control plants in the agricultural processes is one of the effective ways to solve the traditional agricultural synthetic pesticide residues. This study will investigate the effect of bio-control plant Litsea cubeba branch inter-row cover on soil bacterial community, soil-enriched metabolites, and soil mineral nutrition in tea plantation, which will provide a basis for the use of bio-control plant in agroecological farming systems.
Methods
The fruit-bearing (F-Pr) and vegetative (F-Ab) branches of Litsea cubeba were inter-row covered (the use of plants for partial coverage of soil between the rows) in the tea plantation. And we determined the soil microbial community, soil mineral nutriment, and soil-enriched metabolites composition with the methods of 16S rRNA gene sequencing, inductively coupled plasma-atomic emission spectroscopy (ICP-AEC), inductively coupled plasma-optical emission spectrometry (ICP-OES), and gas chromatography-mass spectrometry (GS-MS). We also predict the function of soil bacteria with the Tax4Fun software.
Results
Litsea cubeba inter-row cover modified the soil microbial structure and diversity; Litsea cubeba inter-row cover significantly decreased the relative abundance of Acidobacteria, Chloroflexi, and Planctomycetes while increased the relative abundance of Proteobacteria. Litsea cubeba inter-row cover significantly antagonized the plant pathogen community, and its OTUs number decreased from 907 ± 81 in the control to 337 ± 25 in F-Pr treatment; compared with F-Pr treatment, F-Ab treatment had weaker effect on the abundance of nutrition metabolism and transport, carbohydrate utilization, and nitrate reduction A. The aluminum element in the F-Pr treatment was significantly reduced, while phosphorus was increased. The soil-enriched metabolites of F-Pr treatment contained some antibacterial substance, including 14.2 ± 3.32% citronellol, 10.38 ± 4.79% alpha-terpineol, and 8.25 ± 2.62% (+)-2-bornanone, which was the main environment factor that affects the soil bacterial structure and diversity.
Conclusion
Litsea cubeba inter-row cover significantly affected bacterial structure and diversity, slightly increased the soil pH, and improved soil aluminum and phosphorus status; soil-enriched metabolites were the major environment factor affecting soil bacterial community and should be considered in the application of bio-control plants; Litsea cubeba vegetative branch inter-row cover will be a feasible measure for integrated pest management in tea plantation.
Similar content being viewed by others
Background
Nowadays, pest management in traditional agriculture which based on the synthetic pesticides was successful, but the detrimental effects such as pesticide residue, which pose a serious threat to human and environmental health, are increasingly attracting the public attention (Wei et al. 2017). One of the effective ways to solve the problem of pesticide residues in traditional agricultural is to use bio-control plant intercropping or coverage for integrated pest management. Intercropping of Cinnamomum hupehanum and tea significantly reduced the incidence of disease of tea leaf spot and tea round spot diseases (Sun et al. 2014). Brassicaceae species, as cover crops, have been shown to control various soilborne diseases (Larkin et al. 2010). The root, branches, flowers, and seeds of Marigold L., Tagetes minuta L., Hemp, Capsicum annuum L., and Cupressus lusitanica L. were frequently used for pest control in field and storage crops (Mugisha-Kamatenesi et al. 2008). The reason why bio-control plant could be used in pest management was the role of secondary metabolites that possess similar properties to synthetic pesticides, including toxicity, attractant, and repellency and antifeedant activity (Wang et al. 2020; Pang et al. 2021).
It had been reported that the secondary metabolites of different plant species from developmental stages or organs had also multiple effects on soil microorganisms, such as antimicrobial, attractant, or promoting effect, and even the same secondary metabolite had diametrically opposite effects on different bacteria (Trda et al. 2019; Pang et al. 2021). Benzoxazinoids were shown to attract Chloroflexi and affect the abundance of the maize microbiomes (Hu et al. 2018). The growth of soilborne pathogens was inhibited by scopoletin, whereas the other rhizobacteria were not affected (Stringlis et al. 2019). Rye and white mustard use as cover crops had positive antagonistic effect on the quantity of Bacillus spp., Pseudomonas spp., Clonostachys spp., Myrothecium spp., Penicillium spp., and Trichoderma spp. in the soil under carrot cultivation (Patkowska et al. 2016). Ageratum houstonianum intercropping with pear tree significantly decreased the relative abundances of Actinomycetes, Verrucobacterium, Gemmatimonadetes, Cyanobacteria, Ascomycetes, and Basidiomycetes, while it increased the relative abundances of functional groups of nitrite ammonification, nitrate ammonification, and urea decomposition (Zhang et al. 2021). Chen et al. (2014) found that fruit trees intercropped with basil and mint had a significant impact on the community of soil nitrogen cycling bacteria (Chen et al. 2014). In the integrated pest management system, due to the different composition and content of its secondary metabolites, the impact of bio-control plant on soil microbe may be significantly different, which need in-depth research.
Tea is one of the three soft drinks in the world. There are more than two billion people around the world who like to drink tea (Cheng et al. 2020). However, every drinker may ask: “is the tea safe?” with the fact that pesticide residue contamination is serious in tea leaves. As a result, the demand for organic tea or pesticide-free tea continues to grow. The effective way to meet the market is being laid on integrated pest management with biological plants (intercropping or coverage). Litsea cubeba is the perennial shrub of the genus Lauraceae and is a unique aromatic plant resource in China. The essential oils from fruits of Litsea cubeba have important insect repellent and antibacterial activity and were widely used in condiments, cosmetics, medicine, and other fields (Wu et al. 2013; Pen 2014). In recent years, it was used as an important biological control material in sporadic planting or branch coverage in tea plantation for pest management and has achieved remarkable results (Hao 2019). The secondary metabolites can enter the soil through rainwater leaching, litter decomposition, and root secretion and might affect the soil bacterial community. Soil microbes are an important part of soil ecosystem and the most active biological factors, which have the advantages of nitrogen fixation, phosphorus release, decomposition of organic matter, and enhancement of soil moisture (Zhou and Chen 2014); they also promote the synthesis of more special tea aroma substance, reduce tea pests and diseases, and significantly improve the quality of tea leaves (Zhou and Chen 2014; Hao 2019). Therefore, it was necessary to conduct further experiment to explore the specific response of soil microbe to biological control measures of Litsea cubeba cover in order to comprehensively evaluate its ecological effect.
The soil bioavailability of aluminum and phosphorus is considered to be the main constraints for plant growth and soil microbes in acid soils. Previous studies found that the community of Proteus, Serratia, Azotobacter, etc. was associated with the soil phosphorous and aluminum content (Jacoby et al. 2017; Kour et al. 2021). So, aluminum and phosphorus elements are also key ecological factors when studying the effect of biological control measures on soil microbes in acid soil.
The present study will investigate the effects of two type of branch — fruit-bearing and vegetative of Litsea cubeba inter-row cover — on the structure and diversity of soil bacterial community, soil-enriched metabolites, and soil mineral nutrition in bio-control tea plantation, aiming to explore the following issues: (1) soil bacterial structure and diversity response to Litsea cubeba cover, (2) the composition of soil-enriched metabolites in Litsea cubeba coverage treatment, and (3) what are the key factors affecting the soil bacterial community in Litsea cubeba cover treatment?
Results
Litsea cubeba cover modified the diversity and structure of bacteria community
Rarefaction analysis was performed on each soil sample, and the rarefaction curves tended to the plateau phase, indicating that the sequencing was almost saturated, and the bacterial community diversity in the soil sample can be truly reflected. The sampling was reasonable (SubFig. 1).
The linear mixed model analysis indicated that OTUs number, Chao1, and Shannon index of soil microbes in tea plantation were significantly affected by the covering of Litsea cubeba (Fig. 1). The number of OTUs in the soil of tea plantation was 1470 ± 25.53 in control plot (Fig. 1a). Litsea cubeba cover significantly decreased the OTUs number in soil of tea plantation. The OTUs number was decreased from 1470 ± 25.53 to 943 ± 13.75 and 727 ± 15.58 in the F-Ab and F-Pr treatment, respectively (Fig. 1a). In addition, the change in bacterial diversity was analyzed with the Shannon index, and the total species richness was estimated by the Chao1 index in the tea plantation soil. Shannon and Chao1 indices showed that covering Litsea cubeba reduced the bacterial diversity and the total species richness compared to control soil (Fig. 1b and c).
Estimated number of observed a OTUs counts, b Shannon index, c Chao1 index of soil microbiome of tea plantation across all the covering Litsea cubeba plots. Values are means ± SE (n = 4). The star (*) indicates significant difference between treatments at 0.05 level; CK, control; F-Ab, the vegetative branches cover treatment; F-Pr, the fruit-bearing branches cover treatment
Nonmetric multidimensional scaling was used to detect variation in the community pattern. The two-dimensional figure showed that the bacterial communities were distributed separately, exhibiting differences in the bacterial community pattern in control, F-Ab, and F-Pr treatment (Fig. 2). These results suggest that the structure and diversity of bacterial community changed with the covering treatment.
Litsea cubeba cover showed opposite effects on different bacterial communities
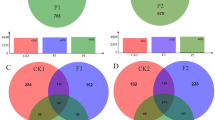
Analysis of the taxonomic groups detected in the soil samples showed that the most dominant phyla across all samples were Proteobacteria, Acidobacteria, Chloroflexi, and Planctomycetes, accounting for about 70% of the phyla (Fig. 3). In addition, Bacteroides, Verrucomicrobia, Gemmatimonadetes, Firmicutes, Saccharibacteria, Armatimonadetes, GAL15, Chlorobi, TM6-Dependentiae, and Deinococcus-thermus were detected in all the samples with low abundance, while the unclassified and rare phyla accounted for about 4.12% in the samples. Litsea cubeba cover had significant impact on the relative abundance of bacterial community (Fig. 3). The relative abundance of Proteobacteria and Acidobacteria was 33.48% and 33.54% in control, respectively (Fig. 3). Compared with the control, the relative abundance of Proteobacteria significantly increased in both Litsea cubeba cover treatment. And an obvious decrease in the relative abundance of Acidobacteria, Chloroflexi, and Planctomycetes was detected in the F-Pr treatment (Fig. 3). Litsea cubeba cover had little effect on the relative abundance of Bacteroides, Verrucomicrobia, Gemmatimonadetes, Firmicutes, Saccharibacteria, Armatimonadetes, GAL15, Chlorobi, TM6-Dependentiae, and Deinococcus-Thermus (Fig. 3).
Litsea cubeba cover antagonized plant pathogens
We conducted a functional analysis of bacterial community with the software of Tax4Fun. The functions mainly involve plant pathogens, carbon cycle and mineral enrich, plant-derived biopolymers degradation, and so on (Table 1). The OTUs number of plant pathogens community in the CK was 907 ± 81 while in the F-Ab and F-Pr treatment was 658 ± 92 and 337 ± 25, respectively (Table 1); the OTUs number of nutrition metabolism and transport were 850 ± 79, 625 ± 48, and 165 ± 37 in CK and F-Ab and F-Pr treatment, respectively. The change trends of other functional group, such as carbon cycle and mineral enrich, nitrate reduction, and carbohydrate utilization, were similar with that of nutrition metabolism and transport (Table 1).
Litsea cubeba cover changed the soil-enriched metabolites composition
The soil-enriched metabolites were obtained by ethyl-acetate extraction from air-dried soil and subsequently analyzed by GC-MS. The identified components with their relative percentages were reported in Table 2. In the F-Pr treatment, (+)-2-bornanone (8.25 ± 2.62%), alpha-terpineol (10.38 ± 4.79%), citronellol (14.2 ± 3.32%), cyclohexanol (20.97 ± 5.64%), and oleamide (35.80 ± 8.99%) were the main constituents (Table 2). The cyclohexene (0.49 ± 0.25%), nickel tetracarbonyl (0.57 ± 0.33%), and phosphine oxide (0.84 ± 0.14%) were the minimum oil (Table 2). The main constituents of control and F-Ab treatment were extraction solvent-ethyl acetate (95.97%) (Table 2).
Litsea cubeba cover reduced soil aluminum and increased phosphorus content
Covering with Litsea cubeba plants had significant effects on the content of aluminum and phosphorus (Fig. 4a and b). The aluminum content in F-Pr treatment was lowest, the content was 23.60 ± 3.54 mg/kg while in the CK and F-Ab treatment was 48.28 ± 3.32 and 26.73 ± 2.17 mg/kg, respectively. In contrast, the phosphorus content increased in Litsea cubeba cover treatment. In F-Pr and F-Ab treatments, the phosphorus content was 18.41 ± 2.42 mg/kg and 5.29 ± 0.97 mg/kg, respectively, which were higher than that in control plots (1.53 ± 0.29 mg/kg).
Effect of Litsea cubeba cover on soil mineral nutrition of tea plantation. a Aluminum. b Phosphorus. Data are means ± SE (n = 4). The star (*) indicates significant difference between treatments at 0.05 level; CK, control; F-Ab, the vegetative branches cover treatment; F-Pr, the fruit-bearing branches cover treatment
Litsea cubeba cover changed soil pH and aluminum forms
Litsea cubeba cover affected the soil pH and different aluminum forms content (Table 3). Compared with the control, Litsea cubeba cover increased the soil pH. In the F-Ab and F-Pr treatment, the soil pH index was 4.76 ± 0.02 and 5.28 ± 0.05, increased by 0.30 and 0.82, respectively. For the different aluminum forms, covering with Litsea cubeba decreased the exchangeable aluminum content. Compare with the control, the content of exchangeable aluminum of F-Ab treatment was 16.32 ± 3.34 mg/kg, and that of F-Pr treatment was 11.27 ± 2.57 mg/kg, reduced by 45.28% and 62.21%, respectively. The trend of aluminum humate was opposite to that of exchangeable aluminum. The aluminum humate content in F-Ab and F-Pr treatments increased by 68.94% and 56.51%, respectively (Table 3). The change trends of aluminum hydrous oxide and hydroxide were similar to that of aluminum humate, while the inorganic adsorption aluminum content was not obviously changed (Table 3).
Soil-enriched metabolites and mineral nutrition affected soil bacteria structure and diversity
RDA analysis results showed that the bacterial community was affected by mineral nutrition and soil-enriched metabolites. The first quadrant showed that the phosphorus distribution affected the structure and diversity of Gemmatimonadetes, Saccharibacteria, and Deinococcus-Thermus; the soil-enriched metabolites, such as oleamide (Ole), cyclohexene (Cyc), and (+)-2-bornanone (Bor), mainly distribute in the second quadrant and affected the structure and diversity of Proteobacteria and Actinobacteria; in the four quadrant, the structure and diversity of Firmicutes, Chloroflexi, Armatimonadetes, and Verrucomicrobia were mainly affected by the aluminum (Fig. 5a).
Redundancy analysis of soil bacterial and environmental factors. Al, aluminum; P, phosphorus; Ole, oleamide; Cyc, cyclohexene; Bor, (+)-2-bornanone; Alp, alpha-terpineol; Cit, citronellol; Amm, ammonia; Al-1, exchangeable aluminum; Al-2, inorganic adsorption aluminum; Al-3, aluminum hydrous oxide and hydroxide; Al-4, aluminum humate. Note: In the plot, red hollow arrows represent environmental factors; solid arrows stand for bacterial community structure information; cosine of the angle between the extension lines of environmental factor and bacterial species equals to the correlation coefficient between the two in numerical value
The environment factors explain showed that aluminum (Al), oleamide (Ole), cyclohexene (Cyc), (+)-2-bornanone (Bor), phosphorus (P), and alpha-terpineol (Alp) were the main impact factors, the value of explains > 95%, and P < 0.05 (Table 4). The redundant analysis of different forms of aluminum and bacterial community showed that the exchangeable aluminum was the main form of aluminum that affects the structure and diversity of soil bacteria (Fig. 5b).
Discussion
Soil bacterial structure and diversity were affected by a variety of factors such as management, soil nutrient, and plant root exudates (Larkin et al. 2010; Patkowska et al. 2016). In our experiment, Litsea cubeba cover could modify the structure and diversity of soil microbial community. The OTUs number, diversity, and total species richness index significantly decreased in the F-Ab and F-Pr treatment, which indicated that the bacterial community were reduced and changed (Fig. 1). But the Litsea cubeba cover did not change the dominant phyla — Proteobacteria, Acidobacteria, Chloroflexi, Planctomycetes, and Verrucomicrobia — in tea plantation (Jiang 2014) (Fig. 3). For the phyla, Litsea cubeba cover showed diametrically opposite effects of antagonism and enrichment (Fig. 3). Compared with the control, the F-Ab and F-Pr treatment significantly decreased the relative abundance of Acidobacteria, Chloroflexi, and Planctomycetes, while they enriched Proteobacteria (Fig. 3). For the functional group, the OTUs number of plant pathogens was decreased significantly in both Litsea cubeba cover treatment (Table 1). The OTUs numbers of carbohydrate utilization community, protein degradation community, were also significantly decreased in F-Pr treatment, while their communities were less affected in the F-Ab treatment. The difference in the effects of F-Ab and F-Pr treatment on soil bacterial community may be related to the difference in soil-enriched metabolites.
Soil-enriched metabolites composition analysis showed that the F-Pr treatment contained a large amount of bactericidal or antibacterial substances, such as citronellol, alpha-terpineol, (+)-2-bornanone, and 4-octene-2,7-diol (Table 2). Previous studies had proved that citronellol had bactericidal or antibacterial effects on the pathogens of Phoma spp. (Caio et al. 2016). Alpha-terpineol fumigation could directly or indirectly modify the soil microbial community by suppressing Ascomycota and enriching antagonistic bacteria (Ye et al. 2021). RDA analysis showed that citronellol, alpha-terpineol, and (+)-2-bornanone significantly affected the bacterial structure and diversity (P < 0.05) (Fig. 5, Table 4). No bactericidal or antibacterial components were detected in soil-enriched metabolites of control and F-Ab treatments, which may be responsible for that the change of the bacterial structure, and diversity was not as significant as that of F-Pr treatment (Table 2). This may be related to the difference in the content and composition of the essential oil of the branches and fruits. The fruit is the main extraction part of the essential oil of Litsea cubeba, which contains strong bactericidal substance citral. Therefore, the bactericidal or antibacterial substances in the soil-enriched metabolites may be an important ecological factor that affects the soil bacterial structure and diversity by covering with Litsea cubeba.
Aluminum and phosphorus were important soil mineral nutrition in red clay soil that affects the bacterial structure and diversity, and their explanatory coefficients were 94.8% and 94.0%, respectively (P < 0.05) (Table 4, Fig. 5). The aluminum and phosphorus contents in soil were changed with covering Litsea cubeba (Fig. 4). Previous studies also showed that there was a significant relationship between soil bacterial community and the content of aluminum and phosphorus (Wang et al. 2011; Jacoby et al. 2017; Kour et al. 2021). In our experiment, RDA analysis showed that aluminum and phosphorus were important ecological factors affecting soil microbes (P < 0.05) (Table 4, Fig. 5), which may partly explain our findings that Litsea cubeba cover significantly affected the structure and diversity of tea plantation microbes especially the functional community, such as pathogens and carbohydrate utilization community (Table 1).
Aluminum is harmful to most organisms; in order to further explain the relationship between aluminum and soil bacteria, we measured the different forms of aluminum. In the experiment, Litsea cubeba cover significantly affected the content of aluminum humate and exchangeable aluminum; for example, the aluminum humate content in F-Pr treatment was 156.51% of that in control (Table 3). This may be due to the increase in soil organic matter content caused by decomposition of the covering material (Zhang et al. 2013; Gou et al. 2020). And the decrease of exchangeable aluminum in the Litsea cubeba cover treatment may be related to the increase of soil pH, for microorganism could affected the release of hydrogen ions (Chen et al. 2007; Liu et al. 2014). RDA analysis also showed that aluminum humate and exchangeable aluminum were important forms that affect the structure and diversity of soil bacteria.
The comprehensive effect of bio-control plant application has always attracted people attention. In our experiment, Litsea cubeba cover treatment changed the aluminum and phosphorus content, decreased the aluminum/phosphorus ratio, increased the soil pH, and improved the soil nutrient status. Previous studies showed that reduction of aluminum/phosphorus ratio was conducive to tea growth, aroma material synthesis, and improving the quality of tea leaves (Flaten 2002; Duan 2012). Litsea cubeba cover significantly reduced the abundance of plant pathogens community and functional group in the F-Pr treatment. The effect of F-Ab treatment on soil bacterial community was mild, which indicates that it will be a relatively feasible method to maintain the balance of ecology and economic benefits. Although the growth of tea trees and tea quality had been improved to a certain extent during our experiment (Hao 2019), the ecological effect of the application of bio-control plants was a long-term process; therefore, long-term monitoring of soil physical and chemical properties, tea tree growth and development, tea leaves quality, etc. is required.
Conclusion
Litsea cubeba cover changed soil bacterial structure and diversity, slightly increased the soil pH, and improved soil aluminum and phosphorus status, the soil of fruit-bearing branch cover (F-Pr) treatment has enriched metabolites with multiple antibacterial or bacteriostatic components which could significantly inhibit soil microbial community without distinguishing bacterial species, and the vegetative branch cover (F-Ab) treatment had less effect on the abundance of functional group of nutrition metabolism and transport, carbohydrate utilization, and nitrate reduction A. Therefore, Litsea cubeba vegetative branch inter-row cover may be a feasible measure for integrated pest management in tea plantation. Our finding suggested that the selection of inter-row covering material in agroecological farming systems should pay attention to the secondary metabolites that enter the soil, especially those with bactericidal or antibacterial effects.
Materials and methods
Study site
The experiment was conducted in Longnan County (114°78′E, 24° 92′N, 600 m a.s.l), Jiangxi province, China. The area is located in subtropical humid monsoon climate zone where the mean annual temperature is 18.9 °C, and long-term annual precipitation is 1526.3 mm. The soil in the area land is red clay soil based on USDA soil taxonomy (USA soil classification system). This area is one of the main production regions of Chinese tea.
Experimental design
In July 2015, when the oil content and composition of Litsea cubeba fruit were optimal, the fruit-bearing and vegetative branches were collected, respectively. According to the principle of randomized block experiment design, 12 plots were selected and divided evenly into 4 groups with 3 treatments in each group: control (CK), vegetative branches cover (F-Ab), and fruit-bearing branches cover (F-Pr). Each plots area was 3 × 4 m, the distance between two plots was 5 m, and there is 1 buffer tea row between 2 groups. The covering thickness of each treatment was 5 cm, the cover material was mixed evenly every 3 months, and the treatment continues for 1 year. In July 2016, the soil samples were collected after the surface debris were removed; sample from 0 to 30 cm was collected.
Each soil sample was about 4–5 kg, the soils were homogenized, and then, the stone and other debris were removed, 0.5 kg soil of each sample was stored at −20 °C for molecular analyses, and the other soil sample was air-dried, ground, and sieved through a 2-mm mesh for soil mineral nutrition, soil pH, and soil-enriched metabolites analysis.
Soil pH determination
Air-dried soil, weighted 10 g, was then placed into a 50 ml flask, added 25 ml potassium chloride solution (1 mol/L) into the soil samples, stirred 1–2 min, stood 30 min, and measured with a pH meter.
Soil ion content measurement
The air-dried soil sample, sieved through a 0.15 mm mesh again, weighted 0.5 g, was placed into a polytetrafluoroethylene tube, and then added 5 ml hydrofluoric acid, 10 ml mixed acid (nitric acid: perchloric acid = 1:1), put the tube in a microwave digestion system for 90 min. Then, the digested samples were placed on a hot plate to dry, 100 °C, 3–5 min. Transferred residues to 50 ml glass tube and the original polytetrafluoroethylene were washed twice with deionized water and were transferred together and finally diluted to 10 ml in volume. The Al3+ and P3+ concentration was determined by an inductively coupled plasma-atomic emission spectroscopy (ICP-AEC) (Song et al. 2011; Hao et al. 2018).
Different forms of aluminum determination
The different forms of aluminum content were determined by using continuous extraction method (Huang and Qu 1996; Pang et al. 1986).
-
1.
Exchangeable aluminum: Air-dried soil 1 g; placed in a 100 ml plastic centrifuge tube; added 10 ml potassium chloride (1 mol/L); shook 30 min; centrifuged at 2000 r/min, 15 min; and collected the supernatant. Using deionized water washes the residue, centrifuged 5 min, and discarded the supernatant.
-
2.
Inorganic adsorption aluminum: Took the residue in 1st step, added 10 ml NH4Ac (1 mol/L), shook 8 h, centrifuged 5 min, and collected the supernatant. Washed the residue with deionizer water, centrifuged 5 min, and discarded the supernatant.
-
3.
Aluminum hydrated oxides and hydroxides: Took the residue in 2nd step, added 10 ml HCl (1 mol/L), shook 1.5 h, centrifuged 5 min, collected the supernatant, washed the residue using deionizer water, centrifuged 5 min, and discarded the supernatant.
-
4.
Aluminum humate: Took the residue in 3nd step, added 10 ml NaOH (0.5 mol/L), shook 3 h, centrifuged 5 min, and collected the supernatant.
The aluminum ions in the supernatant of each step were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES).
Soil-enriched metabolites determination
Gas chromatography-mass spectrometry (GC-MS) method was used to detect soil-enriched metabolites (Duan et al. 2015).
-
Took 2 kg air-dried soil, placed in glass bottle, and added 80% ethyl-acetate solution, soil: solution was 1:3, put the glass bottles in shaking incubator, 20 °C, 200 r/min, 24 h; then completely transferred to 50 ml centrifuge tube, 5 000 r/min, 15 min; then took the supernatant, concentrated to 1–1.5 ml with vacuum rotary evaporator, and condition was 300 hPa, 40–45 °C; then dehydrated with anhydrous sulfuric acid; transferred to an ampoule for storage; and the concentrate was the soil-enriched metabolites.
-
TR-1 silica capillary column (30 m × 0.32 mm × 0.25 μm): carrier gas: He, flow rate 1 ml/min, spitless (split ratio 50:1), and constant pressure mode; injector temperature: 250 °C; gas flow rate is 1.0 ml min−1; programmed oven temperature: initial temperature 70 °C and hold 2 min; and then, the temperature was gradually increased to 280 °C for 20 min. frequency 10 °C min−1.
-
Mass spectrometry conditions: EI source, electron energy 70 ev, and temperature 230 °C; scanning speed 0.2 s, scanning range (m/z) 35–500 amu; and solvent delay time 3 min, the standard mass spectrum library was used to compare the obtained data, and the content of each component of the extract was calculated by the area method.
Sequencing and data analysis
Total DNA was extracted from 0.5 g soil samples (0.5 g/sample) using the SoilGen DNA Kit (CW biotech Corporation, China) following the manufacturer’s instructions. The 515f/806r primer set (GTGCCAGCMGCCGCGGTAA/GGACTACHVGGGTWTCTAAT) was used to amplify the V4 region of the 16S rRNA gene (Peiffer et al. 2013). The reverse primer contained a 6-bp error-correcting barcode unique to each sample. The PCR reaction (30 μL) mixture was composed of 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs), 0.2 μM of each forward and reverse primer, and about 10 ng DNA templates. Thermal cycling conditions were as follows: initial denaturation at 98 °C for 1 min followed by 30 cycles of 98 °C for 10 s, 50 °C for 30 s, 72 °C for 1 min, and a final extension with 72 °C for 5 min. PCR products were purified with GeneJET Gel Extraction Kit (Thermo Scientific). NEBNext Ultra DNA library Prep Kit for Illumina (NEB, USA) was used to generate sequencing libraries following manufacturer’s recommendations, and index codes were added. The library quality was evaluated with the Qubit @ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Finally, sequencing was performed on an Illumina MiSeq platform, and 300 bp paired-end reads were generated.
Paired-end reads from the original DNA fragments were merged with FLASH software (Magoc and Salzberg 2011). The paired-end reads were assigned to samples according to their unique barcode and UPARSE pipeline. The reads were first filtered with the QIIME software package (Quantitative Insights into Microbial Ecology) with the default settings. Then, the operational taxonomic units (OTUS) picking was performed with UPARSE pipeline (Edgar 2013). Sequences with greater than or equal to 97% similarity were assigned to the same OTUs. A representative sequence was picked for each OTUs and was assigned with taxonomic data using the version 2.2 RDP classifier with a confidence threshold of 0.8 (Wang et al. 2007). Singleton OTUs could be potential sequencing errors. The alpha diversity was analyzed by calculating three metrics: Chao1, the observed species, and Shannon index. QIIME was used to do nonmetric multidimensional scaling (NMDS).
Statistical analysis
We conducted separate ANOVA and Dunnett’s tests to determine the bacterial diversity and relative abundance of phyla between CK and treatments (SPSS 16.0). Square root transformation of the OTUs data and arcsine square root transformation of relative abundance of phyla were done before proceeding with ANOVA and post hoc test. The assumptions of normality and homogeneity of variance were checked prior to conduct the statistical tests.
Canoco 5 software, which was the programs for statistical analysis using ordination methods in the field of ecology and related fields, was used to conduct multivariate analysis to study the effects of soil-enriched metabolites and mineral nutrition factors on soil bacterial community using redundant analysis (RDA). Tax4Fun which was a R package that predicts the functional capabilities of bacterial community based on 16 s datasets, was used to predict the soil bacterial community function. It was regarded as significant differences when P-value was less than 0.05.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Caio MCS, Silvio APJ, Tháisda SM, Jaqueline LD, Suzana AM, Herbert JD, Ricardo S, Denise CT, Carlos HGM, Antonio EMC, Maria JSMG, Regina HP (2016) Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Med Mycol 54(5):515–523
Chen WW, Chen CQ, Liu P, Xu GD, He F, Fang M, Chi H (2007) Forms of aluminium in rhizospher soil and its effect on growth of Fagopyrum esculentum and Fagopyrum cymosum. J Soil Water Conserv 21(1):176–179
Chen XX, Song BZ, Yao YC, Wu HY, Hu JH, Zhao LL (2014) Aromatic plants play an important role in promoting soil biological activity related to nitrogen cycling in an orchard ecosystem. Sci Total Environ 472:939–946
Cheng Z, Zhang ZF, Han Y, Wang J, Wang YG et al (2020) A review on 557 anti-cancer effects of green tea catechins. J Funct Foods 74:104172
Duan J, Wang LY, Yang J, Yu CF, Wan JL, Liu Z (2015) Chemical constituents in rhizospheric soil extracts of Pinus massoniana and Liquidambar formosana. Sci Sil Sinic 51(8):8–15
Duan XH (2012) Study on the factors of affecting aluminium cycling of tea plant and qualities of tea leaves. Nan Chang University, Dissertation, Nanchang
Edgar RC (2013) UPARSE: highly accurate OTUs sequences from microbial amplicon reads. Nat Methods 10:996
Flaten TP (2002) Aluminium in tea-concentations, speciation and bioavailability. Coord Chem Rev 228:385–395
Gou XM, Cai Y, Wang CQ, Li B, Zhang RQ, Zhang Y, Tang XY, Chen Q, Shen J, Deng JR, Zhou XY (2020) Effects of different long-term cropping systems on phoD-harboring bacterial community in red soils. J Soils Sediments 21(1):376–387
Hao HP (2019) Effects of intercropping Listsea cubeba and Taxus chinensis on soil microbes and trace element in tea plantation. Institute of Botany, The Chinese Academy of Sciences, Dissertation, Beijing
Hao HP, Li H, Jiang CD, Tang YD, Shi L (2018) Ion micro-distribution in varying aged leaves in salt-treated cucumber seedlings. Plant Physiol Biochem 129:71–76
Hu L, Robert CAM, Cadot S, Zhang X, Ye M, Li B et al (2018) Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 9:2738
Huang YC, Qu CL (1996) Study on the dissolution and morphology of aluminium in soil. Environ Sci 17(1):56–59
Jacoby R, Peukert M, Succurro A, Koprivova A, Kopriva S (2017) The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front Plant Sci 8:1617
Jiang H (2014) The response of soil microbial community structure to cultivating age, fertilization and high temperature in tea orchard. Zhe Jiang University, Dissertation, Hangzhou
Kour D, Rana KL, Kaur T, Yadav N, Yadav AN et al (2021) Biodiversity current developments and potential biotechnological applications of phosphours-solubilizing and -mobilizing microbes: a review. Pedosphere 31(1):43–75
Larkin RP, Griffin TS, Honeycutt CW (2010) Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis 94:1491–1502
Liu SK, Zhou WJ, Miao XL, Yang W, Yang J, Guo ZC (2014) Evolvement of aluminium forms and its effect factors in tea rhizospheric soil. Soil 46:881–885
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Mugisha-Kamatenesi M, Deng AL, Ogendo JO, Omolo EO, Mihale MJ, Otim M, Buyungo JP, Bett PK (2008) Indigenous knowledge of field insect pests and their management around lake Victoria Basin in Uganda. Afr J Environ Sci Technol 2:342–348
Pang SW, Kang DM, Wang YB, Lin T (1986) Study on dissolution and morphology of active aluminium in soil by chemical extraction. Environ Chem 5(3):68–76
Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L, Xu J, Cheng Y (2021) Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci 12:621276
Patkowska E, Baewicz-Woniak M, Konopiński M, Wach D (2016) The effect of cover crops on the fungal and bacterial communities in the soil under carrot cultivation. Plant Soil Environ 62:237–242
Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci USA 110:6548–6553
Pen B (2014) Study on extraction and application of Litsea cubeba essential oil. University of South China, Dissertation, Guangzhou
Song J, Shi GW, Gao B, Fan H, Wang BS (2011) Waterlogging and salinity effects on two Suaeda salsa populations. Physiol Plantarum 141(4):343–351
Stringlis IA, de Jonge R, Pieterse CMJ (2019) The age of coumarins in plant-microbe interactions. Plant Cell Physiol 60:1405–1419
Sun YN, Ran LX, Cai L, Liang MZ, Xu Y, Liu DH, Xia LF (2014) Investigation of major diseases, pests and predators in different intercropping tea plantations. Shandong Agric Sci 46(11):110–113
Trda L, Janda M, Mackova D, Pospichalova R, Dobrev PI, Burketova L et al (2019) Dual mode of the saponin aescin in plant protection: antifungal agent and plant defense elicitor. Front Plant Sci 10:1448
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Wang SQ, Hu CY, Cheng DH, Liao WY, Ge HL, Fang JY (2011) Effect of Al3+ on populations and major physiological groups of microbes in tea garden soils. Environ Pollut Contr 33(6):36–43
Wang W, Yang J, Zhang J, Liu YX, Tian C, Qu B et al (2020) An Arabidopsis secondary metabolite directly targets expression of the bacterial type iii secretion system to inhibit bacterial virulence. Cell Host Microbe 27:601–613
Wei Q, Mu X, Yu HY, Niu ND, Wang LX, Zheng C, Chen Z, Gao C (2017) Susceptibility of Empoasca vitis (Hemiptera: Cicadellidae) populations from the main tea-growing regions of China to thirteen insecticides. Crop Prot 96:204–210
Wu J, Yang QY, Zhao XJ, Kan JQ, Du MY (2013) Study on antimicrobial activities and antimicrobial mechanism of essential oil from Litsea cubeba. Sci Tech Food Ind 34(17):119–125
Ye C, Liu Y, Zhang JX, Li TY, Zhang YJ, Guo CW, Yang M, He XH, Zhu YY, Huang HC, Zhu SS (2021) α-Terpineol fumigation alleviates negative plant-soil feedbacks of Panax notoginseng via suppressing Ascomycota and enriching antagonistic bacteria. Phytopathol Res 3:13
Zhang LS, Liu FT, Zhang YW, Li XW, Li BZ, Xu SR, Gu J, Han MY (2013) Effects of different mulching on soil organic carbon fractions and soil microbial community of apple orchard in loess plateau. Sci Agric Sin 46:3180–3190
Zhang Y, Han M, Song M, Tian J, Song B, Hu Y, Zhang J, Yao Y (2021) Intercropping with aromatic plants increased the soil organic matter content and changed the microbial community in a pear orchard. Front Microbiol 12:616932
Zhou CB, Chen WP (2014) Research progress of soil microorganism in tea plantation. China Tea 3:14–15
Acknowledgements
We thank An-Yang (the Institute of Botany, CAS) and Xiu Qing Li (Agriculture and Agri-Food Canada-Potato Research Centre) for thoughtful feedback and discussions on manuscript.
Funding
This study was funded by the business commissioned projects (No: 70009C6057), National Key R&D Program (No: 2019YFD1000305-09).
Author information
Authors and Affiliations
Contributions
All authors discussed the results and commented on the manuscript. HPH and HTB designed the project. HPH, HTB, and XF collected the samples. HPH, XYY, and LS performed the molecular biology analyses. HPH, XYY, LS, and HXC analyzed the metadata. HPH and XMX conduct of pot experiment, HPH, XF, LS, and XYY wrote the manuscript, while LS revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of authors.
Consent for publication
All authors approved the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: SubFig. 1.
Rarefaction curves of all samples were generated for bacterial OTUs which contained unique sequences and were defined at 97% sequence similarities. CK: Control; F-Ab: The vegetative branches cover treatment; F-Pr: The fruit-bearing branches cover treatment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hao, HP., Xia, F., Bai, HT. et al. Impact of Litsea cubeba inter-row cover on the structure of bacterial community in the tea plantation. Ann Microbiol 72, 37 (2022). https://doi.org/10.1186/s13213-022-01696-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13213-022-01696-2