Abstract
Background
Changes in driving behaviour may start at the preclinical stage of Alzheimer’s disease (AD), where the underlying AD biological process has begun in the presence of cognitive normality. Here, we summarize the emerging evidence suggesting that preclinical AD may impact everyday driving behaviour.
Main
Increasing evidence links driving performance and behaviour with AD biomarkers in cognitively intact older adults. These studies have found subtle yet detectable differences in driving associated with AD biomarker status among cognitively intact older adults.
Conclusion
Recent studies suggest that changes in driving, a highly complex activity, are linked to, and can indicate the presence of, neuropathological AD. Future research must now examine the internal and external validity of driving for widespread use in identifying biological AD.
Similar content being viewed by others
Background
With the ageing population, the proportion of older drivers on the road is also increasing. In many countries such as Canada, the USA, and Australia, driving is essential for daily activities among older adults [1, 2]. Specifically, driving can contribute to older adults’ quality of life by supporting their independence, autonomy, and access to a variety of services [3]. Given that age is the most important risk factor for Alzheimer’s disease (AD), it is anticipated that the number of drivers with AD will continue to grow. Individuals at the early symptomatic stages may be able to safely drive [4]. However, AD will eventually impact the fitness to drive, and people at later stages of AD have to eventually stop driving [5,6,7]. Studies have shown that older drivers with mild to moderate AD are at a 2 to 8 times higher risk of crashes compared to age-matched controls [8, 9]. Additionally, due to navigational deficits, people with AD may become disoriented in different environments and face difficulty finding their way even in familiar environments [10, 11]. While driving, these individuals may forget where they intended to go, not recognize their neighbourhood streets and landmarks, and consequently become lost [12]. Becoming lost may have serious consequences and can place drivers at greater risk of injury and even death [12, 13]. To date, many studies have investigated the interconnectedness between symptomatic AD and driving. Less attention has been paid to changes in driving in the preclinical stage of AD, which occurs in individuals with evidence of AD pathology who have no clinical symptoms. This mini-review summarizes what is known to date about preclinical AD and driving.
Main text
Early evidence from autopsy studies
The earliest evidence that driving may be associated with preclinical AD is from autopsy studies in the late 1990s and early 2000s. The first two of these studies were based on the results of neuropathological examinations on 98 drivers aged 65 years and older, who were killed in traffic accidents between 1992 and 1995 [14, 15]. These two studies demonstrated that 50% and 72% of drivers aged 65–75 years and 75+ years had neuritic plaques, the most prominent pathology found in people with AD, respectively [14] and that among older drivers who died in car accidents, 47–53% have had incipient AD [15]. This evidence was further supported in a later study that examined the brains of older drivers who died as a result of a motor vehicle accident (MVA) and showed that mild neuritic plaque pathology was increased for MVA deaths compared to controls [16].
AD biomarkers and driving: progress to date
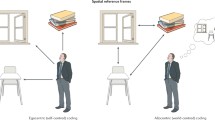
In the late 1900s and early 2000s, positron emission tomography (PET) and cerebrospinal fluid collection (CSF) biomarkers of AD were tested and validated and later became the leading tools to detect AD pathology in vivo at the early stages [17, 18]. These advancements in AD biomarkers inspired research into the associations between driving and in vivo AD. In one of the first studies in this area, an increased number of errors in on-road tests were observed among cognitively normal older adults with higher ratios of CSF tau/Aβ42 and ptau181/Aβ42, as well as mean cortical binding potential (MCBP) for Pittsburgh Compound B (PIB), consistent with the presence of underlying AD pathology [19]. Other studies based on self-reported driving habits questionnaire also indicate that persons with preclinical AD show patterns of risky driving (e.g. higher frequency of traffic violations and accidents) similar to, albeit to a lesser degree, those with very early Alzheimer’s dementia [20, 21]. Furthermore, persons with preclinical AD also have a more rapid time to fail a road test in the future compared to those without the disease [22, 23]. These studies used self-reported questionnaires and on-road tests to characterize driving behaviours (Fig. 1), which present challenges. More specifically, self-reported driving questionnaires do not provide a thorough overview of driving behaviours and may be subject to recall bias, whereas on-road assessments are limited by challenges in availability, generalizability, and affordability [24, 25]. Furthermore, although many studies have evaluated the effects of AD and mild cognitive impairment on driving ability in simulated driving experiments [26,27,28], to the best of our knowledge, no study to date has examined simulated driving in individuals at the preclinical stage of AD.
Everyday driving: the opportunity of mobile technology
A few attempts have been made to address the limitations of on-road assessments and self-reported questionnaires by adopting mobile sensor technologies to enable thorough monitoring of everyday driving behaviours in naturalistic settings. A number of studies that implemented naturalistic driving methodologies, such as the Driving Real-world In-Vehicle Evaluation System (DRIVES), provided additional support for earlier findings on the differences in driving behaviours of cognitively normal older adults with and without preclinical AD [29,30,31]. These studies indicate that persons with preclinical AD already exhibit a pattern of driving restriction similar to those with early Alzheimer’s disease. More specifically, their findings suggest that older adults with preclinical AD are likely to drive less often and have fewer aggressive behaviours such as hard braking, speeding, and sudden acceleration [29,30,31,32]. Furthermore, a 2.5-year longitudinal assessment using DRIVES indicated that persons with preclinical AD also show a greater decline across the follow-up period in the number of days driving per month and the number of trips between 1–5 miles [33]. Most recently, a study evaluated the feasibility of identifying preclinical AD from everyday driving behaviours using machine learning methods on a larger sample of cognitively intact older adults with and without preclinical AD [34]. These findings demonstrated that daily driving behaviours combined with age predict underlying biological AD with high sensitivity (84%), specificity (94%), and accuracy (86%).
These studies suggest that AD, defined using CSF and imaging biomarkers, impacts driving behaviour even among cognitively normal persons. Speculatively, preclinical AD effects on driving may be linked to subtle systemic changes (e.g. cognitive, visual, spatial, motor function) that accompany this stage of the disease [33]. Although such changes may be so subtle as to go unnoticed or undetected, they may in fact be reflected in complex behaviours such as driving [19].
Conclusion
The studies included in this mini-review provide strong evidence of a significant relationship between AD biomarkers and everyday driving, which is a complex instrumental activity of daily living. Such findings are critically needed because they can advance everyday driving, as a digital, cost-effective and accessible biomarker for early AD identification among older adults. It should be noted that the associations in this Minireview come from a small and selected number of studies. Therefore, this needs to remain a research question until far more data have been collected, and the findings should not be used to inform policy. Changes such as driving shorter distances, less often and more cautiously should not be used for decisions related to driving ability or insurance.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- CSF:
-
Cerebrospinal fluid collection
- PET:
-
Positron emission tomography
- GPS:
-
Global Positioning System
- DRIVES:
-
Driving Real-world In-Vehicle Evaluation System
References
Rosenbloom S. Sustainability and automobility among the elderly: an international assessment. Transportation. 2001;28:375–408.
Ross LA, Anstey KJ, Kiely KM, Windsor TD, Byles JE, Luszcz MA, et al. Older drivers in Australia: trends in driving status and cognitive and visual impairment. J Am Geriatr Soc. 2009;57:1868–73.
Spinney JEL, Newbold KB, Scott DM, Vrkljan B, Grenier A. The impact of driving status on out-of-home and social activity engagement among older Canadians. J Transp Geogr. 2020;85:102698.
Man-Son-Hing M, Marshall SC, Molnar FJ, Wilson KG. Systematic review of driving risk and the efficacy of compensatory strategies in persons with dementia. J Am Geriatr Soc. 2007;55:878–84 United States.
Donnelly RE, Karlinsky H. The impact of Alzheimer’s disease on driving ability: a review. J Geriatr Psychiatry Neurol. 1990;3:67–72 United States.
Ali AA, Adler G, Rapoport M. Driving and dementia-an introduction, educational resources, and international perspectives. Am J Geriatr Psychiatry. 2017;25:S19–20 Netherlands: Elsevier B.V.
Ikpeze TC, Elfar JC. The geriatric driver: factors that influence when to stop driving. Geriatr Orthop Surg Rehabil. 2016;7:106–9 United States: SAGE Publications Inc. (E-mail: claims@sagepub.com).
Retchin SM, Hillner BE. The costs and benefits of a screening program to detect dementia in older drivers. Med Decis Making. 1994;14:315–24 SAGE Publications Inc STM.
Friedland RP, Koss E, Kumar A, Gaine S, Metzler D, Haxby JV, et al. Motor vehicle crashes in dementia of the Alzheimer type. Ann Neurol. 1988;24:782–6 United States.
Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route-following and safety errors in early Alzheimer disease. Neurology. 2004;63:832–7 United States.
Rowe MA, Feinglass NG, Wiss ME. Persons with dementia who become lost in the community: a case study, current research, and recommendations. Mayo Clin Proc. 2004;79:1417–22.
Hunt LA, Brown AE, Gilman IP. Drivers with dementia and outcomes of becoming lost while driving. Am J Occup Ther. 2010;64:225–32.
Rowe MA. People with dementia who become lost: preventing injuries and death. AJN Am J Nurs. 2003;103:32–9.
Viitanen M, Johansson K, Bogdanovic N, Berkowicz A, Druid H, Eriksson A, et al. Alzheimer changes are common in aged drivers killed in single car crashes and at intersections. Forensic Sci Int. 1998;96:115–27 Ireland.
Johansson K, Bogdanovic N. Alzheimer’s disease and apolipoprotein E epsilon4 allele in older drivers who died in automobile acc. Lancet. 1997;349:1143–4 Elsevier Science Publishing Company, Inc.
Gorrie CA, Rodriguez M, Sachdev P, Duflou J, Waite PME. Mild neuritic changes are increased in the brains of fatally injured older motor vehicle drivers. Accid Anal Prev. 2007;39:1114–20.
Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–45.
Vlassenko AG, Benzinger TLS, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta BBA - Mol Basis Dis. 2012;1822:370–9.
Roe CM, Barco PP, Head DM, Ghoshal N, Selsor N, Babulal GM, et al. Amyloid imaging, cerebrospinal fluid biomarkers predict driving performance among cognitively normal individuals. Alzheimer Dis Assoc Disord. 2017;31:69–72 United States.
Ott BR, Jones RN, Noto RB, Yoo DC, Snyder PJ, Bernier JN, et al. Brain amyloid in preclinical Alzheimer’s disease is associated with increased driving risk. Alzheimers Dement Diagn Assess Dis Monit. 2017;6:136–42.
Owsley C, Stalvey B, Wells J, Sloane ME. Older drivers and cataract: driving habits and crash risk. J Gerontol Ser A. 1999;54:M203–11.
Roe CM, Babulal GM, Stout SH, Vernon EK, Ghoshal N, Fierberg R, et al. Preclinical Alzheimer’s disease and longitudinal driving decline. Alzheimers Dement Transl Res Clin Interv. 2017;3:74–82 United States: Elsevier Inc. (E-mail: info@wiley.com).
Roe CM, Babulal GM, Mishra S, Gordon BA, Stout SH, Ott BR, et al. Tau and amyloid positron emission tomography imaging predict driving performance among older adults with and without preclinical Alzheimer’s disease. J Alzheimers Dis. 2018;61:509–13 Netherlands.
Kay L, Bundy A, Clemson L, Jolly N. Validity and reliability of the on-road driving assessment with senior drivers. Accid Anal Prev. 2008;40:751–9 England.
Sawada T, Tomori K, Hamana H, Ohno K, Seike Y, Igari Y, et al. Reliability and validity of on-road driving tests in vulnerable adults: a systematic review. Int J Rehabil Res Int Z Rehabil Rev Int Rech Readaptation. 2019;42:289–99.
Frittelli C, Borghetti D, Iudice G, Bonanni E, Maestri M, Tognoni G, et al. Effects of Alzheimer’s disease and mild cognitive impairment on driving ability: a controlled clinical study by simulated driving test. Int J Geriatr Psychiatry. 2009;24:232–8 England.
Anderson SN, McGehee DV, Dawson JD, Rizzo M. Simulated car crashes at intersections in drivers with Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15:10–20 United States: Lippincott Williams and Wilkins (530 Walnut Street, P O Box 327, Philadelphia PA 19106-3621, United States).
Armstrong MA, Mercier O, Stinchcombe A, Yamin S, Knoefel F, Gagnon S. Using video replay of simulated driving to estimate driving safety and cognitive status. Safety. 2021;7 Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85108252689&doi=10.3390%2fsafety7020045&partnerID=40&md5=d9d114c4adcbd9247ded212f77f3d9de.
Babulal GM, Stout SH, Benzinger TLS, Ott BR, Carr DB, Webb M, et al. A naturalistic study of driving behavior in older adults and preclinical Alzheimer disease: a pilot study. J Appl Gerontol. 2019;38:277–89 SAGE Publications Inc.
Babulal GM, Johnson A, Fagan AM, Morris JC, Roe CM. Identifying preclinical Alzheimer’s disease using everyday driving behavior: proof of concept. J Alzheimers Dis. 2021;79:1009–14 IOS Press.
Davis JD, Babulal GM, Papandonatos GD, Burke EM, Rosnick CB, Ott BR, et al. Evaluation of naturalistic driving behavior using in-vehicle monitoring technology in preclinical and early Alzheimer’s disease. Front Psychol. 2020;11 Available from: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.596257. Cited 2022 Jul 18.
Babulal GM, Ghoshal N, Stout SH, Vernon EK, Roe CM, Addison A, et al. Development and interval testing of a naturalistic driving methodology to evaluate driving behavior in clinical research. F1000Res. 2016;5:1716 United Kingdom: Faculty of 1000 Ltd.
Roe CM, Stout SH, Rajasekar G, Ances BM, Jones JM, Head D, et al. A 2.5-year longitudinal assessment of naturalistic driving in preclinical Alzheimer’s disease. J Alzheimers Dis. 2019;68:1625–33.
Bayat S, Babulal GM, Schindler SE, Fagan AM, Morris JC, Mihailidis A, et al. GPS driving: a digital biomarker for preclinical Alzheimer disease. Alzheimers Res Ther. 2021;13:115.
Acknowledgements
Not applicable.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
SB and CR both contributed equally to the conception, drafting, and revision of this manuscript. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bayat, S., Roe, C.M. Driving assessment in preclinical Alzheimer’s disease: progress to date and the path forward. Alz Res Therapy 14, 168 (2022). https://doi.org/10.1186/s13195-022-01109-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-022-01109-1