Abstract
An adverse intrauterine or periconceptional environment, such as hyperglycemia during pregnancy, can affect the DNA methylation pattern both in mothers and their offspring. In this study, we explored the epigenetic profile in maternal peripheral blood samples through pregnancy to find potential epigenetic biomarkers for gestational diabetes mellitus (GDM), as well as candidate genes involved in GDM development. We performed an epigenome-wide association study in maternal peripheral blood samples in 32 pregnant women (16 with GDM and 16 non-GDM) at pregnancy week 24–28 and 36–38. Biochemical, anthropometric, and obstetrical variables were collected from all the participants. The main results were validated in an independent cohort with different ethnic origin (European = 307; South Asians = 165). Two hundred and seventy-two CpGs sites remained significantly different between GDM and non-GDM pregnant women across two time points during pregnancy. The significant CpG sites were related to pathways associated with type I diabetes mellitus, insulin resistance and secretion. Cg01459453 (SELP gene) was the most differentiated in the GDM group versus non-GDM (73.6 vs. 60.9, p = 1.06E−11; FDR = 7.87E−06). Three CpG sites (cg01459453, cg15329406, and cg04095097) were able to discriminate between GDM cases and controls (AUC = 1; p = 1.26E−09). Three differentially methylated positions (DMPs) were replicated in an independent cohort. To conclude, epigenetic marks during pregnancy differed between GDM cases and controls suggesting a role for these genes in GDM development. Three CpGs were able to discriminate GDM and non-GDM groups with high specificity and sensitivity, which may be biomarker candidates for diagnosis or prediction of GDM.
Similar content being viewed by others
Introduction
GDM is defined as a hyperglycemia with onset during pregnancy. The prevalence of this pathology varies between 1.8% and 31.5%, depending on the diagnostic criteria used and the population studied [1]. For women, GDM may increase the risk of prematurity, C-section delivery, preeclampsia, long-term risk of type 2 diabetes mellitus (T2DM), metabolic syndrome, renal disease, and cardiovascular disease (CVD). Women with a previous history of GDM have up to 10 times higher risk of developing T2DM, and half of them will develop some level of impaired glucose metabolism in the next 10 years after having GDM [2, 3]. The offspring of women with GDM have increased risk of fetal macrosomia, higher infant fat mass, and obesity [4]. Therefore, pregnancy is a unique window of opportunity to identify women and children at increased long-term risk of T2DM, obesity, and other cardiometabolic pathologies [5].
Although the severely increased long-term risk of T2DM after GDM is well-documented, the underlying molecular mechanisms are unclear. In last decades, epigenetic has been proposed as a potential link between genetic and environmental risk factors for GDM and T2DM [6]. Epigenetics refer to changes to the DNA that alter gene expression without altering the DNA sequence. These changes include DNA methylation, histone modification and, recently, non-coding RNA such as micro-RNA (miRNAs) [7].
Currently, most epigenetic studies in GDM have been carried out in offspring exposed to GDM [8,9,10]. However, few studies have explored the effect of GDM on the epigenome of the mothers and potential genes involved in the development of diabetes in pregnancy [11,12,13]. Further, none have so far tested the association with GDM across time points during pregnancy. Early identification of pregnant women with high risk of GDM and knowledge about the mechanisms responsible for development of GDM would help to improve diagnosis and treatment and to stop the vicious cycle of obesity and T2DM [14].
In this study, we explored the epigenetic profile in maternal peripheral blood samples through pregnancy to find potential epigenetic biomarkers for GDM, as well as candidate genes involved in GDM development.
Results
Characteristics of the discovery cohort (EPI-DG)
The main characteristics of the studied population are shown in Table 1. GDM and non-GDM pregnant women did not differ in age, gestational age, pre-pregnant weight and BMI, biochemical variables such as cholesterol, HDL-Chol, triglycerides, and homeostatic model of insulin resistance (HOMA-IR) (Table 1). However, the GDM group had a lower weight gain during the last trimester of pregnancy compared with the control group. This observation is probably due to the good metabolic control and adherence to the lifestyle recommendations of the GDM pregnant women after diagnosis. After dietetic recommendations were implemented, 37.5% of the GDM cases required additional pharmacological treatment (insulin).
DNA methylation pattern in pregnant women with GDM and non-GDM
A total of 1141 CpGs and 465 CpGs sites (FDR < 0.05, deltaBeta > 5% and B ≥ 0) were differentially methylated at diagnostic (T0) and antenatal visits (T1), respectively. The majority (66%) of DMPs were hypermethylated at both visits (T0 = 757, T1 = 311) in GDM compared to non-GDM group. Based on genome position, most of these DMPs were located in Open Sea, and according to gene context, mainly in body and IGR regions (Additional file 1: Fig. S1). Additionally, we evaluated if the epigenetic profile changed during pregnancy in both groups. We observed a total of 267 DMPs in the control group throughout time, whereas in the GDM group, we did not find any CpGs site differentially methylated over time (Additional file 2: Table S1). Lastly, we observed only two CpGs which responded differentially over time in the GDM group relative to the non-GDM group (Additional file 3: Fig. S2).
Using a Venn diagram, we observed that 272 CpGs sites were differentially methylated between GDM cases and controls across the two different time points (Fig. 1). From these CpGs sites, we selected the 20 most significant for further analysis. The top 20 DMPs were related to 12 genes (Table 2). Cg01459453, annotated to Selectin-P (SELP) gene was the most differentially methylated between both groups (12.7%), being hypermethylated in the GDM group. NBL1 gene was specially enriched with three CpGs sites (cg18923740, cg15589641, and cg14579430) differentially methylated along the genome (body, TSS1500 and 5’UTR). No differentially methylated regions (DMRs) were found using the algorithm of DMRcate Bioconductor Package.
Gene ontology analysis
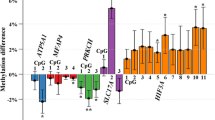
Gene set enrichment analysis was performed using EnrichR and GO [15,16,17]. The 272 CpGs sites were annotated to 140 genes. Of them, 10% contained more than one probe differentially methylated (Additional file 4: Fig. S3). KEEG analysis identified six significant pathways related to Axon Guidance, ErbB signaling pathway, and calcium signaling pathway, among others. Moreover, relevant pathways related to type I diabetes mellitus (PTPRN2), insulin resistance (RPS6KA2), and insulin secretion (ADCYAP1R1) were also identified (Fig. 2). Analysis based on the Tissue Protein Expression database revealed that a group of genes annotated to our DMPs, such as CHSY1, ERBB4, TRIP6, DIP2C, AGRN and LGR6, showed higher expression levels in placenta than in other tissues (Fig. 2).
ROC curve analysis of DMPs
We selected the 272 CpGs and performed ROC curve analysis to identify whether these could differentiate GDM cases from non-GDM. From the 272 CpG sites, 104 were statistically able to discriminate between GDM cases and controls (AUC ≥ 0.8) at diagnostic visit. The same approach was performed with the following clinical variables: HOMA-IR, age, gestational age, pre-pregnant BMI, and the glucose values at each point of the OGTT. Between the clinical variables, only the glucose values of OGTT had an AUC statistically significant ≥ 0.8 (AUCSOG_60 = 0.047; AUCSOG_120 = 0.025; and AUCSOG_180 = 0.043). We performed a logistic regression analysis to identify the variables that best discriminated between GDM cases and non-GDM, including all CpGs sites and glucose values from OGTT that had significant ROC curves. Of all the variables, three CpGs (cg01459453, cg15329406, and cg04095097) stood out as the most significant discriminators (Table 3). ROC figures are shown in Additional file 5: Fig. S4. Pregnant women with GDM showed a significantly higher DNA methylation in cg01459453 (SELP) compared with non-GDM women (73.6% vs. 60.9%, p = 1.10–07). The same significant trend was observed for cg15329406 and cg04095097 (Fig. 3).
Box plots of the three CpGs statistically significantly included in the logistic regression model. Legend: GDM_0: Gestational diabetes mellitus group at T0. GDM_1: Gestational diabetes mellitus group at T1. Non_GDM_0: Non-gestational diabetes mellitus group at T0, Non_GDM_1: Non-gestational diabetes mellitus group at T1
Validation in EPIPREG cohort
The characteristics of EPIPREG are shown in Table 1. In EPIPREG, we attempted to replicate the top 20 DMPs and the three DMPs identified as the best discriminators of GDM. Cg04095097 was replicated in the full sample (p = 0.004), and this persisted after adjustment for covariates (Table 4). Of the top 20 DMPs, two CpGs were statistically different between GDM and non-GDM in the full EPIPREG sample (cg27603605 and cg12080079) (Table 4). Like the EPI-DG cohort, DNA methylation levels of these DMPs were higher in GDM pregnant women in comparison with non-GDM (Fig. 4). When stratifying by ethnicity, we observed statistically significant differences only for cg04095097 in the South Asians pregnant women (0354 non-GDM vs. 0.397 GDM; p = 0.026), and a trend in Europeans (Additional file 2: Table S2). Finally, a mixed models logistic regression showed that pregnant women with higher methylation levels of cg04095097 had a greater odd for GDM than women with lower methylation levels in the EPIPREG sample (OR = 1.25; 95% CI = 1.01–1.52).
Finally, when we performed ROC curve analysis with these CpGs in EPIPREG cohort, we obtained AUC statistically significant, although with an AUC lower than EPI-DG, for the following CpGs sites: cg04095097 (AUC = 0.570, p = 0.015), cg04095097 + cg27603605 (AUC = 0.597, p = 0.0009) and a combination with the three CpGs (AUC = 0.605, p = 0.0003) (Additional file 6: Fig. S5).
mQTLs
From look-ups in GoDMC, we found 21 mQTLs for the three CpG sites replicated. According to Phenoscanner, the mQTL for cg12080079, rs585075, was associated with GDM (p = 0.03) (Table 5). Likewise, the other mQTLs were nominally related to T2DM, several cardiometabolic traits and outcomes, and inflammatory processes such as hypertension, psoriasis, Crohn’s disease, or rheumatoid arthritis (Table 5).
Discussion
To the best of our knowledge, this is the first study analyzing the epigenetic profile in peripheral blood samples of pregnant women with and without GDM in different times during pregnancy. We identified 272 DMPs that were differently methylated between GDM and non-GDM across two time points during pregnancy, and several genes that could be involved in the pathophysiology of GDM such as SELP, LGR6, NBL1, RPS6KA2 or ADCYAP1R1. We also detected three DMPs, cg01459453, cg15329406, and cg04095097, that adequately discriminated GDM occurrence and replicated one of these in an independent cohort.
Most epigenetic studies of gestational diabetes research have been carried out in placenta and cord blood samples [18]. Only a few studies have evaluated the epigenetic pattern in blood samples from pregnant women with GDM and non-GDM [19]. Although this approach cannot infer causality, the findings are very interesting as biomarkers and potential genes involved in the pathogenesis of GDM.
Potential pathways and genes involved in GDM
We identified several CpGs annotated to genes related with relevant metabolic pathways. The CpGs most differentially methylated (cg01459453) between both groups of pregnant women was annotated to SELP gene. SELP gene encode for P-Selectin, a granular membrane protein and a cellular adhesion molecule that mediates the interaction of activated endothelial cells or platelets with leukocytes. Several epidemiological studies suggest that the endothelial dysfunction is closely related to insulin resistance, preceding the development of T2DM [20, 21]. This impaired function can decrease the delivery of insulin to the interstice, limiting the insulin action [20]. Likewise, other authors have found that high levels of P-selectin are associated with metabolic syndrome, and its expression and secretion have been related to low HDL cholesterol and elevated fasting glucose [22]. Another of the most significantly CpG associated with GDM in our study was cg04802986 located within the leucine-rich repeat-containing G-protein coupled receptor 6 (LGR6). CpG sites from this gene in the offspring’ epigenome were recently associated with maternal GDM, fasting glucose, 1-h, and 2-h glucose levels following an OGTT [23]. These epigenetic marks were attenuated by an intervention with diet and physical activity during pregnancy [23], suggesting that the effect of high maternal glucose levels on DNA methylation in the offspring could be modified by a lifestyle intervention.
NBL1 and DIP2C had several DMPs differentially methylated. NBL1 showed four CpGs sites differentially methylated, three of them within the top 20 DMPs. NBL1 belongs to the DAN gene family, whose proteins are secreted and act as bone morphogenetic protein (BMP) antagonists avoiding the interaction with their receptors. Thus, they can play an important role during growth and development [24]. Just like SELP, several CpGs sites have been associated with age-related processes [25] as well as with gestational age [26]. Moreover, CpGs from NBL1 has also been associated with pre-pregnancy maternal BMI in a meta-analysis performed within the pregnancy and childhood epigenetics cohort (PACE) [27]. Regarding to DIPC2, this gene encodes a member of the disco-interacting protein homolog 2 family although its function is not fully understood. According to the literature, DIP2c gene is very susceptible to be modified by external factors [28, 29], so the hyperglycemia during pregnancy could be another modifier factor. Finally, our enrichment analysis identified within our DMPs, relevant pathways related to type I diabetes mellitus (PTPRN2) insulin resistance (RPS6KA2) and secretion (ADCYAP1R1). These results have been confirmed by several studies. For example, RPS6KA2 has been related with insulin signaling pathways and T2DM complications [30, 31].
Few studies have analyzed the DNA methylation in maternal blood in women with and without GDM. In line with our findings, Wu et al. [11] evaluated changes in the epigenome from pregnant women prior to GDM development by genome-wide 450 K array. They identified five CpGs (COPS8, PIK3R5, HAAO, C5orf34, and CCDC124 genes) as potential clinical biomarkers for early detection of GDM and therapeutic intervention. These authors validated these results by pyrosequencing, finding a good correlation with β-values, but they did not replicate the findings in other cohorts. Dias et al. [12] examined the relationship between GDM and DNA methylation profile in peripheral blood cells in South African women. They compared the epigenome by EPIC Illumina array in pregnant women with GDM and non-GDM at the first trimester. Just like the study by Wu et al., several CpGs sites were differentially methylated, and the associated genes were involved in pathways of metabolic regulation. Additionally, the top five CpGs were correlated with relevant metabolic variables such as glucose and insulin concentrations. Kang et al. [32] analyzed the epigenome in maternal and cord blood samples from 16 pregnant women (eight with GDM) and their offspring [33]. They found a differentially methylated pattern between GDM and unexposed group in both types of samples, supporting the hypothesis that the GDM has epigenetic effect on both mothers and their offspring.
Potential epigenetic biomarkers of GDM
Due to the great controversial and variety about the diagnostic criteria for GDM, there is an increasing need to establish biomarkers able to identify in early pregnancy the occurrence of GDM. Currently, the Gold Standard is made with an OGTT at around 26–28 weeks of gestation but it is known that differences in fetal growth occur even at early pregnancy (at 12 weeks) between mothers who will be diagnosed with GDM and who will be not [34]. Finding out accessible and stable biomarkers for predicting GDM would allow an early intervention and the subsequent reduction risk both in mothers and their offspring. A recent study based on integration of EWAs and machine learning has identified 62 specific CpGs sites related to GDM, and six of them located in the promoter region, which were included in a predictive model for GDM whose AUC were for training and testing set, 0.8138 and 0.7576, respectively [35]. Just like us, CpGs sites within DIP2C and PTPRN2 genes were differentially methylated between GDM and non-GDM pregnant women. Another group, led by Enchun et al. [36], has identified DNA methylation sites as potential biomarkers of GDM diagnostic, integrating high-throughput methylation and gene expression data . Although they identified ten genes related with GDM development, the study has several limitations, such as the absence of experimental validation and lacking confounding variables. Wang et al. [37] measured specific CpGs sites, previously published in literature, in 80 GDM cases and 80 matched controls in the first trimester to test if those target CpGs could be associated with GDM pathophysiology in order to be considered as potential predictor of the disease. Overall, a total of 13 CpGs sites showed DNA methylation levels statistically significant between GDM and non-GDM, and the AUC ranged from 0.593 to 0.650 to predict the risk of GDM. Some of the annotated genes, such as NFATC4 or the family of ARHGAP, have been also associated in our study. Recently, a study carried out by Megía et al. [13] has identified several CpGs sites as possible biomarker to detect risk for development glucose abnormalities later in life in women with GDM . The CpGs sites were associated with LINC00917, TRAPPC9, and LEF1 genes.
We identified three CpGs sites in the EPI-DG cohort with high capacity to discriminate between GDM and non-GDM pregnant women, whereof cg04095097 was replicated in the EPIPREG sample. An important characteristic from our study is that we have found stable epigenetic marks during pregnancy able to differentiate GDM and non-GDM groups, suggesting that maybe these marks could be at early pregnancy. In EPI-DG, pregnant women with GDM and non-GDM from the discovery cohort were matched by age, gestational age, and pre-pregnant BMI, whereas the replication cohort was not matched by any variables. Hence, the replication cohort is more heterogeneous than the discovery cohort, as suggested by the statistically significant differences in several variables between women with and without GDM. Maybe this could explain why these DMPs had less capacity to discriminate GDM versus non-GDM in EPIPREG cohort. However, the replicated CpG sites seem robustly associated with GDM, as they were common in two independent cohorts using different GDM criteria and across ethnic origin. Epigenetic marks could help to find a universal diagnostic criterion for GDM. Additionally, mQTLs found suggest that these CpGs are influenced by genetic variants associated with GDM, cardiometabolic traits and autoimmune disease [38,39,40].
Our study has important strengths. Firstly, we have analyzed DNA methylation at two different points during pregnancy. In the discovery cohort, GDM cases and non-GDM were very homogeneous, reducing the likelihood of bias or confounding variables to drive our results. A limitation of our study is that the replication cohort used different GDM criteria and women with and without GDM were not matched. Hence, a replication cohort with a more similar design could have provided better replication results. As limitation, we could not analyze the effect of DNA methylation on gene expression due to lack of RNA samples. Finally, further studies with higher sample size and at early pregnancy are needed to confirm our results and the potential of these epigenetic marks as biomarkers of GDM occurrence.
Conclusion
We identified differentially methylated marks between GDM and non-GDM pregnant women at two different time points during pregnancy. Several of these DMPs are within genes associated with metabolic pathways related to insulin and glucose metabolism, pointing out a possible role in the GDM development. Furthermore, we have identified three CpGs sites that may pose as potential biomarkers for diagnosis of GDM.
Methods
Subjects
Pregnant women who attended Unit of Diabetes and Pregnancy at University Hospital Virgen de la Victoria, after a positive O’Sullivan test, were eligible for recruitment. GDM was diagnosed using a two-step strategy according to National Diabetes Data Group NDDG criteria [41]. Firstly, a screening test in pregnant women between 24–29 weeks of pregnancy, with a 50 g oral glucose load, was done in primary centers. An oral glucose tolerance test (OGTT-100 g) was carried out in those women with a positive screening test (> 7.7 mmol/L). Patients were diagnosed with GDM if glucose values were higher than the threshold, at least in two points: fasting > 5.8 mmol/L; after 1 h > 10.6 mmol/L; after 2 h > 9.2 mmol/L; and after 3 h > 8.0 mmol/L. Those pregnant women with normal OGTT-100 (NGT) were considered as controls (non-GDM). After GDM diagnosis, women were recommended to make lifestyle changes and self-monitoring of blood glucose (SBGM) at fasting and 1-h postprandial breakfast, lunch and dinner (Bayer, Contour® Next Glucose test strips, XT or USB meters). Diet recommendations included 175 g of carbohydrate, a minimum of 71 g of protein, and 28 g of fiber, avoiding saturated fat and simple carbohydrates and preferring a moderate consumption of complex carbohydrates. These recommendations were maintained during the whole study. After 1 week, glycemic controls were analyzed by the endocrinologist. If ≥ 2 glucose fasting values were ≥ 95 mg/dl (5.3 mmol/L) and/or 1-h postprandial ≥ 140 mg/dl (7.8 mmol/L) despite lifestyle changes, the addition of pharmacological treatment (insulin) was indicated.
Blood samples were collected in two different time, at diagnostic visit T0 (24–29 weeks) and at antenatal visit T1 (36–38 weeks). This cohort is part of the EPI-DG study which started at the beginning of 2019. Characteristics of this cohort has been recently published [42].
An epigenome-wide DNA methylation analysis (EWAS) was performed in 16 pregnant women with GDM and 16 non-GDM matched by age, gestational age, and pre-pregnant BMI to avoid confounding factors in the methylation data analysis. Clinical, anthropometric, and biochemical variables were collected in each visit. Weight gain was calculated as the difference between the weight at the antenatal visit (T1) and at diagnostic visit (T0).
All patients gave their consent to participate in the study. The study was approved by the Institutional review board at the Hospital Universitario Virgen de la Victoria de Málaga, Spain.
Samples extraction, DNA isolation and bisulfite conversion
Blood samples were collected in each visit (T0 and T1) after a 12-h fast and stored at -80ºC until DNA isolation. Peripheral blood DNA was isolated using Qiamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Quality and concentration of DNA was measured using Qubit 3.0 Fluorometer with Qubit dsDNA HS Assay Kit Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). A total of 500 ng of genomic DNA was bisulfite treated with Epitect Bisulfite Kit (Qiagen, Germany) for posterior DNA methylation analysis.
Epigenome-wide DNA methylation analysis
An Epigenome-wide DNA methylation analysis (EWAS) was performed in a total of 32 pregnant women, 16 GDM and 16 non-GDM (discovery cohort). DNA was hybridized in the Infinitum MethylationEPIC Bead Chip and 850.000 CpGs sites were analyzed. Raw data files were processed using R package ChAMP version 2.9.10 [43], filtering probes is performed in probes with a detection p value above 0.01 in one or more samples, probes with a beadcount less than 3 in at least 5% of samples, probe non-CpG, probes with SNPs [44], probes that align to multiple locations [45], and probes on the X or Y.
Intra-cell type normalization was done using beta-mixture quantile normalization (BMIQ) method. To correct for the differences in methylation resulting from differences in cellular heterogeneity, the Houseman correction was used [46].
Methylation data analysis
The differentially methylated positions (DMPs) were obtained using eBayes moderated t statistic with limma package [47] for R statistical software (4.0.4). β-values and M-values were calculated to obtain the methylation levels. While β-values is the estimate of methylation level using the ratio of the methylation probe intensity and the overall intensity, it is used for report results. M-value is a logarithmic transformation of β-value, and it is necessary to perform the differential methylation analysis. Linear models were used to identify differentially methylated CpGs sites (DMPs) between GDM and non-GDM samples (FDR-adjusted p value < 0.05, and deltabelta ≥|5|%.) at both times. A Venn diagram was used to select those DMPs common in both visits (Venny 2.1 https://bioinfogp.cnb.csic.es/tools/venny/). All models were adjusted by age, pre-pregnant BMI, newborn sex, weight gain during pregnancy, gestational age, HOMA-IR, and required treatment. These variables have been previously associated with GDM in the literature. Additionally, weight gain was also included due to this variable was statistically different between groups.
A gene ontology (GO) and enrichment analysis were performed with those CpGs that were differentially methylated both T0 and T1 visits. Gene ontology tool website [17] and EnrichrR [24] were used for GO and enrichment analysis, respectively. Tissue protein expression database was used to explore gene expression levels in different tissues [48].
ROC analysis and Logistic regression
Receiver operating characteristics (ROC) curves were performed to determine the AUC of the differentially methylated CpGs between GDM and non-GDM pregnant women. Variables with AUC ≥ 0.8 and p < 0.05 were selected. To generate the predictive models, binomial logistic regression was used as the dependent variable GDM. The ROC (receiver operating characteristic) analysis has allowed evaluating the generated models using different metrics such as sensitivity, specificity, precision, and area under the curve (AUC). We used R software (4.0.4) for this statistical analysis.
Replication
For replication, we used the EPIPREG sample, which is a sub-study of the population-based STORK Groruddalen (STORK G) cohort [49].
In EPIPREG, we quantified DNA methylation in maternal peripheral blood leukocytes in gestational week 28 ± 2 in all Europeans (n = 312) and South Asians (n = 168) participating in STORK G who were genotyped and had fasting glucose data recorded, with Infinium MethylationEPIC BeadChip (Illumina, San Diego, CA, USA). Three hundred and seven Europeans and 165 South Asians passed the quality control. Details about the EPIPREG sample have been described previously [50].
All women completed a 75 g oral glucose tolerance test in gestational week 28 ± 2. Fasting and 2-h glucose was analyzed with a point-of-care instrument (HemoCue, Angelholm, Sweden). We classified GDM in retrospect with a modified version of the WHO 2013 criteria (fasting glucose ≥ 5.1–6.9 mmol/l or 2-h glucose ≥ 8.5–11 mmol/l, no data for 1-h glucose) [51].
We performed an eBayes moderated t statistic with the limma package, adjusted for the same variables as in the main analysis of EPI-DG cohort: age, pre-pregnant BMI, fetal sex, gestational age, and HOMA-IR. In EPIPREG, the analysis was additionally adjusted for ethnicity. P < 0.05 was considered statistically significant.
mQTL
We performed look-ups in Genetics of DNA Methylation Consortium (GoDMC) [52] of the CpGs replicated in EPIPREG to identify methylation quantitative trait loci (mQTL). The mQTLs were filtered with LD-link web-tool [53] and variants with R2 < 0.2 were kept. We used Phenoscanner [54] to search for phenotypes nominally associated (p < 0.05) with the mQTLs that survived filtering.
Availability of data and materials
The data sets from EPI-DG used during the current study are available from the corresponding author on reasonable request. Regarding EPIPREG due to strict regulations for genetic data and privacy protection of patients in Norway, all requests for data access are processed by the STORK G project’s steering committee. Please contact the principal investigator of STORK G (on.oiu.nisidem@dnarb.l.m.a) or the principal investigator of EPIPREG (on.oiu.nisidem@remmos.enitsirhc).
References
Eades CE, Cameron DM, Evans JMM. Prevalence of gestational diabetes mellitus in Europe: a meta-analysis. Diabetes Res Clin Pract. 2017;1(129):173–81.
Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9.
Lowe WL, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005–16.
Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75(3):221–8.
Saravanan P, Magee LA, Banerjee A, Coleman MA, Von Dadelszen P, Denison F, et al. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabet Endocrinol. 2020;8:793–800.
Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62(10):1789–801.
Goyal D, Limesand SW, Goyal R. Epigenetic responses and the developmental origins of health and disease. J Endocrinol. 2019;242(1):T105–19.
Ruchat SM, Houde AA, Voisin G, St-Pierre J, Perron P, Baillargeon JP, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013;8(9):935–43.
El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, Korenkov M, et al. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62(4):1320–8.
Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, et al. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring.
Wu P, Farrell WE, Haworth KE, Emes RD, Kitchen MO, Glossop JR, et al. Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics. 2016.
Dias S, Adam S, Rheeder P, Louw J, Pheiffer C. Altered genome-wide dna methylation in peripheral blood of south african women with gestational diabetes mellitus. Int J Mol Sci. 2019;20:23.
Ballesteros M, Gil-Lluís P, Ejarque M, Diaz-Perdigones C, Martinez-Guasch L, Fernández-Veledo S, et al. DNA methylation in gestational diabetes and its predictive value for postpartum glucose disturbances. J Clin Endocrinol Metab. 2022
Hjort L, Novakovic B, Grunnet LG, Maple-Brown L, Damm P, Desoye G, et al. Diabetes in pregnancy and epigenetic mechanisms-how the first 9 months from conception might affect the child’s epigenome and later risk of disease. Lancet Diabetes Endocrinol. 2019;7(10):796–806.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–7.
Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles G V, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013; 14.
Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–26.
Moen GH, Sommer C, Prasad RB, Sletner L, Groop L, Qvigstad E, et al. Mechanisms in endocrinology: epigenetic modifications and gestational diabetes: a systematic review of published literature. Eur J Endocrinol. 2017;176(5):R247–67.
Elliott HR, Sharp GC, Relton CL, Lawlor DA. Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia. 2019;62(12):2171–8.
Pletsch-Borba L, Watzinger C, Fortner RT, Katzke V, Schwingshackl L, Sowah SA, et al. Biomarkers of vascular injury and type 2 diabetes: a prospective study, systematic review and meta-analysis. J Clin Med. 2019;8:12.
Pankow JS, Decker PA, Berardi C, Hanson NQ, Sale M, Tang W, et al. Circulating cellular adhesion molecules and risk of diabetes: the multi-ethnic study of atherosclerosis (MESA). Diabet Med. 2016;33(7):985.
Ruiz-Ojeda FJ, Olza J, Gil Á, Aguilera CM. Oxidative stress and inflammation in obesity and metabolic syndrome. Obes Oxidative Stress Diet Antioxidants. 2018;1:1–15.
Antoun E, Kitaba NT, Titcombe P, Dalrymple KV, Garratt ES, Barton SJ, et al. Maternal dysglycaemia, changes in the infant’s epigenome modified with a diet and physical activity intervention in pregnancy: secondary analysis of a randomised control trial. PLoS Med. 2020;5(17):11.
Opazo JC, Hoffmann FG, Zavala K, Edwards SV. Evolution of the DAN gene family in vertebrates. Dev Biol. 2022;1(482):34–43.
Mulder RH, Neumann A, Cecil CAM, Walton E, Houtepen LC, Simpkin AJ, et al. Epigenome-wide change and variation in DNA methylation in childhood: trajectories from birth to late adolescence. Hum Mol Genet. 2021;30(1):119.
Bohlin J, Håberg SE, Magnus P, Reese SE, Gjessing HK, Magnus MC, et al. Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biol. 2016;7(17):1.
Sharp GC, Salas LA, Monnereau C, Allard C, Yousefi P, Everson TM, et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet. 2017;26(20):4067–85.
Shorey-Kendrick LE, McEvoy CT, O’Sullivan SM, Milner K, Vuylsteke B, Tepper RS, et al. Impact of vitamin C supplementation on placental DNA methylation changes related to maternal smoking: association with gene expression and respiratory outcomes. Clin Epigenet. 2021;1(13):1.
England-Mason G, Merrill SM, Moore S, Giesbrecht GF, Letourneau N, MacDonald A, et al. Prenatal exposure to phthalates and DNA methylation in infant blood and buccal samples: an epigenome-wide association study. prep. 2021;163:107183.
Ustinova M, Peculis R, Rescenko R, Rovite V, Zaharenko L, Elbere I, et al. Novel susceptibility loci identified in a genome-wide association study of type 2 diabetes complications in population of Latvia. BMC Med Genom. 2021;1(14):1.
Balakrishnan A, Guruprasad KP, Satyamoorthy K, Joshi MB. Interleukin-6 determines protein stabilization of DNA methyltransferases and alters DNA promoter methylation of genes associated with insulin signaling and angiogenesis. Lab Investig. 2018;98(9):1143–58.
Jessica Kang, Chien-Nan Lee, Hung-Yuan Li, Kai-Han Hsu SYL. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population.
Kang J, Lee CN, Li HY, Hsu KH, Lin SY. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res Clin Pract. 2017;1(132):127–36.
Elenis E, Wikström AK, Simic M. Accelerated fetal growth in early pregnancy and risk of preterm birth: a prospective cohort study. BMC Pregnancy Childbirth. 2020;20(1):1–8.
Liu Y, Geng H, Duan B, Yang X, Ma A, Ding X. Identification of diagnostic CpG signatures in patients with gestational diabetes mellitus via epigenome-wide association study integrated with machine learning. Biomed Res Int. 2021;2021.
Li E, Luo T, Wang Y. Identification of diagnostic biomarkers in patients with gestational diabetes mellitus based on transcriptome gene expression and methylation correlation analysis. Reprod Biol Endocrinol. 2019;17(1).
Wang X, Huang J, Zheng Y, Long S, Lin H, Zhang N, et al. Study on the relationship between DNA methylation of target CpG sites in peripheral blood and gestational diabetes during early pregnancy. Sci Rep. 2021;11(1).
Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017;66(11):2888–902.
Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009 Jul;5(7).
Liu JZ, Van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86.
National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57.
Molina-Vega M, Picón-César MJ, Lima-Rubio F, Gutiérrez-Repiso C, Linares-Pineda TM, Suárez-Arana M, et al. Insulin requirement for gestational diabetes control is related to higher vitamin D levels up to 1 year postpartum: a prospective cohort study. Antioxidants. 2022;11(11):2230.
Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, et al. ChAMP: 450k chip analysis methylation pipeline. Bioinformatics. 2014;30(3):428–30.
Zhou W, Laird PW, Shen H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 2017;45(4):e22–e22.
Weng X, Liu F, Zhang H, Kan M, Wang T, Dong M, et al. Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Res Clin Pract. 2018;142:10–8.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinf. 2012;13(1):1–16.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, et al. A draft map of the human proteome. Nature. 2014;509(7502):575–81.
Jenum AK, Sletner L, Voldner N, Vangen S, Mørkrid K, Andersen LF, et al. The STORK Groruddalen research programme: a population-based cohort study of gestational diabetes, physical activity, and obesity in pregnancy in a multiethnic population. Rationale, methods, study population, and participation rates. Scand J Public Health. 2010;38(5):60–70.
Fragoso-Bargas N, Opsahl JO, Kiryushchenko N, Böttcher Y, Lee-Ødegård S, Qvigstad E, et al. Cohort profile: epigenetics in pregnancy (EPIPREG) – population-based sample of European and South Asian pregnant women with epigenome-wide DNA methylation (850k) in peripheral blood leukocytes. PLoS One. 2021;16(8 August).
Jenum AK, Mrøkrid K, Sletner L, Vange S, Torper JL, Nakstad B, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified international association of diabetes and pregnancy study groups criteria: a population-based cohort study. Eur J Endocrinol. 2012;166(2):317–24.
Min JL, Hemani G, Hannon E, Dekkers KF, Castillo-Fernandez J, Luijk R, et al. Genomic and phenotypic insights from an atlas of genetic effects on DNA methylation. Nat Genet. 2021;53(9):1311–21.
Myers TA, Chanock SJ, Machiela MJ. LDlinkR: An R package for rapidly calculating linkage disequilibrium statistics in diverse populations. Front Genet. 2020;11.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Funding
This study was supported by the Juan Rodés program from “Instituto de Salud Carlos III” (JR20-00040 to MM-V), Miguel Servet Type I program from the ISCIII-Madrid, Spain (CP20/00066 to CG-R), PFIS program (FI19/00178 to TML-P), Garantía Juvenil program (POEJ-0039-18) from Ministerio de trabajo y economía social de España, and the Nicolas Monardes Program from the “Servicio Andaluz de Salud, Junta de Andalucía,” Spain (RC-0008-2021 to SM). In addition, this study was supported by the “Centros de Investigación Biomédica en Red” (CIBER) of the Institute of Health Carlos III (ISCIII) (CB06/03/0018), and research Grants from the ISCIII (PI18/01175, PI21/01864) and from “Servicio Andaluz de Salud,” Junta de Andalucía (PI-0283-2018, PI-0419-2019). This study has been co-funded by FEDER funds (“A way to make Europe”).
Author information
Authors and Affiliations
Contributions
TML-P involved in methodology, analysis, and wrote original draft; NP-M processed the samples and performed the experiments prior to the DNA methylation; NF-B involved in analysis of EPIPREG cohort; CG-R participated in the design of the work; FL-M involved in sample collection; MS-A involved in data collection; AS-P involved in revision of the manuscript; FJ-T involved in supervision and revision of the paper; MM-V involved in conceptualization and acquired data from the patients and collected samples; MJ-P involved in conceptualization, funding acquisition, and contributed to the discussion; CS involved in conceptualization and design of the EPIPREG sample and in-depth revision of the manuscript; and SM involved in conception/design, funding acquisition, interpretation of the data, and has substantively revised the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Hospital Universitario Virgen de la Victoria, Málaga.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Data distribution, at baseline and antenatal visits, based on Genomic position: open sea, shore, island, and shelf; based on gene context: TSS, exon IGR, 5'UTR, and 3'UTR.
Additional file 2: Table S1.
Summary of number of differentially methylated probes. Table S2. Validation CpGs in EPIPREG cohort.
Additional file 3: Fig. S2.
DNA methylation levels of both CpGs which responded differentially over time in the GDM group relative to the non-GDM group, at T0 and T1.
Additional file 4: Fig. S3.
Bar plot of gene mostly enriched by significant DMP. The number in each bar indicates how many hyper- or hypo-differential methylated CpGs are included in that gene.
Additional file 5: Fig. S4.
Receiver operating characteristic models of the best three CpGs that discriminate between GDM and non-GDM: A) Model with only one CpG cg01459453. B) Model with two CpGs: cg01459453 and cg15329406. C) Model with 3 CpGs: cg01459453, cg15329406 and cg04095097. AUC: area under the curve.
Additional file 6: Fig. S5.
Receiver operating characteristic models of the three CpGs validated in EPIPREG cohort. A) One CpG model: cg04095097, B) Two CpG model: cg04095097 and cg27603605, C) Three CpG model: cg04095097, cg27603605, and cg12080079. AUC: area under the curve.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Linares-Pineda, T., Peña-Montero, N., Fragoso-Bargas, N. et al. Epigenetic marks associated with gestational diabetes mellitus across two time points during pregnancy. Clin Epigenet 15, 110 (2023). https://doi.org/10.1186/s13148-023-01523-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-023-01523-8