Abstract
Background
Cystoscopy is the gold standard for bladder cancer detection, but is costly, invasive and has imperfect diagnostic accuracy. We aimed to identify novel and accurate DNA methylation biomarkers for non-invasive detection of bladder cancer in urine, with the potential to reduce the number of cystoscopies among hematuria patients.
Results
Biomarker candidates (n = 32) were identified from methylome sequencing of urological cancer cell lines (n = 16) and subjected to targeted methylation analysis in tissue samples (n = 60). The most promising biomarkers (n = 8) were combined into a panel named BladMetrix. The performance of BladMetrix in urine was assessed in a discovery series (n = 112), consisting of bladder cancer patients, patients with other urological cancers and healthy individuals, resulting in 95.7% sensitivity and 94.7% specificity. BladMetrix was furthermore evaluated in an independent prospective and blinded series of urine from patients with gross hematuria (n = 273), achieving 92.1% sensitivity, 93.3% specificity and a negative predictive value of 98.1%, with the potential to reduce the number of cystoscopies by 56.4%.
Conclusions
We here present BladMetrix, a novel DNA methylation urine test for non-invasive detection of bladder cancer, with high accuracy across tumor grades and stages, and the ability to spare a significant number of cystoscopies among patients with gross hematuria.
Similar content being viewed by others
Background
Bladder cancer accounts for around 570,000 new cases and over 210,000 deaths each year worldwide [1]. The most common symptom of bladder cancer is gross hematuria, i.e., visible blood in the urine, which is present in almost 80% of newly diagnosed patients [2]. However, hematuria may also be caused by a variety of other genitourinary conditions, and its specificity to detect bladder cancer is rather low [3]. Indeed, the incidence rate for bladder cancer among patients with gross hematuria has been estimated to 17% [4].
The standard procedure for bladder cancer detection in hematuria patients and other patients with a suspected bladder tumor is cystoscopy, which is an endoscopic examination of the bladder mucosa. Although considered the gold standard, cystoscopy is invasive, can be uncomfortable for the patients, and its diagnostic accuracy is stage- and operator-dependent [5]. It is also costly and contributes to make bladder cancer one of the most expensive cancers to manage [6]. In the USA, it is has been estimated that around 20,000 cancer cases are missed among patients with hematuria by cystoscopy each year, and that 230,000 unnecessary cystoscopies are performed [7]. Cytology, i.e., a visual inspection of urine cells under the microscope, is commonly used in combination with cystoscopy in high-risk patients. Cytology is non-invasive and has high specificity, but suffers from poor sensitivity, particularly for low-grade cancers [3, 5].
Identifying non-invasive urine biomarkers as an alternative to cystoscopy that could differentiate benign and malignant causes of hematuria would be of great benefit for both the patients and the society. Six urine tests have obtained approval from the US Food and Drug Administration (FDA) for bladder cancer detection and/or surveillance, but unfortunately they show varying performance across studies, and the accuracy is often poor for low-stage and low-grade tumors [8]. Consequently, none of these tests are currently recommended in routine clinical practice [9, 10]. In addition, a variety of urinary molecular biomarkers have been reported and reviewed elsewhere [11,12,13], but small and unrepresentative clinical series, absence of proper control groups, suboptimal sensitivity, in particular for low-grade tumors, and lack of validation are factors that typically prevent translation of biomarkers into clinical practice.
Aberrant DNA methylation is a frequent and early event in bladder carcinogenesis and has promising biomarker potential [14]. Still, only a handful of methylation biomarkers have been reported with both high sensitivity and specificity (> 90%) for urine-based detection of bladder cancer [15, 16].
The aim of the present study was to develop a highly accurate urine DNA methylation test for detection of bladder cancer, with the potential to reduce the number of costly and uncomfortable cystoscopy examinations for hematuria patients.
Results
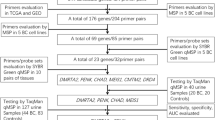
The overall strategy used for biomarker discovery and evaluation of biomarker performance in urine, including in a large series of hematuria patients, is shown in Fig. 1.
The overall strategy for biomarker discovery and evaluation of the BladMetrix test performance in urine. The figure gives an overview of the overall strategy for identification of accurate biomarkers for bladder cancer detection (upper part), and evaluation of the best performing biomarkers as a panel, i.e., the BladMetrix test, in urine (lower part). BlCa Bladder cancer; NBM Normal bladder mucosa; HC Healthy controls; PrCa Prostate cancer; ReCa Renal cancer; NPV Negative predictive value; PPV Positive predictive value. Figure created with BioRender.com
Methylome-wide discovery of DNA methylation biomarker candidates
The workflow established for identification of differentially methylated regions (DMRs) from raw RRBS (reduced representation bisulfite sequencing) data is explained in details in Additional file 1: Methods. After quality control and trimming of the RRBS data, samples were left with on average 106 million reads (Additional file 1: Fig. S1 and Table S1). About 71% of the reads were uniquely mapped and kept for downstream analyses (Additional file 1: Fig. S2). The average coverage and mean methylation level per sample were 111.6X and 37.2%, respectively (Additional file 1: Table S1). Calculation and filtering of DMRs resulted in 214 windows fulfilling the selection criteria (Additional file 1: Fig. S1), including 64 200-bp windows and 150 1000-bp windows. Of note, the 200-bp and 1000-bp windows were overlapping substantially, indicating that the 1000-bp window strategy was possibly redundant. Overlapping windows were considered as a single biomarker candidate, resulting in 32 candidates (Additional file 1: Fig. S3 and Table S2). Almost 60% (19/32) of the candidates were located more than 1,500 bp away from the nearest transcription start site (TSS; Additional file 1: Table S2).
Selection of the most promising biomarker candidates
A stepwise selection of the most promising biomarkers among the 32 candidates identified from methylome sequencing was performed (Additional file 1: Fig. S4). In brief, qMSP assays were designed for 28 of the 32 biomarker candidates, the four remaining candidates having too few CpG sites to allow assay design. Nineteen assays passed the quality control, all displaying high concordance with the RRBS data. Vimentin (VIM), identified as a promising bladder cancer methylation biomarker in our previous study [17], was included in subsequent analyses. In tissue samples (n = 60), the sensitivities of these 20 biomarker candidates ranged from 25 to 100%, and the specificities from 92 to 100% (Additional file 1: Table S3). When prostate and renal cancer samples were included in the control group, the specificity of the biomarkers remained high (92.5–100%; median = 97.5%; Additional file 1: Table S3). Biomarker candidates with > 50% sensitivity and a significant AUC (area under the ROC curve; n = 12) were considered for further analysis in urine using droplet digital PCR (ddPCR).
BladMetrix performance in urine—the discovery series
Eight biomarker candidates demonstrated good technical performance using ddPCR (Additional file 1: Fig. S4). The sensitivities of the individual biomarkers in the urine discovery series (n = 112) ranged from 54 to 73% (Additional file 1: Table S4). The specificities ranged from 95 to 96% and the area under the ROC curves (AUCs) from 0.68 to 0.85, considering only healthy individuals in the control group (Additional file 1: Fig. S5 and Table S4). Including prostate- and renal cancers in the control group resulted in specificities ranging from 94 to 98% (Additional file 1: Table S4). Testing for potential age dependent methylation showed no significant difference when stratifying the bladder cancers in two equally sized age groups (Additional file 1: Table S5). Given an overall high performance, all eight biomarkers were combined into a panel named BladMetrix. Various approaches for defining scoring thresholds were tested, including generalized linear models using individual biomarkers as covariates or by integrating all 8 biomarkers (data not shown). Of these approaches, individual scoring of the biomarkers had the best performance. A cutoff of ≥ 2/8 methylated biomarkers was found to give the most optimal combination of sensitivity, specificity and number of conclusive test results (Additional file 1: Table S6), and was used to define a positive test. A negative test was defined as 0/8 methylated biomarkers, and samples with 1/8 methylated biomarkers were considered inconclusive. Figure 2 illustrates the suggested clinical use and scoring of the BladMatrix urine test for a patient with a suspected bladder cancer. In the discovery series, BladMetrix achieved a sensitivity of 95.7% (22/23) and a specificity of 94.7% (71/75), considering the conclusive test results, while 12.5% (14/112) of the samples were scored inconclusive (Table 1 and Additional file 1: Table S7).
Suggested clinical use and scoring of the BladMetrix test. The BladMetrix test, which is an 8-biomarker panel, is scored negative, positive or inconclusive depending on the number of methylated biomarkers. A negative test will spare the patient for a clinical intervention, while both a positive and an inconclusive test will require the patient to undergo cystoscopy, which is in line with todays’ standard routine. Figure created with BioRender.com
BladMetrix performance among hematuria patients—a blinded analysis of a prospectively collected urine series
The cutoff to score a positive test, identified from the discovery series (Fig. 2; Additional file 1: Table S6), was quality controlled and validated in the independent prospective and blinded hematuria urine series by subsampling (n = 273; Additional file 1: Table S8). In the complete hematuria urine series, 93.0% of the patients (254/273) had a conclusive test result. Among those, BladMetrix achieved a sensitivity of 92.1% (82/89), a specificity of 93.3% (154/165), and a negative predictive value (NPV) of 98.1% for detection of bladder cancer (Table 1 and Additional file 1: Table S7). Finally, considering that BladMetrix identified 154 true negatives across the whole series of 273 patients, the test shows potential to reduce the number of cystoscopies by 56.4%.
BladMetrix performance across tumor stages and grades
All low-stage and low-grade lesions were detected in the discovery urine series (100% sensitivity). In the hematuria series, Ta tumors and low-grade lesions were detected with a sensitivity of 84.6% and 85.2%, respectively (Table 2). All T2–T4 lesions were correctly scored in both urine series. False negative cases included one high-grade carcinoma in situ (CIS) in the discovery series, as well as 6 Ta tumors (4 low-grade and 2 high-grade) and 1 T1 tumor (high-grade) in the hematuria series.
Discussion
We here present BladMetrix, a novel urine DNA methylation test for detection of bladder cancer with high diagnostic accuracy and potential to spare a significant number of cystoscopies. When analyzed in a large, blinded and prospective series of urine from patients with gross hematuria, BladMetrix achieved 92.1% sensitivity, 93.3% specificity and a NPV of 98.1%. Importantly, the sensitivity remained high across all tumor grades and stages, detecting Ta and low-grade tumors with around 85% sensitivity. In addition, BladMetrix showed potential to reduce the number of cystoscopies by 56.4% among gross hematuria patients.
Cystoscopy remains the gold standard for detection of bladder cancer, including in hematuria patients, but is a costly and invasive procedure that can be uncomfortable for the patients. In addition, the diagnostic accuracy is suboptimal. In several meta-analyses, the pooled sensitivity and specificity of the routinely used white light cystoscopy (WLC) has been estimated to around 70% [5, 18]. Variants of WLC include narrow band imaging (NBI) and photodynamic diagnosis (PDD), both methods shown to have higher sensitivity (96% and 93%, respectively), but lower specificity (65% and 63%, respectively) compared to WLC [18]. Screening of patients with an accurate molecular urine test such as BladMetrix, possibly followed by NBI or PDD, could represent a highly beneficial alternative to the current standard using WLC. Interestingly, it has been reported that the bladder cancer detection rate using cystoscopy improves if the urologist has been informed about a positive test result upfront [19]. A urine test stratifying for cystoscopy examination would be particularly useful among patients with gross hematuria, a patient group where the incidence rate of bladder cancer is as low as 17% [4], meaning that an unnecessary high number of cystoscopies are being performed.
Despite a handful of FDA-approved urinary tests and a variety of studies in the literature, accurate, robust and reliable biomarkers are still lacking, and no urine-based molecular tests are routinely used in the clinic [20]. Relevant criteria for a urine test with potential for clinical use in hematuria patients are improved accuracy compared to cystoscopy, high sensitivity across tumor grades, and high specificity considering other urological cancers. Moreover, the great majority of bladder cancer patients report that they are not willing to replace cystoscopy with a urinary test that has less than 90% sensitivity [21, 22]. These criteria are fulfilled by BladMetrix, including both sensitivity and specificity above 90% in a prospective series of hematuria patients, high sensitivity for low-grade tumors (85%) and high specificity against other urological cancers (95%). This level of accuracy across tumor stages and grades is rare among urinary tests for bladder cancer detection, and has to the best of our knowledge not been reached by any stand-alone commercially available urine test [11,12,13]. Of note, AssureMDX have been reported in the literature with 93–97% sensitivity for detection of bladder cancer in patients with gross hematuria, but has lower specificity (83–86%) compared to BladMetrix [23, 24]. Among non-commercial molecular tests, highly promising results have been published for UroMark, with 98% sensitivity and 97% specificity for detecting bladder cancer in hematuria patients, and clinical trials are ongoing to validate the results [25]. Also of note, Dahmcke et al. has reported a urine-DNA test with 97% sensitivity in the same prospective and blinded hematuria series as analyzed in this study, however with lower specificity (77%) [26].
Among bladder cancer patients, low-grade Ta tumors represent the majority of cases at diagnosis [9], and a test with high accuracy for these lesions is thus of great clinical interest. While cytology [5], several of the FDA-approved tests [8] and the majority of other published urinary biomarkers [12] typically have poor sensitivity for low-stage and low-grade tumors, BladMetrix show high accuracy also for these lesions with a detection rate of around 85% (Table 2). Importantly, this is comparable to WLC, which has been shown to miss up to 17% of early stage Ta tumors [27].
A limited number of hematuria urine samples (7%; 19/273) analyzed in the present study were scored inconclusive (i.e., 1/8 methylated biomarkers). The consequence for these patients in a clinical scenario would be to undergo cystoscopy (Fig. 2), which is in line with the current standard routine for hematuria patients. Importantly, despite a small percentage of inconclusive tests, we show that over half of the patients could have been spared an unnecessary cystoscopy using the BladMetrix test.
Our strategy for biomarker discovery is based on methylome sequencing of cancer cell lines. Cell lines have previously been shown to be highly suited for identification of accurate cancer biomarkers [17, 28, 29], which was confirmed in the present study, where the biomarker panel had high accuracy in both tissue and urine. As expected from a genome-wide approach, biomarkers were not restricted to areas of the genome with known biological function such as promoters. The majority of the candidates (59%) were located more than 1,500 bp away from the nearest TSS, indicating that intergenic regions are relevant sources for novel biomarkers.
The use of various material sources (cancer cell lines, tissue, urine), distinct clinical settings (patients scheduled for surgery vs. standard urological evaluation of hematuria patients), independent sample collection procedures in two countries and different methods for urine processing (centrifugation vs. filtration [30]) demonstrates the robustness of BladMetrix in various contexts. It also opens up for application in other clinical settings. A Norwegian multi-center trial is ongoing, aiming at following 500 patients with non-muscle-invasive bladder cancer over a two-year period to evaluate the clinical utility of BladMetrix for surveillance.
Conclusions
In conclusion, we here present BladMetrix, a novel urine DNA methylation test for accurate detection of bladder cancer. In a large urine series from patients with gross hematuria, BladMetrix achieved high sensitivity and specificity across tumor stages and grades, and showed potential to spare over half of the cystoscopies that are routinely performed in this patient group. Given the high accuracy, cost efficiency, non-invasive nature and straightforward implementation, BladMetrix shows promise as a clinical test for urine-based detection of bladder cancer in hematuria patients.
Methods
Discovery of biomarker candidates from methylome sequencing
Sixteen urological cancer cell lines—eight bladder cancer, four prostate cancer and four renal cancer—were obtained from the American Type Culture Collection (ATCC). All cell lines were STR-tested and authenticated (Additional file 1: Table S9). RRBS library construction and sequencing were performed at Beijing Genomics Institute as previously described [31]. The data were processed according to the bioinformatics workflow described in Additional file 1: Methods, Fig. S1 and Table S10. DMRs were identified using a sliding window approach as illustrated in Additional file 1: Fig. S3.
Identification of the most promising biomarker candidates in tissue
The biomarker candidates identified from RRBS were evaluated in 60 tissue samples, including 20 bladder cancer, 10 prostate cancer, 10 renal cancer and 20 normal bladder mucosa samples (Additional file 1: Materials). Biomarker candidates displaying > 50% sensitivity and having a significant AUC were considered for methylation analysis in urine using ddPCR, and the best performing biomarkers were combined into a panel, named BladMetrix.
Analysis of the biomarker panel in urine – discovery and hematuria series
BladMetrix was first analyzed in the urine discovery series (n = 112; 26 bladder cancers, 18 prostate cancers, 12 renal cancers, 56 healthy controls). Different cutoffs for scoring a positive urine test were investigated, varying from ≥ 1/8 to 8/8 methylated biomarkers. The cutoff providing the most optimal combination of sensitivity, specificity, and number of conclusive samples was selected and applied for analyses in an independent urine series including 273 patients with gross hematuria, of which 93 had a clinically confirmed bladder cancer [26]. All patients considered for inclusion underwent TURB with biopsy and histological evaluation, and histological diagnosis was used as the standard reference.
Clinical data for the bladder cancer patients in both urine series (discovery- and hematuria series) is shown in Table 3. See Additional file 1: Materials and Table S11 for more information.
Urine processing, DNA isolation and bisulfite conversion
Urine samples in the discovery series were processed within 4 h using a standard centrifugation protocol, and DNA was isolated from urine pellets using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Urine samples in the hematuria series were processed using a urine filtration device, and DNA was purified from lysed cell samples using the Oragene DNA purifying solution (DNA Genotek, Ottawa, Canada) as previously described [30]. For all samples, the EpiTect Bisulfite Kit (Qiagen) was used for DNA bisulfite conversion. See Additional file 1: Methods for details.
Targeted methylation analysis—qMSP and ddPCR
Bisulfite-treated DNA from tissue samples was analyzed by quantitative methylation-specific PCR (qMSP) using the 7900HT Real-Time PCR System (Life Technologies, Carlsbad, CA, USA) as previously described [32]. Urine DNA was analyzed by ddPCR using the QX200™ Droplet Digital™ PCR System (BioRad, Hercules, CA, USA) as previously described [33], and positive droplets were called using our PoDCall software [33] (https://bioconductor.org/packages/PoDCall/). See Additional file 1: Methods for details. All primer and probe sequences are listed in Additional file 1: Table S12. All analyses were performed according to the updated digital MIQE-guidelines (Additional file 1: Table S13) [34]. The “Standards for Reporting Diagnostic accuracy studies” (STARD) checklist [35] is included as Additional file 1: Table S14.
Statistics
Statistical analyses were performed using IMB SPSS Statistics 25 and GraphPad Prism 9.1.2. Receiver operating characteristics (ROC) curve were used to evaluate the performance of the individual biomarker candidates. The area under the ROC curves (AUC), sensitivities, specificities and 95% confidence intervals were calculated considering conclusive test results [36]. The methylation concentrations providing the highest possible sensitivities with specificities > 95% (based on the discovery series: bladder cancer and healthy controls) were chosen as thresholds for scoring a sample as positive or negative for the individual biomarkers. Negative and positive predictive value (NPV and PPV) were calculated considering the sensitivity, specificity and a disease prevalence of 20% in hematuria patients [26].
Availability of data and materials
The methylome sequencing data are available from the corresponding author upon reasonable request. All other data that supports the findings of this study are available in the article and in the Additional file 1.
Abbreviations
- ATCC:
-
American type culture collection
- AUC:
-
Area under the ROC curve
- CIS:
-
Carcinoma in situ
- ddPCR:
-
Droplet digital PCR
- DMR:
-
Differentially methylated region
- FDA:
-
The US food and drug administration
- MIQE:
-
Minimum information for publication of quantitative PCR experiments
- NBI:
-
Narrow band imaging
- NPV:
-
Negative predictive value
- PDD:
-
Photodynamic diagnosis
- PPV:
-
Positive predictive value
- qMSP:
-
Quantitative methylation-specific PCR
- ROC:
-
Receiver operating characteristics
- RRBS:
-
Reduced representation bisulfite sequencing
- TSS:
-
Transcription start site
- WLC:
-
White light cystoscopy
References
Global Cancer Statistics 2020 [Internet]. World Health Organization. 2020. Available from: http://gco.iarc.fr/.
Ramirez D, Gupta A, Canter D, Harrow B, Dobbs RW, Kucherov V, et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. 2016;117(5):783–6.
Shirodkar SP, Lokeshwar VB. Bladder tumor markers: from hematuria to molecular diagnostics–where do we stand? Expert Rev Anticancer Ther. 2008;8(7):1111–23.
Rai BP, Luis Dominguez Escrig J, Vale L, Kuusk T, Capoun O, Soukup V, et al. Systematic review of the incidence of and risk factors for urothelial cancers and renal cell carcinoma among patients with haematuria. Eur Urol. 2022;82(2):182–92.
Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TR, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14(4):1–331.
Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66(2):253–62.
David SA, Patil D, Alemozaffar M, Issa MM, Master VA, Filson CP. Urologist use of cystoscopy for patients presenting with hematuria in the United States. Urology. 2017;100:20–6.
Chou R, Gore JL, Buckley D, et al. Urinary biomarkers for diagnosis of bladder cancer: a systematic review and meta-analysis. Ann Intern Med. 2015;163(12):922–31.
Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–61.
Witjes JA, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–75.
Batista R, Vinagre N, Meireles S, Vinagre J, Prazeres H, Leão R, et al. Biomarkers for bladder cancer diagnosis and surveillance: a comprehensive review. Diagnostics (Basel). 2020;10(1):39.
Laukhtina E, Shim SR, Mori K, D’Andrea D, Soria F, Rajwa P, et al. Diagnostic accuracy of novel urinary biomarker tests in non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4(6):927–42.
Sathianathen NJ, Butaney M, Weight CJ, Kumar R, Konety BR. Urinary biomarkers in the evaluation of primary hematuria: a systematic review and meta-analysis. Bladder Cancer. 2018;4(4):353–63.
Kandimalla R, van Tilborg AA, Zwarthoff EC. DNA methylation-based biomarkers in bladder cancer. Nat Rev Urol. 2013;10(6):327–35.
Bosschieter J, Lutz C, Segerink LI, Vis AN, Zwarthoff EC, Avm RJ, et al. The diagnostic accuracy of methylation markers in urine for the detection of bladder cancer: a systematic review. Epigenomics. 2018;10(5):673–87.
Larsen LK, Lind GE, Guldberg P, Dahl C. DNA-methylation-based detection of urological cancer in urine: overview of biomarkers and considerations on biomarker design, source of DNA, and detection technologies. Int J Mol Sci. 2019;20(11):2657.
Costa VL, Henrique R, Danielsen SA, Duarte-Pereira S, Eknaes M, Skotheim RI, et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin Cancer Res. 2010;16(23):5842–51.
Russo GI, Sholklapper TN, Cocci A, Broggi G, Caltabiano R, Smith AB, et al. Performance of narrow band imaging (NBI) and photodynamic diagnosis (PDD) fluorescence imaging compared to white light cystoscopy (WLC) in detecting non-muscle invasive bladder cancer: a systematic review and lesion-level diagnostic meta-analysis. Cancers (Basel). 2021;13(17):4378.
van der Aa MN, Steyerberg EW, Bangma C, van Rhijn BW, Zwarthoff EC, van der Kwast TH. Cystoscopy revisited as the gold standard for detecting bladder cancer recurrence: diagnostic review bias in the randomized, prospective CEFUB trial. J Urol. 2010;183(1):76–80.
Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European association of urology guidelines on non–muscle-invasive bladder cancer (Ta, T1, and carcinoma in Situ). Eur Urol. 2022;81(1):75–94.
Yossepowitch O, Herr HW, Donat SM. Use of urinary biomarkers for bladder cancer surveillance: patient perspectives. J Urol. 2007;177(4):1277–82.
Vriesema JL, Poucki MH, Kiemeney LA, Witjes JA. Patient opinion of urinary tests versus flexible urethrocystoscopy in follow-up examination for superficial bladder cancer: a utility analysis. Urology. 2000;56(5):793–7.
van Kessel KE, Van Neste L, Lurkin I, Zwarthoff EC, Van Criekinge W. Evaluation of an epigenetic profile for the detection of bladder cancer in patients with hematuria. J Urol. 2016;195(3):601–7.
van Kessel KE, Beukers W, Lurkin I, Ziel-van der Made A, van der Keur KA, Boormans JL, et al. Validation of a DNA methylation-mutation urine assay to select patients with hematuria for cystoscopy. J Urol. 2017;197(3):590–5.
Feber A, Dhami P, Dong L, de Winter P, Tan WS, Martinez-Fernandez M, et al. UroMark-a urinary biomarker assay for the detection of bladder cancer. Clin Epigenetics. 2017;9:8.
Dahmcke CM, Steven KE, Larsen LK, Poulsen AL, Abdul-Al A, Dahl C, et al. A prospective blinded evaluation of urine-DNA testing for detection of urothelial bladder carcinoma in patients with gross hematuria. Eur Urol. 2016;70(6):916–9.
Grossman HB, Gomella L, Fradet Y, Morales A, Presti J, Ritenour C, et al. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol. 2007;178(1):62–7.
Lind GE, Danielsen SA, Ahlquist T, Merok MA, Andresen K, Skotheim RI, et al. Identification of an epigenetic biomarker panel with high sensitivity and specificity for colorectal cancer and adenomas. Mol Cancer. 2011;10:85.
Andresen K, Boberg KM, Vedeld HM, Honne H, Jebsen P, Hektoen M, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61(5):1651–9.
Andersson E, Dahmcke CM, Steven K, Larsen LK, Guldberg P. Filtration device for on-site collection, storage and shipment of cells from urine and its application to DNA-based detection of bladder cancer. PLoS ONE. 2015;10(7): e0131889.
Wang L, Sun J, Wu H, Liu S, Wang J, Wu B, et al. Systematic assessment of reduced representation bisulfite sequencing to human blood samples: a promising method for large-sample-scale epigenomic studies. J Biotechnol. 2012;157(1):1–6.
Andresen K, Boberg KM, Vedeld HM, Honne H, Hektoen M, Wadsworth CA, et al. Novel target genes and a valid biomarker panel identified for cholangiocarcinoma. Epigenetics. 2012;7(11):1249–57.
Pharo HD, Andresen K, Berg KCG, Lothe RA, Jeanmougin M, Lind GE. A robust internal control for high-precision DNA methylation analyses by droplet digital PCR. Clin Epigenetics. 2018;10:24.
Group TD, Huggett JF. The digital MIQE guidelines update: minimum information for publication of quantitative digital PCR experiments for 2020. Clin Chem. 2020;66(8):1012–29.
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clin Chem. 2015;61(12):1446–52.
Shinkins B, Thompson M, Mallett S, Perera R. Diagnostic accuracy studies: how to report and analyse inconclusive test results. BMJ. 2013;346: f2778.
Acknowledgements
We are truly thankful to the patients and healthy individuals that provided urine samples for this study. Moreover, the bioinformatic analyses were performed on the TSD (Tjeneste for Sensitive Data) facilities, owned by the University of Oslo, operated and developed by the TSD service group at the University of Oslo, IT-Department (USIT) (tsd-drift@usit.uio.no) and on resources provided by UNINETT Sigma2—the National Infrastructure for High Performance Computing and Data Storage in Norway.
Funding
This work was supported by a grant from the South-Eastern Norway Regional Health Authority (project number 40093, to GEL), a Cancer-BIOTEK Grant No. (254834), and innovation funding from the South-Eastern Norway Regional Health Authority and the University of Oslo, Norway. The funding sources had no role in this study.
Author information
Authors and Affiliations
Contributions
GEL and PG conceived and designed the study. GEL obtained funding and supervised the study. HDP, HMV, HH, SBW, MBF, AKS, CD, LKL, SMR, RH, CJ, KS and RW contributed in acquisition of the data. HDP, MJ, EAW, HMV, HH, SBW, MBF, PG and GEL participated in analysis and interpretation of the data. MJ, EAW, HDP and HMV performed the statistical analyses. HDP and MJ drafted the article. GEL, PG, RW, HMV, CD and LKL critically revised the manuscript and provided important comments to improve intellectual content. All authors read and approved the final version of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out according to the Helsinki declaration, and the included research biobanks have been registered according to national legislation. The study has been approved by the Regional Committee for Medical and Health Research Ethics, which requires that informed consent is obtained from patients enrolled in the study.
Consent for publication
Not applicable.
Competing interests
GEL certifies that all conflicts of interest are the following: GEL, CJ and RH are named on a patent (WO/2012/052844) proposing VIM as a biomarker for detection of bladder cancer, granted in US (US9797016) and EP (2,630,261). GEL, RW, CJ and HDP are named on a pending PCT patent application (WO/2020/099938) covering the biomarkers published in this study. GEL, MJ and HDP are named on two pending US national applications (US/2019/0221286 and WO/2019/106149) covering the 4Plex control and PoDCall algorithm used in the present study for normalization of DNA methylation ddPCR results. The remaining authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pharo, H.D., Jeanmougin, M., Ager-Wick, E. et al. BladMetrix: a novel urine DNA methylation test with high accuracy for detection of bladder cancer in hematuria patients. Clin Epigenet 14, 115 (2022). https://doi.org/10.1186/s13148-022-01335-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-022-01335-2