Abstract
Background
Tumour DNA methylation profiling has shown potential to refine disease subtyping and improve the diagnosis and prognosis prediction of breast cancer. However, limited data exist regarding invasive lobular breast cancer (ILBC). Here, we investigated the genome-wide variability of DNA methylation levels across ILBC tumours and assessed the association between methylation levels at the variably methylated regions and overall survival in women with ILBC.
Methods
Tumour-enriched DNA was prepared by macrodissecting formalin-fixed paraffin embedded (FFPE) tumour tissue from 130 ILBCs diagnosed in the participants of the Melbourne Collaborative Cohort Study (MCCS). Genome-wide tumour DNA methylation was measured using the HumanMethylation 450K (HM450K) BeadChip array. Variably methylated regions (VMRs) were identified using the DMRcate package in R. Cox proportional hazards regression models were used to assess the association between methylation levels at the ten most significant VMRs and overall survival. Gene set enrichment analyses were undertaken using the web-based tool Metaspace. Replication of the VMR and survival analysis findings was examined using data retrieved from The Cancer Genome Atlas (TCGA) for 168 ILBC cases. We also examined the correlation between methylation and gene expression for the ten VMRs of interest using TCGA data.
Results
We identified 2771 VMRs (P < 10−8) in ILBC tumours. The ten most variably methylated clusters were predominantly located in the promoter region of the genes: ISM1, APC, TMEM101, ASCL2, NKX6, HIST3H2A/HIST3H2BB, HCG4P3, HES5, CELF2 and EFCAB4B. Higher methylation level at several of these VMRs showed an association with reduced overall survival in the MCCS. In TCGA, all associations were in the same direction, however stronger than in the MCCS. The pooled analysis of the MCCS and TCGA data showed that methylation at four of the ten genes was associated with reduced overall survival, independently of age and tumour stage; APC: Hazard Ratio (95% Confidence interval) per one-unit M-value increase: 1.18 (1.02–1.36), TMEM101: 1.23 (1.02–1.48), HCG4P3: 1.37 (1.05–1.79) and CELF2: 1.21 (1.02–1.43). A negative correlation was observed between methylation and gene expression for CELF2 (R = − 0.25, P = 0.001), but not for TMEM101 and APC.
Conclusions
Our study identified regions showing greatest variability across the ILBC tumour genome and found methylation at several genes to potentially serve as a biomarker of survival for women with ILBC.
Similar content being viewed by others
Introduction
Invasive lobular breast cancer (ILBC) is the second most common histological subtype of breast cancer accounting for 10–15% of all cases [1,2,3]. ILBCs are typically oestrogen receptor (ER) and progesterone receptor (PR) positive and human epidermal growth factor receptor 2 (HER2) negative and are strongly associated with hormonal risk factors for breast cancer [4,5,6,7]. The incidence of ILBC increased sharply in the late 1990s as a consequence of the increased use of hormone replacement therapy (HRT) [8,9,10,11,12,13]. Awareness of the increased risk of breast cancer associated with HRT led to reduced use and a decline in ILBC incidence [14], but it has been shown to increase again recently [15, 16].
ILBCs display an obscure growth pattern with small, round and discohesive cells growing in a single file without forming any distinct clusters [17]. This is likely to be related to a loss of E-cadherin protein which is common in ILBC tumourigenesis and is a hallmark of this subtype [18]. Compared with other breast cancer types, ILBCs are less likely to form a firm and distinct lump and often present as undefined palpable masses on mammography [19, 20]. This poses a significant challenge for its early detection by routine mammographic screening [19, 21,22,23]. This occult nature may explain the detection of ILBC cases at advanced stages [4, 24,25,26]. ILBCs display a unique metastatic behaviour and often metastasis to the gastrointestinal tract [27, 28], colon [29], ovaries [30] and uterus [31], which is uncommon for other breast cancers types.
ILBC is biologically and histologically heterogeneous with several histological subtypes described that show distinct clinical behaviour and outcomes [3, 17, 32,33,34,35]. Aberrant tumour DNA methylation is a hallmark of cancer that occurs early in cancer development and is thus a potentially valuable marker of tumour progression and patient survival. Alterations in tumour DNA methylation have been investigated in detail for many types of cancer, including breast cancer but ILBCs are largely underrepresented in these studies [36, 37]. Studies focusing on ILBC-specific DNA methylation alterations have mainly used a candidate gene approach and have reported aberrant promoter methylation status for specific genes such as CDH1 [38,39,40,41], RASSF1A, HIN-1, RAR-β, cyclin-D2, TWIST [42], ADAM33 [43], SFRP1 [44] and DAPK1 [45]. Moelans et al. (2015) compared the methylation profiles of classic ILBC (n = 20), pleomorphic ILBC (n = 16) and IDBC (n = 20) for 24 established and putative tumour suppressor genes and found lower TP73 and MLH1 promoter methylation and higher RASSF1 promoter methylation in pleomorphic compared with classic ILBC [46]. Bae et al. (2004) compared the methylation profiles of ILBC (n = 19), IDBC (n = 60) and mucinous breast cancer (n = 30) for a panel of 12 genes and found BRCA1 promoter hypermethylation in 92% of mucinous breast cancer compared with 39% in ILBC and 28% in IDBC. They also reported ILBC and mucinous breast cancer samples to be more frequently methylated for other genes in the panel compared with IDBC [47].
In this study, we hypothesised that genome-wide variations in DNA methylation patterns within the ILBC group may guide or reflect different tumour biologies leading to subgroups of tumours that differ in their clinical behaviour. Our aims were twofold: i) to investigate the genome-wide DNA methylation variability within the ILBC group and ii) to assess associations between tumour methylation at the most variable methylated regions and overall survival for women with ILBC.
Results
Study participants
The median age at breast cancer diagnosis in the MCCS was 65 years with tumours being diagnosed at stage 1A/1B (50%), 2A/2B (37%) and 3A/3C/4 (9%). There were 37 deaths observed during follow-up (median [IQR]: 13 [9–18] years). The tumours were mainly ER-positive, PR-positive and HER2-negative (47%). In TCGA data, the median age at diagnosis was 62 years. In both datasets, older women (aged 60 years or older at diagnosis) formed the majority of the cases (65%, in the MCCS and 58%, in TCGA). There was a higher proportion of young women at diagnosis (age less than 50 years: 21%) in TCGA compared with the MCCS (5%). The proportion of later-stage tumours (3A/3B/3C/4) was also higher in TCGA (33%) compared with the MCCS (9%). A total of 14 deaths were recorded during the follow-up (median [IQR]: 2 [1.5–5] years) in TCGA dataset. The clinical and pathological features of the study participants in the MCCS and TCGA and a comparison of the two studies are summarised in Table 1.
Variably methylated regions in ILBC
We identified 2,771 regions across the genome that showed substantially variable methylation (P < 10−8) across ILBCs in the MCCS (Additional file 2: Table S1). These VMRs corresponded to 2,208 genes and 563 intergenic regions. The most significant regions (P < 10−8) and the genes associated with these regions were chr20:13199787–13201844 (ISM1, 29 CpGs), chr5:112073348–112074043 (APC, 16 CpGs), chr17:42091713–42093050 (TMEM101, 16 CpGs), chr11:2290953–2293552 (ASCL2, 41 CpGs), chr10:134598496–134602228 (NKX6, 39 CpGs) and chr1:22844750–228647248 (HIST3H2A/HIST3H2BB, 28 CpGs). The average methylation level (beta-values) ranged between 0.09 and 0.63 at ISM1, 0.08 and 0.82 at APC, 0.15 and 0.83 at TMEM101, 0.15 and 0.77 at ASCL2, 0.07 and 0.70 at NKX6, and 0.05 and 0.58 at HIST3H2A/ HIST3H2BB (Fig. 1). There was some tendency for VMRs including more CpGs to be more highly ranked (Additional file 1: Fig. S1). We found a significant enrichment for CpG island-associated regions compared to all probes included in the HM450K array (Fig. 2a). Gene annotation also showed that 62% of the VMRs were located in gene promoter regions (1st Exon, 5 prime UTR, TSS1500 and TSS200) compared with 20% in gene body regions and 23% in enhancer regions (Fig. 2b). The pathway enrichment analysis showed that the genes associated with the VMRs were enriched for 1,973 terms (FDR-adjusted P < 0.05) including 54 KEGG pathways with stronger evidence for neuroactive ligand-receptor interaction (hsa04080), breast cancer (hsa05224), pathways in cancer (hsa05200), hippo signalling pathway (hsa04390), Rap1 signalling pathway (hsa04015) and PI3K-Akt signalling pathway (hsa04151). Figure 3 shows the twenty most significant KEGG pathways enriched in the VMRs.
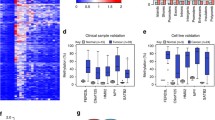
Methylation pattern of invasive lobular breast cancer (ILBC) samples. Heatmaps show the methylation patterns of invasive lobular breast cancer (ILBC) samples in the Melbourne Collaborative Cohort Study (MCCS) across the ten most significant variably methylated regions (VMRs): a ISM1, b APC, c TMEM101, d ASCL2, e NKX6, f HIST3H2A, g HCG4P3, h HES5, i CELF2 j EFCAB4B. Annotation of CpGs by genomic position and location in the context of gene are marked on the maps. Annotation of samples by age at diagnosis and tumour characteristics are shown in the colour bars as indicated in the legend on the top-right. The methylation beta-value of the CpG positions shown in the heatmap is indicated in the colour key on the top-right corner
Genomic distribution of the variably methylated regions (VMRs). Bar plots show the distribution of 2771 variably methylated regions (VMRs) identified within invasive lobular breast cancer (ILBC) samples in the Melbourne Collaborative Cohort Study (MCCS) a relative to CpG islands, shores (0–2 kb from island), shelves (2–4 kb from island) and open sea and b in relation to the gene. Different genomic locations are shown on the x-axis and the percentage of CpG positions related to the VMRs is shown on the y-axis. The distribution of the HM450K probes relative to each CpG context is also indicated. P-values (Chi-square test) assessing significant enrichment in a given category relative to the HM450K array composition are indicated (*P < 0.001)
Twenty most significantly enriched KEGG pathways. Bar plot shows twenty most significantly enriched KEGG pathways in the variably methylated region (VMRs) identified within invasive lobular breast cancer (ILBC) samples in the Melbourne Collaborative Cohort Study (MCCS). The enriched terms are shown on the y-axis and the P-values (log transformed) assessing significant enrichment are shown on the x-axis
Replication of the VMR analysis in TCGA dataset (n = 168), identified 2760 VMRs, of which 763 (28%) overlapped with the MCCS. The ten most significant VMRs identified in the MCCS ranked highly in the TCGA dataset (Table 2).
Pathway enrichment analysis of the 763 overlapping VMRs resulted in 416 enriched functional terms (FDR-adjusted P < 0.05) including nine enriched KEGG pathways. Of these, 369 overlapped with pathways identified for all MCCS VMRs; neuroactive ligand-receptor interaction (hsa04080) and hippo signalling pathway (hsa04390) were among the KEGG pathways that were also found to be significantly enriched using all MCCS VMRs.
VMRs and association with overall survival
In the MCCS, higher tumour methylation showed association with shorter overall survival for APC (HR = 1.28, 95% CI: 1.07–1.53), HIST3H2A/HIST3H2BB (HR = 1.28, 95% CI: 1.02–1.62), CELF2 (HR = 1.30, 95% CI: 1.07–1.58) and TMEM101 (HR = 1.21, 95% CI: 1.00–1.48). Weak evidence of association was also observed for ISM1 (HR = 1.34, 95% CI: 0.97–1.85), NKX6 (HR = 1.25, 95% CI: 0.98–1.60) and HCG4P3 (HR = 1.24, 95% CI: 0.93–1.67). After adjusting for age at diagnosis and tumour stage, the association remained consistent for APC (HR = 1.24, 95% CI: 1.04–1.49), TMEM101 (HR = 1.22, 95% CI: 0.99–1.51) and HCG4P3 (HR = 1.25, 95% CI: 0.91–1.72) (Table 3). As shown in Table 3, all VMRs had an average methylation level below 0.5 and the direction of association was positive (gains in methylation associated with shorter survival).
In TCGA dataset, the crude HRs were all positive, consistent with the MCCS dataset, albeit generally greater, in particular for ISM1 (HR = 1.48, 95% CI: 0.91–2.41), ASCL2 (HR = 1.28, 95% CI: 0.74–2.20), NKX6 (HR = 2.06, 95% CI: 1.32–3.21), HIST3H2A/HIST3H2BB (HR = 1.35, 95% CI: 1.00–1.83), HCG4P3 (HR = 2.04, 95% CI: 1.32–3.15), CELF2 (HR = 1.50, 95% CI: 1.06–2.12) and EFCAB4B (HR = 1.41, 95% CI: 1.05–1.89). Associations remained consistent after adjustment for age at diagnosis and tumour stage for all VMRs except those located at APC and HES5. The pooled HRs after adjustment for age at diagnosis and tumour stage showed that methylation was associated with overall survival for four genes: APC (HR = 1.18, 95% CI: 1.02–1.36), TMEM101 (HR = 1.23, 95% CI: 1.02–1.48), HCG4P3 (HR = 1.37, 95% CI: 1.05–1.79) and CELF2 (HR = 1.21, 95% CI: 1.02–1.43) (Table 4).
Correlation with gene expression
A relatively strong negative correlation between DNA methylation and gene expression was observed for six of the nine tested VMRs in TCGA (Fig. 4). These included EFCAB4B (R = − 0.5, P = 1.4 × 10−10), CELF2 (R = − 0.25, P = 0.001), HIST3H2A (R = − 0.41, P = 1 × 10−7), ASCL2 (R = − 0.24, P = 0.002), ISM1 (R = − 0.24, P = 0.002) and HES5 (R = − 0.15, P = 0.04). No or slightly positive correlation between DNA methylation and gene expression levels was observed for APC, TMEM101 and NKX6. The feature-by-feature analysis of correlations with gene expression was very consistent with the analysis using average methylation, virtually all associations being in the same direction, with only moderate variation in effect estimates (Additional file 3: Table S2).
Correlation between methylation levels and gene expression. The graphics show the correlation between average DNA methylation (on the x-axis) and gene expression (on the y-axis) levels of invasive lobular breast cancer (ILBC) cases at the corresponding genes associated with nine of ten strongest variably methylated regions (VMRs) with available gene expression data in The Cancer Genome Atlas (TCGA) in the invasive lobular breast cancer (ILBC) cases in TCGA dataset: a ISM1, b APC, c TMEM101, d ASCL2, e NKX6, f HIST3H2A, g HES5, h CELF2, i EFCAB4B
Discussion
We investigated the genome-wide DNA methylation pattern of ILBC tumours, with the aim of identifying methylation markers predictive of patient outcome. Scanning of the ILBC methylome revealed regions of variable methylation in ILBC tumours. The VMRs were primarily located in CpG island regions and were significantly enriched in pathways such as breast cancer (hsa05224), pathways in cancer (hsa05200), hippo signalling pathway (hsa04390), Rap1 signalling pathway (hsa04015) and PI3K-Akt signalling pathway (hsa04151). These pathways have previously been found to be dysregulated in cancer tissue [48,49,50,51,52,53]. Some of the key genes involved in the enriched pathways included APC, DAPK1, BMP2 and CCND2. DAPK1 is an important regulator of cell apoptotic pathways [54] and DAPK1 promoter hypermethylation has previously been reported in ILBCs with a potential role in tumour progression [45, 55]. BMP2 is a member of the TGF-ß superfamily and is involved in cell proliferation and differentiation during tumour formation [56]. Promoter methylation of BMP2 has been associated with breast cancer progression and drug resistance [57]. CCND2 promoter methylation was previously reported to be a common event in breast cancer and have prognostic value [58]. We found a similar DNA methylation variability profile in TCGA dataset, in particular for the VMRs showing strongest variability in the MCCS.
Several previous studies have reported tumour DNA methylation to have prognostic value in cancer [59,60,61,62,63,64]. Methylation at many gene promoters has been reported to have independent prognostic value in breast cancer including HOXA11 [65], ESR1 and PITX2 [66], HOXD13 [67] CDH22 [68] BRCA1 and RASSF1 [69, 70]. Tumour DNA methylation and its prognostic significance has also been investigated for certain breast cancer subtypes, in particular gene expression-based subtypes. Thomas et al. (2017) used hierarchical clustering based on DNA methylation to further segregate luminal A tumours into two subgroups and found that the subgroup with lower relative methylation showed better prognosis [71], similar to the findings of our study. Another study using whole-genome methylation sequencing stratified triple-negative breast cancers into three methylation-defined clusters and found the hypomethylated cluster to show better prognosis compared with the other two highly methylated clusters [72], also consistent with our results. However, to our knowledge, no study has reported on the overall tumour methylation variability in ILBC and tested the potential for the variably methylated regions to be used as prognostic markers. The assessment of VMRs was genome-scale but only the highest ranking VMRs were tested for their association with survival. Although many of the tested VMRs showed a significant association with overall survival, there could be other VMRs or individual CpG sites for which methylation is associated with survival. We found that promoter hypermethylation at APC, TMEM101 and HCG4P3 was associated with shorter overall survival in the MCCS after adjustment for age and tumour stage. The results in TCGA were largely consistent with the MCCS, although associations generally appeared stronger; this might suggest that the prognostic value of these DNA methylation markers is greater for women with more advanced ILBC. In the pooled analysis, DNA methylation at four genes (APC, TME101, HCG4P3 and CELF2) was associated with shorter overall survival. All the highest-ranking VMRs had an average methylation level below 0.5, and the direction of association with survival was virtually always positive, which indicates that methylation gains (i.e. loss of the normal hypomethylation state) were associated with worse survival. APC is a well-known tumour suppressor gene and this finding is in agreement with previous reports [73, 74]. Debouki et al. (2017) found a significant correlation between APC promoter methylation and aggressive behaviour of both non-familial and familial breast cancer in the Tunisian population [73]. The association of APC promoter methylation with reduced survival has also been reported for other cancer types, such as non-small cell lung cancer [75] and prostate cancer [76, 77]. CELF2, an RNA binding protein involved in alternative splicing, has also been reported to be involved in breast cancer growth and progression. Piqué et al., (2019) found that CELF2 promoter methylation led to a loss of CELF2 expression that had a growth promoter effect in breast tumours. They also found that CELF2 promoter methylation was associated with worse patient outcome [78]. In TCGA data, we found a strong, negative correlation between CELF2 promoter methylation and the gene expression levels. TMEM101 is a transmembrane protein that has been shown to activate NF-kappa-beta signalling pathways. There is to our knowledge no previous literature suggesting a role of TMEM101 promoter methylation in relation to cancer progression/survival. HCG4P3 is also known as HLA complex group 4 pseudogene 3, and there is to our knowledge no record of this gene being involved in cancer.
The main limitation of this study was the relatively small sample size that limited our analysis to all-cause death as an endpoint. The MCCS and TCGA data had different characteristics in terms of their study design and sample variation. The two studies had different follow-up times, and TCGA data had more young women and generally higher tumour stage (Table 1). Our findings for both the VMR and survival analysis were nevertheless consistent across the two studies. We considered the main factors that we thought could impact methylation profiles in tumours and ILBC survival, i.e. age and stage. Factors such as smoking, alcohol consumption or diabetes, and perhaps family history (via underlying genetic sequence) likely play some role, but it is presumably less important, so we did not include them in the analysis. These variables are not systematically collected with precision (questionnaires) in the clinical setting. In this context, our study identified methylation biomarkers, and it is likely that many factors worthy of investigation (genetic and lifestyle and environmental) play a role in explaining the observed associations. Finally, while we identified a large number of regions across the ILBC genome that showed substantial variable methylation pattern, only the strongest ten VMRs were tested for association with survival to minimise the multiple testing burden. If replicated by other studies, the methylation markers identified in our study may contribute to the development of molecular signatures for enhanced prediction of ILBC survival.
Conclusions
Our study indicates that methylation levels at the most variable regions across the genome may explain differences in tumour prognosis within the ILBC subtype. We identified APC, TMEM101, HCG4P3 and CELF2 promoter methylation as possibly relevant prognostic biomarkers for women with ILBC. Further studies are required to confirm our findings and to assess their utility in a clinical setting.
Methods
Study samples
The samples included in this study were obtained from the Melbourne Collaborative Cohort Study (MCCS) [79]. The MCCS was set up in 1990 with the aim of investigating the role of diet and lifestyle in cancer and other diseases. Between 1990 and 1994, 41,513 participants, aged 40–69, were recruited in the study, and baseline information on lifestyle, health and diet was collected through interviews. Women with ILBC included in this study were diagnosed between 1993 and 2011, based on the International Classification of Diseases for Oncology (ICD-O) codes 8520 (73%), 8522 (26%) and 8500 (1 case). Clinical and pathological characteristics of the study participants are listed in Table 1.
Endpoints
Incidences of cancer cases and deaths in the MCCS participants are regularly updated by linkage to the Victorian and national cancer and death registries, which are considered to be virtually complete. The latest linkage was completed on 31 March 2017 and death data were considered to be complete up to 31 December 2016. Overall survival was defined as the time (in years) from breast cancer diagnosis to death (from any cause) or end of follow-up.
DNA extraction from formalin-fixed paraffin embedded breast tumour tissue
Pathology material related to each ILBC case had previously been retrieved from the diagnostic service laboratory and reviewed by qualified pathologists. Unstained sections had been prepared and stored desiccated at 4 °C for up to 20 years. DNA extraction was conducted as described in Wong et al. [80]. Briefly, the tumour areas most suitable for macrodissection were identified by a qualified pathologist using the WHO classification of tumours of the Breast Criteria (WHO Classification of Tumours of the Breast (2012). 4th edn. International Agency for Research on Cancer (IARC), Lyon) [81] and recorded by directly marking up representative H&E stained sections. An average of two corresponding 3um methyl green stained FFPE sections were macrodissected as described in Wong et al. [82], and DNA was extracted using the QIAamp DNA FFPE protocol.
Tumour purity was estimated using the R tool InfiniumPurify [83] that takes methylation beta-values of the tumour samples and uses the methylation levels of pre-selected informative differentially methylated CpG sites (iDMCs) identified from TCGA data (when data from normal-adjacent tissue are not available) to estimate tumour purity for each tumour sample by density evaluation of Gaussian kernel. Tumour purity estimates, obtained as the proportion of tumour cells in each sample, were high, ranging from 37 to 88% across samples; 88% of the samples had an estimated tumour purity greater than 50%.
Genome-wide DNA methylation profiling
Genome-wide DNA methylation was measured using the HumanMethylation450K (HM450K) BeadChip array (Illumina). For each sample, a total of 300–500 ng of tumour DNA was bisulfite-converted using Zymo Gold EZ-DNA kit (Irvine, CA) and restored using the DNA Restoration Kit as per the manufacturer’s instructions (Illumina, CA, United States). Sample DNA quantity was assessed using an in-house modified quality control protocol [80]. Samples that passed the final quality check were run on the HM450K array (Illumina) according to manufacturer’s instructions.
Data pre-processing and normalisation
Raw intensity files (IDAT files) were imported into the R computing environment using the Bioconductor package minfi [84], and all samples were pre-processed and normalised together. Data quality was first evaluated by assessing the detection P-value, which was obtained for every CpG site in every sample. Samples with an average detection P-value > 0.01 were considered poor quality and were removed from further analysis. CpG probes with a detection P-value > 0.05 in at least one sample were considered unreliable and were removed from further analysis. Data were normalised using the minfi functional normalisation (FNORM) method to correct for both within-array (technical bias between type I and type II probes) as well as between-array unwanted variations [85]. After data pre-processing and normalisation, a total of 449,005 CpG sites remained for analysis. Beta-values (ratio of the methylated probe intensity and the sum of methylated and unmethylated probe intensity) and M-values (log2 beta-value) were calculated. M-values were used in all statistical analyses, while beta-values were used for data exploration and visualisation, as suggested in [86].
TCGA data
Raw DNA methylation data (IDAT files) for 168 ILBC cases were downloaded from the TCGA legacy database (Study Accession: phs000178) using the R package TCGABiolinks [87]. Methylation data were pre-processed and normalised similarly to the MCCS, and methylation values (beta-values and M-values) were calculated for 168 ILBCs at 440,380 CpG positions across the genome. Survival data were retrieved for 159 (95%) ILBC cases. Gene expression data in the form of normalised counts (RNA sequencing-Illumina Hi-Seq) were retrieved for 159 (95%) ILBC cases. Cases of ILBC in the TCGA dataset were diagnosed between 1992 and 2013. Clinical characteristics of the TCGA samples are listed in Table 1.
Statistical analysis
Variable methylation analysis
Variable methylation analysis was performed using the DMRcate package in R [88]. To identify the variably methylated regions (VMRs), the variance of M-values was computed across 130 ILBCs in the MCCS, and Gaussian smoothing was applied to the resulting per-CpG-site test statistics using the default DMRcate options. DMRcate uses the method of Satterthwaite to smooth test statistics and derive respective P-values. Nearby significant CpG sites were collapsed in clusters using a bandwidth of 1000 base pairs (bp). The clusters that showed the highest variability in DNA methylation (i.e. regions with a minimum adjusted P-value (minfdr) of less than 10−8) were defined as the VMRs. There were 396 solo CpGs that were not included in the VMR calculation. This analysis was replicated using the 168 ILBC samples from TCGA.
Gene set enrichment analysis
Gene set enrichment analysis was performed on all the genes associated with the VMRs using the web-based tool Metaspace using the default settings [89]. Pathway and gene set enrichment analysis were carried out using the KEGG Pathway database [90]. All genes in the human genome were used as the enrichment background. Pathways and biological terms with a P-value < 0.01, a minimum count of 3 and an enrichment factor > 1.5 (the ratio between the observed counts and the counts expected by chance) were selected and grouped into clusters.
Survival analysis
Survival analyses were undertaken for the ten most variably methylated regions identified across the MCCS ILBC samples. Follow-up started at the date of diagnosis and ended at the date of death or end of follow-up, whichever came first. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CI) for the association between DNA methylation levels (M-values) and risk of death. Three models were fitted: (1) univariable, with DNA methylation as a crude predictor, and multivariable, (2) with additional adjustment for age at diagnosis and (3) with adjustment for age at diagnosis and tumour stage. For each VMR, the methylation level was defined as the average methylation value across all CpG sites covering the VMR. The same analysis was carried out using the 168 ILBC samples from TCGA. Survival analyses were undertaken using the R package Survival [91]. HRs from the two individual studies were then pooled using fixed-effects meta-analysis with inverse variance weights.
Association with gene expression
To test if DNA methylation correlated with gene expression at the ten strongest VMRs (identified in the MCCS), we assessed the correlation between average methylation levels (average M-values for all CpGs covering a VMR) and gene expression levels using Pearson’s correlation; we used matching gene expression and DNA methylation data available in the TCGA dataset for nine of the ten strongest VMRs. The correlations with gene expression were also assessed for individual CpG sites of each VMR.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- ER:
-
Oestrogen receptor
- FFPE:
-
Formalin fixed paraffin embedded
- FNORM:
-
Functional normalisation
- HM450K:
-
HumanMethylation 450K
- HER2:
-
Human epidermal growth factor receptor 2
- HRT:
-
Hormone replacement therapy
- HR:
-
Hazard ratio
- ILBC:
-
Invasive lobular breast cancer
- ICD-O:
-
International Classification of Diseases for Oncology
- IDBC:
-
Invasive ductal breast cancer
- MCCS:
-
Melbourne Collaborative Cohort Study
- MRI:
-
Magnetic resonance imaging
- PR:
-
Progesterone receptor
- TCGA:
-
The Cancer Genome Atlas
- VMR:
-
Variably methylated region
References
Reed AEM, McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ’omics. Breast Cancer Res. 2015;17(1):12.
Lee J-H, Park S, Park HS, Park B-W. Clinicopathological features of infiltrating lobular carcinomas comparing with infiltrating ductal carcinomas: a case control study. World J Surg Oncol. 2010;8:34.
Orvieto E, Maiorano E, Bottiglieri L, Maisonneuve P, Rotmensz N, Galimberti V, et al. Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single institution. Cancer. 2008;113(7):1511–20.
Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149–56.
Mersin H, Yildirim E, Gülben K, Berberoğlu U. Is invasive lobular carcinoma different from invasive ductal carcinoma? Eur J Surg Oncol. 2003;29(4):390–5.
Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, Clavel-Chapelon F, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128(1):144–56.
Flesch-Janys D, Slanger T, Mutschelknauss E, Kropp S, Obi N, Vettorazzi E, et al. Risk of different histological types of postmenopausal breast cancer by type and regimen of menopausal hormone therapy. Int J Cancer. 2008;123(4):933–41.
Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer. 2000;88(11):2561–9.
Verkooijen HM, Fioretta G, Vlastos G, Morabia A, Schubert H, Sappino A-P, et al. Important increase of invasive lobular breast cancer incidence in Geneva, Switzerland. Int J Cancer. 2003;104(6):778–81.
Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomark Prev. 2005;14(4):1008–11.
Portschy PR, Marmor S, Nzara R, Virnig BA, Tuttle TM. Trends in incidence and management of lobular carcinoma in situ: a population-based analysis. Ann Surg Oncol. 2013;20(10):3240–6.
Louwman MWJ, Vriezen M, van Beek MWPM, Nolthenius-Puylaert MCBJET, van der Sangen MJC, Roumen RM, et al. Uncommon breast tumors in perspective: incidence, treatment and survival in the Netherlands. Int J Cancer. 2007;121(1):127–35.
Rosenberg LU, Magnusson C, Lindström E, Wedrén S, Hall P, Dickman PW. Menopausal hormone therapy and other breast cancer risk factors in relation to the risk of different histological subtypes of breast cancer: a case–control study. Breast Cancer Res. 2006;8(1):R11.
Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomark Prev. 2007;16(12):2773–80.
Wachtel MS, Yang S, Dissanaike S, Margenthaler JA. Hormone replacement therapy, likely neither Angel Nor Demon. PLoS ONE. 2015;10(9):e0138556.
Ward EM, DeSantis CE, Lin CC, Kramer JL, Jemal A, Kohler B, et al. Cancer statistics: breast cancer in situ. CA Cancer J Clin. 2015;65(6):481–95.
Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979;3(6):467–88.
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–19.
Le Gal M, Ollivier L, Asselain B, Meunier M, Laurent M, Vielh P, et al. Mammographic features of 455 invasive lobular carcinomas. Radiology. 1992;185(3):705–8.
Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics. 2009;29(1):165–76.
Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol. 1993;161(5):957–60.
Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–49.
Munot K, Dall B, Achuthan R, Parkin G, Lane S, Horgan K. Role of magnetic resonance imaging in the diagnosis and single-stage surgical resection of invasive lobular carcinoma of the breast. Br J Surg. 2002;89(10):1296–301.
Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau S-W, Broglio K, Theriault RL, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41–8.
Molland JG, Donnellan M, Janu NC, Carmalt HL, Kennedy CW, Gillett DJ. Infiltrating lobular carcinoma—a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast. 2004;13(5):389–96.
Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26(18):3006–14.
Mosiun JA, Idris MSB, Teoh LY, Teh MS, Chandran PA, See MH. Gastrointestinal tract metastasis presenting as intussusception in invasive lobular carcinoma of the breast: a case report. Int J Surg Case Rep. 2019;64:109–12.
Montagna E, Pirola S, Maisonneuve P, De Roberto G, Cancello G, Palazzo A, et al. Lobular metastatic breast cancer patients with gastrointestinal involvement: features and outcomes. Clin Breast Cancer. 2018;18(3):e401–5.
Viso Vidal D, Villanueva Pavón R, Jorquera PF. Linitis plastica of the colon due to metastases of invasive lobular breast carcinoma. Rev Esp Enferm Dig. 2019;111(4):326–8.
Ferlicot S, Vincent-Salomon A, Médioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. 2004;40(3):336–41.
Briki R, Cherif O, Bannour B, Hidar S, Boughizane S, Khairi H. Uncommon metastases of invasive lobular breast cancer to the endometrium: a report of two cases and review of the literature. Pan Afr Med J. 2018;30:268.
Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133(2):713–23.
Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast: clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996;77(1):113–20.
Butler D, Rosa M. Pleomorphic lobular carcinoma of the breast: a morphologically and clinically distinct variant of lobular carcinoma. Arch Pathol Lab Med. 2013;137(11):1688–92.
Eusebi V, Magalhaes F, Azzopardi JG. Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol. 1992;23(6):655–62.
Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002;21(35):5462–82.
Jovanovic J, Rønneberg JA, Tost J, Kristensen V. The epigenetics of breast cancer. Mol Oncol. 2010;4(3):242–54.
Droufakou S, Deshmane V, Roylance R, Hanby A, Tomlinson I, Hart IR. Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer. 2001;92(3):404–8.
Sarrió D, Moreno-Bueno G, Hardisson D, Sánchez-Estévez C, Guo M, Herman JG, et al. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer. 2003;106(2):208–15.
Caldeira JRF, Prando ÉC, Quevedo FC, Neto FAM, Rainho CA, Rogatto SR. CDH1promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer. 2006;6(1):48.
Zou D, Yoon H-S, Perez D, Weeks RJ, Guilford P, Humar B. Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CDH1) as a target for chemoprevention of lobular neoplasia. J Pathol. 2009;218(2):265–72.
Fackler MJ, McVeigh M, Evron E, Garrett E, Mehrotra J, Polyak K, et al. DNA methylation of RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107(6):970–5.
Seniski GG, Camargo AA, Ierardi DF, Ramos EAS, Grochoski M, Ribeiro ESF, et al. ADAM33 gene silencing by promoter hypermethylation as a molecular marker in breast invasive lobular carcinoma. BMC Cancer. 2009;9:80.
Lo P-K, Mehrotra J, D’Costa A, Fackler MJ, Garrett-Mayer E, Argani P, et al. Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol Ther. 2006;5(3):281–6.
Lehmann U, Celikkaya G, Hasemeier B, Länger F, Kreipe H. Promoter hypermethylation of the death-associated protein kinase gene in breast cancer is associated with the invasive lobular subtype. Cancer Res. 2002;62(22):6634–8.
Moelans CB, Vlug EJ, Ercan C, Bult P, Buerger H, Cserni G, et al. Methylation biomarkers for pleomorphic lobular breast cancer-a short report. Cell Oncol. 2015;38(5):397–405.
Bae YK, Brown A, Garrett E, Bornman D, Fackler MJ, Sukumar S, et al. Hypermethylation in histologically distinct classes of breast cancer. Clin Cancer Res. 2004;10(18 Pt 1):5998–6005.
Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Can Res. 2010;70(21):8517–25.
Yin M, Zhang L. Hippo signaling: a hub of growth control, tumor suppression and pluripotency maintenance. J Genet Genom. 2011;38(10):471–81.
Li N, Xie C, Lu N. Crosstalk between Hippo signalling and miRNAs in tumour progression. FEBS J. 2017;284(7):1045–55.
Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Can Res. 2009;69(12):4962–8.
McSherry EA, Brennan K, Hudson L, Hill AD, Hopkins AM. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011;13(2):R31.
Ma X-L, Shen M-N, Hu B, Wang B-L, Yang W-J, Lv L-H, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37.
Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Sabanay H, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15(12):1875–86.
Tserga A, Michalopoulos NV, Levidou G, Korkolopoulou P, Zografos G, Patsouris E, et al. Association of aberrant DNA methylation with clinicopathological features in breast cancer. Oncol Rep. 2012;27(5):1630–8.
Thawani JP, Wang AC, Than KD, Lin C-Y, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66(2):233–46.
Du M, Su XM, Zhang T, Xing YJ. Aberrant promoter DNA methylation inhibits bone morphogenetic protein 2 expression and contributes to drug resistance in breast cancer. Mol Med Rep. 2014;10(2):1051–5.
Hung C-S, Wang S-C, Yen Y-T, Lee T-H, Wen W-C, Lin R-K. Hypermethylation of CCND2 in lung and breast cancer is a potential biomarker and drug target. Int J Mol Sci. 2018;19(10):3096.
Buffart TE, Overmeer RM, Steenbergen RD, Tijssen M, van Grieken NC, Snijders PJ, et al. MAL promoter hypermethylation as a novel prognostic marker in gastric cancer. Br J Cancer. 2008;99(11):1802–7.
Hu CY, Mohtat D, Yu Y, Ko Y-A, Shenoy N, Bhattacharya S, et al. Kidney cancer is characterized by aberrant methylation of tissue-specific enhancers that are prognostic for overall survival. Clin Cancer Res. 2014;20(16):4349–60.
Ellinger J, Bastian PJ, Jurgan T, Biermann K, Kahl P, Heukamp LC, et al. CpG island hypermethylation at multiple gene sites in diagnosis and prognosis of prostate cancer. Urology. 2008;71(1):161–7.
Guo W, Zhu L, Yu M, Zhu R, Chen Q, Wang Q. A five-DNA methylation signature act as a novel prognostic biomarker in patients with ovarian serous cystadenocarcinoma. Clin Epigenet. 2018;10(1):142.
Sailer V, Gevensleben H, Dietrich J, Goltz D, Kristiansen G, Bootz F, et al. Clinical performance validation of PITX2 DNA methylation as prognostic biomarker in patients with head and neck squamous cell carcinoma. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0179412.
de Almeida BP, Apolónio JD, Binnie A, Castelo-Branco P. Roadmap of DNA methylation in breast cancer identifies novel prognostic biomarkers. BMC Cancer. 2019;19(1):219.
Xia B, Shan M, Wang J, Zhong Z, Geng J, He X, et al. Homeobox A11 hypermethylation indicates unfavorable prognosis in breast cancer. Oncotarget. 2017;8(6):9794.
Sheng X, Guo Y, Lu Y. Prognostic role of methylated GSTP1, p16, ESR1 and PITX2 in patients with breast cancer: a systematic meta-analysis under the guideline of PRISMA. Medicine. 2017;96(28):e7476.
Zhong Z, Shan M, Wang J, Liu T, Xia B, Niu M, et al. HOXD13 methylation status is a prognostic indicator in breast cancer. Int J Clin Exp Pathol. 2015;8(9):10716.
Martín-Sánchez E, Mendaza S, Ulazia-Garmendia A, Monreal-Santesteban I, Córdoba A, Vicente-García F, et al. CDH22 hypermethylation is an independent prognostic biomarker in breast cancer. Clin Epigenet. 2017;9(1):7.
Wu L, Wang F, Xu R, Zhang S, Peng X, Feng Y, et al. Promoter methylation of BRCA1 in the prognosis of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2013;142(3):619–27.
Jiang Y, Cui L, Shen S, Ding L. The prognostic role of RASSF1A promoter methylation in breast cancer: a meta-analysis of published data. PLoS ONE. 2012;7(5):e36780.
Fleischer T, Klajic J, Aure MR, Louhimo R, Pladsen AV, Ottestad L, et al. DNA methylation signature (SAM40) identifies subgroups of the Luminal A breast cancer samples with distinct survival. Oncotarget. 2017;8(1):1074.
Stirzaker C, Zotenko E, Song JZ, Qu W, Nair SS, Locke WJ, et al. Methylome sequencing in triple-negative breast cancer reveals distinct methylation clusters with prognostic value. Nat Commun. 2015;6(1):1–11.
Debouki-Joudi S, Trifa F, Khabir A, Sellami-Boudawara T, Frikha M, Daoud J, et al. CpG methylation of APC promoter 1A in sporadic and familial breast cancer patients. Cancer Biomark. 2017;18(2):133–41.
He K, Zhang L, Long X. Quantitative assessment of the association between APC promoter methylation and breast cancer. Oncotarget. 2016;7(25):37920.
Brabender J, Usadel H, Danenberg KD, Metzger R, Schneider PM, Lord RV, et al. Adenomatous polyposis coli gene promoter hypermethylation in non-small cell lung cancer is associated with survival. Oncogene. 2001;20(27):3528–32.
Henrique R, Ribeiro FR, Fonseca D, Hoque MO, Carvalho AL, Costa VL, et al. High promoter methylation levels of APC predict poor prognosis in sextant biopsies from prostate cancer patients. Clin Cancer Res. 2007;13(20):6122–9.
Richiardi L, Fiano V, Vizzini L, De Marco L, Delsedime L, Akre O, et al. Promoter methylation in APC, RUNX3, and GSTP1 and mortality inprostate cancer patients. J Clin Oncol. 2009;27:3161–8.
Piqué L, de Paz AM, Piñeyro D, Martínez-Cardús A, de Moura MC, Llinàs-Arias P, et al. Epigenetic inactivation of the splicing RNA-binding protein CELF2 in human breast cancer. Oncogene. 2019;38(45):7106–12.
Milne RL, Fletcher AS, MacInnis RJ, Hodge AM, Hopkins AH, Bassett JK, et al. Cohort Profile: The Melbourne Collaborative Cohort Study (Health 2020). Int J Epidemiol. 2017;46(6):1757–i.
Wong EM, Joo JE, McLean CA, Baglietto L, English DR, Severi G, et al. Tools for translational epigenetic studies involving formalin-fixed paraffin-embedded human tissue: applying the Infinium HumanMethyation450 Beadchip assay to large population-based studies. BMC Res Notes. 2015;8:543.
Lakhani SR. WHO Classification of Tumours of the Breast: International Agency for Research on Cancer; 2012.
Wong EM, Joo JE, McLean CA, Baglietto L, English DR, Severi G, et al. Analysis of the breast cancer methylome using formalin-fixed paraffin-embedded tumour. Breast Cancer Res Treat. 2016;160(1):173–80.
Qin Y, Feng H, Chen M, Wu H, Zheng X. InfiniumPurify: an R package for estimating and accounting for tumor purity in cancer methylation research. Genes Dis. 2018;5(1):43–5.
Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–9.
Fortin J-P, Labbe A, Lemire M, Zanke BW, Hudson TJ, Fertig EJ, et al. Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol. 2014;15(12):503.
Du P, Zhang X, Huang C-C, Jafari N, Kibbe WA, Hou L, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinform. 2010;11:587.
Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71.
Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, Lord R, et al. De novo identification of differentially methylated regions in the human genome. Epigenet Chromatin. 2015;8(1):6.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Therneau T. A package for survival analysis in S. R package version 2.37-7. 2014.
Acknowledgements
The authors thank the staff of the Presidion Medicine Biorepository, School of Clinical Sciences at Monash Health, Monash University and all the participants and contributors to the Melbourne Collaborative Cohort Study.
Funding
MS was the recipient of a Beaney Scholarship in Pathology from The University of Melbourne (2018). TN-D a National Breast Cancer Foundation (Australia) Career Development Fellow (ECF-17-001). MCS is a National Health and Medical Research Council (NMHRC, Australia) Senior Research Fellow (APP1155163). This work was supported by an NHMRC Program Grant (APP1074383) and a project grant from Cancer Australia (APP1047347). Melbourne Collaborative Cohort Study (MCCS) cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further augmented by Australian National Health and Medical Research Council Grants 209057, 396414 and 1074383 and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database.
Author information
Authors and Affiliations
Contributions
MS generated data, conducted analyses and drafted the manuscript; P-AD led the statistical analyses and contributed to the writing of the manuscript; EMW generated data and contributed to manuscript preparation; JHJ generated data, conducted analyses, and contributed to the writing of the manuscript; JLH contributed to the preparation of the manuscript; TN-D conducted analyses and contributed to the drafting of the manuscript; GGG contributed data and contributed to the preparation of the manuscript; RLM contributed data and contributed to the preparation of the manuscript; CMcL generated data and contributed to the drafting of the manuscript; MCS conceived the study, supervised the laboratory work, conducted analyses and contributed to drafting the manuscript; All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The participants of the Melbourne Collaborative Cohort Study provided informed consent. This study was approved by the Cancer Council Victoria Human Research Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Relation between the number of CpGs related to each VMR and the VMR ranking
Additional file 2.
List of variable methylated regions identified in ILBC.
Additional file 3.
Correlation between DNA methylation and gene expression (feature-by-feature analysis).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Suman, M., Dugué, PA., Wong, E.M. et al. Association of variably methylated tumour DNA regions with overall survival for invasive lobular breast cancer. Clin Epigenet 13, 11 (2021). https://doi.org/10.1186/s13148-020-00975-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-020-00975-6