Abstract
Objective
Several pathological conditions trigger the formation of microvesicles (MVs), including infectious diseases such as COVID-19. The shedding of MVs increases the levels of inflammatory factors (e.g., interleukin-6; IL-6) and ultimately leads to an inflammatory cascade response, while also increasing the procoagulant response. The current study aimed to evaluate the level of circulating MVs and their procoagulant activity as well as the serum level of IL-6 in patients with COVID-19 and healthy controls. In this case-control study, 65 patients with COVID-19 and 30 healthy individuals were sampled after obtaining written informed consent. MVs counting was measured using conjugated CD61, CD45, CD235a, and Annexin-V antibodies. Additionally, the procoagulant activity of MVs and the IL-6 level were estimated using enzyme-linked immunosorbent assay (ELISA).
Results
The majority of MVs were platelet-derived MVs (PMVs). Patients with COVID-19 had significantly higher levels of MVs, procoagulant MVs, and IL-6 compared to healthy controls (p < 0.001). MVs were significantly correlated with procoagulant MVs, D-Dimer levels, fibrinogen, and IL-6, but not with platelet, lymphocyte, and neutrophil counts.
Conclusion
Elevated levels of procoagulant MVs and their association with inflammatory and coagulation markers in patients with COVID-19 are suggested as a novel circulatory biomarker to evaluate and predict the procoagulant activity and severity of COVID-19.
Similar content being viewed by others
Introduction
COVID-19 is a pro-inflammatory and pro-thrombotic disease, with numerous immune mechanisms resembling other viral infections, but exhibiting distinct changes in pathophysiological reactions [1, 2]. Herein, it induces systemic hyper-inflammation, raising the risk of thromboembolism. The presence of thrombosis is considered a significant factor in the severity of the disease [3]. Endothelial cell damage, vasculitis, alterations in blood flow and viscosity, activation of the coagulation cascade, and MVs formation are only a few pathomechanisms contributing to endothelial damage and thrombosis formation [4].
In addition, this infectious respiratory virus disrupts and changes the normal responses of immune systems [5]. COVID-19 mainly affects lymphocytes, especially T cells, and is associated with lymphopenia [6]. Moreover, cytokines especially IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IFN-γ, monocyte chemical protein 1 (MCP1), macrophage inflammatory protein 1 alpha (MIP-a1) and tumor necrosis factor-alpha (TNF-α) are significantly increased in patients with a severe type of the disease [7]. On the other hand, IL-6 is released from neutrophils and leads to cytokine storm and eventually lung damage [8]. Hence, cytokine storm plays an essential role in the deterioration of patients’ condition due to COVID-19 [7].
MVs or microparticles (MPs) are a group of small-sized vesicles (ranging from 0.1 to 1 μm) which released from different cell types followed by various triggers such as viral infections, oxidative stress, cell activation, injury, inflammation, and apoptosis [9,10,11]. The clinical importance of MVs in different diseases is growing steadily, emphasizing the necessity for further research to explore the biological characteristics of MVs and their role in various diseases and pathological conditions [9, 10]. Circulating MVs increased in some pathological processes such as inflammation and infection and their production increased the level of inflammatory factors such as IL-1, IL-6, IL-8, and TNF-a and finally caused inflammatory waterfall response due to further activations of inflammation [12]. Moreover, MVs lead to the enhancement of procoagulant response through tissue factor exposure and phospholipids with a negative charge such as phosphatidylserine (PS) that finally cause thrombin generation [13]. This study aimed to assess the levels of procoagulant MVs and IL-6 in patients with COVID-19 to improve our understanding of the potential role of circulating procoagulant MVs in predicting the procoagulant activity and severity of COVID-19 disease.
Materials and methods
Study design
The case-control study was confirmed by the Ethics Committee of Jahrom University of Medical Sciences (IR.JUMS.REC.1399.049), and conducted in accordance with the Declaration of Helsinki. This study was carried out from July 2021 to August 2022. Before recruiting any participant in this study, all of them signed a written consent form after being explained the study. In this study, 65 COVID-19 patients with proven real-time PCR (RT-PCR) test who were referred to Hospital Peymanieh of Jahrom city were selected as the case group. In addition, 30 sex- and age-matched healthy participants with negative screening reports who had no history of previous exposure to COVID-19, with normal coagulation screening tests, and without any underlying coagulation or platelet disorders consisted the control group. Also, a history of qualitative and quantitative platelet disorders, anti-platelet or anti-coagulant medications, and anti-inflammation medications, were defined as the exclusion criteria.
Data collection
Variables comprising demographics, medical history, major comorbidities, clinical data, dates of onset, and main biological findings on the day of admission were collected from the patient’s medical records. In addition, data on the patient’s hemodynamic status, disease severity, presence of fever, need for ventilatory support, and daily use of specific therapies were also collected.
Blood sample collection
After obtaining written consent, venous blood samples were collected from the case (who confirmed with COVID-PCR test) and control groups from their antecubital vein through a 21-gauge needle (BD Vacutainer needles). Blood samples were collected from each participant into tubes containing ethylenediaminetetraacetic acid (EDTA, 1.5 mg/ml), sodium citrate (0.2 ml 3.8%), and anticoagulant-free tubes. Serum samples for IL-6 analysis were collected by centrifuging clotted blood samples at 1200 g for 10 min and subsequently stored at -20 °C until analysis.
Laboratory tests
Other Laboratory tests included baseline complete blood count (CBC), prothrombin time (PT), and partial thromboplastin time (PTT) coagulation test, fibrinogen, D-Dimer, and ferritin were performed on patients and healthy subjects. The routine CBC and white blood cell (WBC) differential count were performed on the date of COVID-19 diagnosis or admission date on Sysmex hematology analyzers (Sysmex XT 2000i, Diamond Diagnostics, USA) as a part of routine clinical care. Also, by using the semi-automated coagulation analyzer (Start Max, Stago, France), fibrinogen (STA-Liquid Fib, Stago, France, Cat. No. 00673), PT (STA-NeoPTimal 5, Stago, France, Cat. No. 01163), and PTT (STA-C.K. Prest, Stago, France, Cat. No. 00597), tests were evaluated based on the clot formation method. D-Dimer was also measured enzymatically (D-Dimer AccuBind ELISA Kit, Lake Forest, USA, Cat. No. 12025–300 A).
Microvesicle isolation
To evaluate the presence of circulating MVs in samples, MVs were separated from the taken samples with centrifugation. At first, plasma was separated immediately (within 30 min) by centrifugation of a blood sample containing citrate anticoagulant at 2000 g for 10 min. Plasma was removed and mixed with phosphate-buffered saline (PBS; pH 7.4, ratio 2.5:1) and centrifuged again at 2500 g for 10 min. After removal of the top two-thirds of the double-centrifuged plasma, the residual plasma was stored at − 70 °C until MVs analysis.
Microvesicle analysis by flow cytometry
CyFlow Space flow cytometer (Partec PAS, Germany) was used to determine the count and phenotype of MVs in the supernatant of blood samples, and the collected data were analyzed using Flomax software. This instrument consists of two parts including laser and standard optical filter and the voltages of the photomultiplier were set in the desired range for cellular analysis. Also, forward scatter (FSC) and side scatter (SSC) parameters, which determine the cell size and granularity, respectively, are adjusted logarithmically.
Yellow-Green (YG) Carboxylate Microspheres 1.0 μm (Polysciences, Warrington, Philadelphia) were applied to identify the size events. The bead vial contains a precise number of fluorescent beads (4.5 × 1010 particles/mL), enabling the comparison of measured MVs between different samples.
Fluorescein isothiocyanate (FITC) anti-human CD45 (BD Pharmingen, San Diego, CA, USA), FITC anti-human CD235a (BD), and Phycoerythrin (PE) anti-human CD61 (BD) were used to tag leukocyte- (LMVs), red blood cell- (RMVs), and platelet-derived MVs (PMVs), respectively. Moreover, FITC human Annexin V (BD) was used to tag phosphatidylserine (PS) on procoagulant MVs.
To determine the cell origin and concentration of MVs in plasma, samples (50 μl) were labeled with anti-CD45 (5 μl), anti-CD235a (5 μl), and anti-CD61 (5 μl) for 30 min at 4 °C in the dark. Also, 50 μl of plasma samples were added to test tubes containing 300 μl binding buffer (0.1 mol/l HEPES / pH 7.4, 25 mmol/l CaCl2, and 1.4 mol/l NaCl), and then FITC-Annexin V (5 μl) was added. After a 30 min incubation at 25 °C in the dark, samples were analyzed. Before analysis, 5 μl of well-homogenized beads that were diluted 1:500 in double-distilled water, was added to each sample. The MVs enumeration was measured in relation to ten thousand bead events. Finally, the MVs absolute count was calculated as follows [14, 15]:
In addition, the control tube containing beads and buffer only without human plasma was used to evaluate the background noise.
Microvesicle procoagulant activity assay
To determine the procoagulant activity of MVs, a functional ELISA test (Zymuphen MP-activity, Hyphen BioMed, Neuville-sur-Oise, France) based on PS expression was used. Firstly, calibrator (100 μl), and 1/20 diluted plasma (100 μl) along with calcium, factor Xa, and thrombin inhibitor were added to microplate wells that pre-coated with streptavidin and biotinylated Annexin V, and then incubated for 1 h at 37 °C. Following a washing step, the mixture of factors Xa-Va containing calcium (100 μl), and then pure prothrombin (50 μl) were added and kept plate for 10 min at 37 °C. If there are MVs in the sample, the MVs are connected to annexin V through PS and the FXa-FVa complex can convert prothrombin into active thrombin in the presence of calcium.
After incubation time, the specific chromogenic substrate (50 μl) was used to detect the amount of generated thrombin that correlated with the number of procoagulant MVs. The final reaction was stopped with citric acid (2%, 50 μl), and the absorbance of the solution was measured at 405 nm. According to the calibration curve, results were calculated and expressed as nano-molar PS (nM PS) equivalents [14, 15].
Measuring the serum level of IL-6
The Single Molecule Counting (SMC™) Human IL-6 High Sensitivity Immunoassay (SMC™ Human IL-6 High Sensitivity Immunoassay Kit, Merck, Darmstadt, Germany, Cat. No. 03-0155-00) was used to measure the serum level of IL-6 on the stored samples. The process was conducted following the manufacturer’s recommended procedure with a detection limit of 0.08 pg/mL, an intra-assay variation of 7.8%, and an inter-assay variation of 18% (Fig. 1).
Statistical analysis
All statistical analyses were performed by GraphPad Prism (version 8.0) and SPSS software version 24 (IBM SPSS Statistics version 24, USA). The normal distribution of the data was determined using the Kolmogorov-Smirnov test. The descriptive data were presented as mean ± standard deviation, and the analytical data were compared using the one-way analysis of variance (ANCOVA) and Duncan’s test. For all statistical analyses, a p-value < 0.05 was considered statistically significant.
Results
Demographic and clinical findings
In this case-control study, A total of 65 COVID-19 patients and 30 healthy individuals were included. In this study, the mean age of patients and healthy volunteers was 48.1 ± 11.3, and 47.5 ± 10.4 years, respectively. There were 38 men and 27 women in the patient group, and 17 men and 13 women in the healthy group.
Demographic and clinical characteristics of the patients are detailed in Table 1. Of these patients, four (06.15%) had underlying cardiovascular disease, two (03.07%) had kidney disorders, six (09.23%) had high blood pressure, seven (10.76%) had diabetes, sixteen (24.61%) suffered from obesity, and ten patients (15.38%) had two or more underlying disorders.
Most patients were hospitalized after 6.8 days of symptoms, with fatigue and cough being the most prevalent complaints. Thirty patients were admitted in general ward (32.30%), and ICU (13.84%) due to the pneumonia with hypoxemia. patients received respiratory support with either non-invasive oxygen therapy (40%) or mechanical ventilation (10.76%). Out of all the patients, five deaths were observed.
Laboratory findings
A statistically significant difference in hemoglobin, hematocrit, RBC, WBC, platelet, neutrophil count, and lymphocyte count was observed between patients and healthy individuals (p < 0.01). The results of CBC indices in both studied groups are shown in Table 2. In the coagulation tests panel, we observed a significant increase in PT and PTT in the patient group (P < 0.01). Also, the plasma levels of fibrinogen (P < 0.01) and D-Dimer (P < 0.001) increased significantly in the patient group (Table 2). The variable level of fibrinolytic system in patients’ group was significantly higher than healthy one. In the inflammatory panel, there was a significant increase in ferritin levels in the patient group compared to healthy individuals (P < 0.001).
MVs enumeration
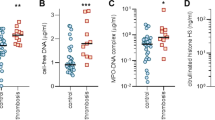
Flow cytometric analysis revealed that all samples tested had MVs. At first, MVs were gated based on their specific FSC and SSC signals. MVs were seen as the population slightly smaller and with lower SSC than the 1.0 μm beads (Fig. 2). The number of total MVs counted based on the FSC and SSC signals was significantly higher in patients compared to the control (Fig. 3).
Flowcytometric graphs of MVs: FSC and SSC represent the size and granularity of MVs, respectively. Using 1.0 μm beads, the size range of MVs was determined. (a) The two specified ranges under the headings R1 and R2 represent MVs and 1.0 μm beads, respectively. According to FSC and SSC, the MVs gate was lower than beads. (b) Logarithmic histogram of FSC versus MVs count, which shows the distribution of MVs compared to beads. (c) A graph of beads and buffer only without plasma
Afterward, MVs were further identified by labeling with fluorescent CD61, CD45, CD235, and Annexin-V markers. Flow cytometric results showed that the majority of MVs originated from platelets (CD61+). The number of CD61 + MVs (PMVs) was significantly different between patients and the control group (p < 0.001) (Fig. 3). Also, there was a statistically significant difference in the level of RMVs and LMVs between patients and the control group (p < 0.001).
Moreover, MVs also showed positive results for Annexin-V staining. Annexin-V serves as a valuable marker for assessing the overall levels of procoagulant MVs. In the study, patients exhibited higher levels of procoagulant MVs compared to the control group (p < 0.001) (Fig. 3).
MVs procoagulant activity
The procoagulant activity assay results are shown in Fig. 4. In this study, the procoagulant activity of MVs was significantly higher in patients than in the control group (p < 0.001) (Fig. 4).
Serum level of IL-6 assay
The mean concentration of IL-6 levels in the serum patients with COVID-19 and control groups was 581.0 ± 312.5, and 486.6 ± 307.8 pg/mL, respectively. In this study, the serum level of IL-6 was significantly higher in patients than in the control group (p < 0.001).
Correlation studies
Pearson correlation analysis was carried out to determine the relationship between the concentrations of MVs with other studied parameters in COVID-19 patients (Table 3). Correlation studies revealed a significant positive correlation between MVs and Annexin-V positive MVs (r = 0.52, p = 0.001), procoagulant activity of MVs (r = 0.55, p = 0.000), IL-6 (r = 0.49, p = 0.001), D-Dimer level (r = 0.61, p = 0.001), and neutrophil count (r = 0.24, p = 0.038). On the other hand, no significant correlation was observed between MVs levels and platelet count (r = -0.09, p = 0.112), and lymphocyte count (r = -0.31, p = 0.051) (Table 3).
Discussion
Quantitation of new blood biomarkers is crucial for the understanding of diseases, which can lead to exploration into new therapeutic and prognostic applications [16]. The evaluation of procoagulant circulating MVs in patients with COVID-19 is an important area of research as it can provide valuable insights into the pathophysiology of the disease and potentially serve as a new biomarker for disease progression and severity [9]. Many studies have shown that the COVID-19 disease is associated with abnormalities in cytokine markers and procoagulant MVs. In this study, we aimed to assess the procoagulant MVs and IL-6 levels in patients with COVID-19 to improve our comprehension of the potential role of circulating MVs and IL-6 in treatment approaches and disease prognosis.
Our findings revealed significant differences between the patient and control groups in hematological parameters. It showed a higher count of leukocytes, neutrophils, and platelets, as well as higher hemoglobin levels in patients compared to the control group. On the other hand, the lymphocyte counts in patients showed a significant decrease. These findings are consistent with previous reports in COVID-19 patients [6, 7].
Decreased lymphocyte counts are common in patients with COVID-19 [17, 18]. A meta-analysis study showed a significant decrease in lymphocyte counts in severe patients with COVID-19 [18]. This change in lymphocyte counts is associated with oxygen demand, and also coagulopathy in patients with COVID-19 [19]. In the study by Yang et al., [20] which examined the association between thrombocytopenia and COVID-19, a significant decrease in platelet count was observed in patients, similar to our study. Maybe, the main reason for the patient’s thrombocytopenia was DIC.
In line with our findings, it has been reported that many patients displayed varying degrees of reduction in RBC count and hemoglobin levels, with more pronounced effects observed in severe and critically ill patients [21].
The coagulation panel also showed a significant increase in PT, PTT, and fibrinogen levels in the patient group. Several studies have indicated that PT and PTT levels in COVID-19 patients are elevated compared to those in healthy individuals [22, 23]. However, this observation may be influenced by potential confounders, as the use of low molecular weight heparin (LMWH) and unfractionated heparin (UFH) is common in COVID-19 treatment and could affect PTT results. PTT-dependent coagulation factors, particularly 8, 9, 11, and 12, are reduced by heparin [23].
The most commonly used test associated with coagulopathy is plasma D-Dimer, and the present study indicated a significant increase in the patient’s D-Dimer level. Some studies have indicated that D-Dimer levels at admission can predict the prognosis of COVID-19 [24]. Another study demonstrated that D-Dimer levels were elevated in critically ill patients, leading to an increased risk of thrombotic events and mortality [25].
A key feature of viral infections is the development of an inflammatory environment around infected cells, and the release of MPs can be prompted by viral pathogens and is closely linked to cell activation, as well as the onset of apoptosis and necrosis in these infected cells [9]. Our flow cytometry results demonstrated that the level of total MVs, LMVs, RMVs, and PMVs increased significantly in the patient group compared to the healthy subjects. Consistent with our findings, Zahran et al., [11] demonstrated a high level of MVs in COVID-19 patients, suggesting these MVs as a promising prognostic factor for COVID-19 patients [9].
MVs are released by various cell types and possess proinflammatory and procoagulant properties [14, 15]. In situations of hemostasis imbalance, like in a COVID-19 infection, MVs are likely to be involved, even in the initial stages of sepsis, as a mechanism to counteract the body’s systemic inflammation. It is noteworthy that MVs can also induce detrimental changes in the activity of enzymes associated with inflammation and oxidative stress, a scenario that may occur at different stages of a COVID-19 infection [5].
To measure general levels of procoagulant MVs, Annexin-V, which binds to PS, is a useful marker. In our study, flow cytometry analysis revealed that around 61.1% of total MVs expressed PS on their surface, and this MV subpopulation was higher in patients compared to healthy volunteers, which is similar to previously published findings related to COVID-19 [26]. In the context of COVID-19, the virus causes direct harm to cells and triggers the release of MVs that are positive for PS (PS + MVs). Additionally, it indirectly activates the immune system, including monocytes, neutrophils, and lymphocytes, leading to a cytokine storm and extensive cell damage and death. It is crucial to note that the destruction of vascular endothelial cells by the COVID-19 virus and the resulting immune inflammation plays a central role in the development of clot formation [6]. The MVs, are believed to have a significant role in coagulation due to their PS content. PS acts as a binding site for attaching tenase and prothrombinase complexes that support robust amounts of thrombin generation [7]. Moreover, consistent with the studies by Balbi et al. [27] and Zahran et al., [9], the current study demonstrated a significant increase in the procoagulant activity of MVs. The presence of MVs and their PS content is thought to play a significant role in the formation of blood clots in individuals with COVID-19, leading to the formation of vascular thrombosis [10].
The current study found a significantly increased serum level of IL-6 in the COVID-19 patient group compared to the control. During episodes of acute inflammation, stimulated macrophages and neutrophils produce IL-6 [11]. IL-6 supports the accumulation of inflammatory cells by preventing T cells from undergoing cell death, facilitating the development of T cells into active effector cells, and causing blood vessels to become more permeable [28]. Moreover, the release of MVs resulting from endothelial cell damage caused by viral infection can trigger an elevation in pro-inflammatory cytokines such as IL-6. Consequently, elevated MV levels can play a role in the advancement of a disease. It has been shown recently that the presence of MVs can induce the production of inflammatory cytokines such as IL-6 and IL-8, exacerbating the symptoms of COVID-19 [29].
Conclusion
The current study’s findings revealed significantly elevated levels of MVs, Annexin-V positive MVs, and IL-6 in patients with COVID-19; these heightened levels are linked to increased procoagulant activity of MVs in COVID-19 patients, thereby raising the risk of hypercoagulability in COVID-19 and subsequently increasing the tendency towards prothrombotic events. Further research is needed to explain the exact mechanism of MVs and IL-6 in COVID-19, which could potentially lead to the development of new therapeutic and prognostic markers in predicting inflammatory response and thrombotic events.
Data availability
The datasets that were analyzed during the current study are available through the corresponding author upon reasonable request.
Abbreviations
- CBC:
-
Complete blood count
- EDTA:
-
Ethylene diamine tetra acetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FSC:
-
Forward scatter
- G-CSF:
-
Granulocyte colony stimulating factor
- IFN-γ:
-
Interferonγ
- IL-6:
-
Interleukin-6
- LMVs:
-
Leukocyte-derived Microvesicles
- MCP1:
-
Monocyte chemical protein 1
- MIP-a:
-
Macrophage inflammatory protein 1 alpha
- MPs:
-
Microparticles
- MVs:
-
Microvesicles
- PBS:
-
Phosphate-buffered saline
- PMVs:
-
Platelet-derived Microvesicles
- PS:
-
Phosphatidyl serine
- PT:
-
Prothrombin time
- PTT:
-
Partial thromboplastin time
- RMVs:
-
Red blood cell-derived Microvesicles
- SSC:
-
Side scatter
- TNF-α:
-
Tumor necrosis factor alpha
- WBC:
-
White blood cell
References
Jonigk D, Werlein C, Acker T, et al. Organ manifestations of COVID-19: what have we learned so far (not only) from autopsies? Virchows Arch. 2022;481:139–59.
Wadowski PP, Panzer B, Józkowicz A, Kopp CW, Gremmel T, Panzer S, Koppensteiner R. Microvascular thrombosis as a critical factor in severe COVID-19. Int J Mol Sci. 2023;24(3):2492.
Gonzalez-Gonzalez FJ, Ziccardi MR, McCauley MD. Virchow’s triad and the role of thrombosis in COVID-related stroke. Front Physiol. 2021;12:769254.
Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–29.
Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, et al. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct Target Therapy. 2020;5(1):128.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–2.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Garnier Y, Claude L, Hermand P, Sachou E, Claes A, Desplan K, et al. Plasma microvesicles of intubated COVID-19 patients cause endothelial cell death, neutrophil adhesion and netosis, in a phosphatidylserine‐dependent manner. Br J Haematol. 2022;196(5):1159–69.
Zahran AM, El-Badawy O, Ali WA, Mahran ZG, Mahran EEM, Rayan A. Circulating microvesicles and activated platelets as novel prognostic biomarkers in COVID-19; relation to cancer. PLoS ONE. 2021;16(2):e0246806.
Ardoin S, Shanahan J, Pisetsky D. The role of microvesicles in inflammation and thrombosis. Scand J Immunol. 2007;66(2–3):159–65.
Che Mohd Nassir CMN, Hashim S, Wong KK, Abdul Halim S, Idris NS, Jayabalan N, et al. COVID-19 infection and circulating microvesicles—reviewing evidence as microthrombogenic risk factor for cerebral small vessel disease. Mol Neurobiol. 2021;58(8):4188–215.
Sun H, Du Y, Kumar R, Buchkovich N, He P. Increased circulating microparticles contribute to severe infection and adverse outcomes of COVID-19 in patients with diabetes. Am J Physiol Heart Circ Physiol. 2022;323(6):H1176–93.
Morel O, Marchandot B, Jesel L, Sattler L, Trimaille A, Curtiaud A, et al. Microparticles in COVID-19 as a link between lung injury extension and thrombosis. ERJ Open Res. 2021;7(2):00954–2020.
Hashemi Tayer A, Amirizadeh N, Ahmadinejad M, Nikougoftar M, Deyhim MR, Zolfaghari S. Procoagulant activity of red blood cell-derived microvesicles during red cell storage. Transfus Med Hemother. 2019;46(4):224–30.
Hashemi Tayer A, Ranjbaran R, Kamravan M, Abbasi M, Zareian R. Association of circulating procoagulant microvesicles with painful vaso-occlusive crisis in sickle cell disease. Transfus Med Hemother. 2023;50(5):448–55.
Battaglini D, Lopes-Pacheco M, Castro-Faria-Neto HC, Pelosi P, Rocco PRM. Laboratory biomarkers for diagnosis and prognosis in COVID-19. Front Immunol. 2022;13:857573.
Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95(7):834–47.
Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive care. 2020;8:1–10.
Mao J, Dai R, Du R-C, Zhu Y, Shui L-P, Luo X-H. Hematologic changes predict clinical outcome in recovered patients with COVID-19. Ann Hematol. 2021;100:675–89.
Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–72.
Yuan X, Huang W, Ye B, Chen C, Huang R, Wu F, et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol. 2020;112:553–9.
Jin X, Duan Y, Bao T, Gu J, Chen Y, Li Y, et al. The values of coagulation function in COVID-19 patients. PLoS ONE. 2020;15(10):e0241329.
Araya S, Mamo MA, Tsegay YG, Atlaw A, Aytenew A, Hordofa A, et al. Blood coagulation parameter abnormalities in hospitalized patients with confirmed COVID-19 in Ethiopia. PLoS ONE. 2021;16(6):e0252939.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Chocron R, Duceau B, Gendron N, Ezzouhairi N, Khider L, Trimaille A, et al. D-Dimer at hospital admission for COVID-19 are associated with in-hospital mortality, independent of venous thromboembolism: insights from a French multicenter cohort study. Arch Cardiovasc Dis. 2021;114(5):381–93.
Hamali HA, Saboor M, Dobie G, Madkhali AM, Akhter MS, Hakamy A, Al-Mekhlafi HM, Jackson DE, Matari YH, Mobarki AA. Procoagulant microvesicles in COVID-19 patients: possible modulators of inflammation and prothrombotic tendency. Infect Drug Resist. 2022;15:2359–68.
Balbi C, Burrello J, Bolis S, Lazzarini E, Biemmi V, Pianezzi E et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine. 2021;67.
Burnouf T, Chou M-L, Goubran H, Cognasse F, Garraud O, Seghatchian J. An overview of the role of microvesicles/microvesicles in blood components: are they clinically beneficial or harmful? Transfus Apheres Sci. 2015;53(2):137–45.
Guo D-Z, Lv Y, Pan S. Increased circulating microvesicles and inflammatory factors aggravate coronavirus disease 2019 (COVID-19). 202.
Acknowledgements
This study has been supported by the Jahrom University of Medical Sciences. We would particularly like to thank all volunteers who participated in this study.
Funding
The research was funded by Jahrom University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. AHT and MK contributed to all parts of the study. AHT, FF, and HKJ contributed to the study implementation. AHT, MHK, and TA collaborated in the analysis and interpretation of data. TA and AHT collaborated on the manuscript writing and revision. All the authors commented on the drafts of the manuscript and approved the final version of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study complied with the guidelines for human studies. The procedures of study were approved by Ethical Committee of Jahrom University of Medical Sciences (IR.JUMS.REC.1399.049). Written informed consent was obtained from patients and healthy volunteers (or their parent or legal guardian in the case of children under 17) prior to participation in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tayer, A.H., Jahromi, H.K., Kamravan, M. et al. Evaluation of circulating microvesicles and their procoagulant activity in patients with COVID-19. BMC Res Notes 17, 233 (2024). https://doi.org/10.1186/s13104-024-06875-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-024-06875-9