Abstract
Objective
Biliary atresia (BA) is a progressive fibro-obliterative disease of the biliary tract, which results in end-stage liver disease. However, liver fibrosis progression may continue even after Kasai surgery. Recent evidence showed that collagen plays a pivotal role in the progression of liver fibrosis in BA. However, most studies were conducted in developed countries. We investigated the expressions of the collagen gene cluster (COL6A1, COL6A2, COL6A3, and COL1A1) in BA patients in Indonesia.
Results
There was a significant down-regulated expression of COL6A1 (ΔCT 9.06 ± 2.64 vs. 5.42 ± 2.41; p = 0.0009), COL6A2 (ΔCT 8.25 ± 2.07 vs. 5.77 ± 3.51; p = 0.02), COL6A3 (ΔCT 11.2 ± 6.08 vs. 6.78 ± 3.51; p = 0.024), and COL1A1 (ΔCT 3.26 ± 1.71 vs. 0.19 ± 2.76; p = 0.0015) in BA patients compared to controls. Interestingly, the collagen gene cluster expressions were significantly associated with the presence of cirrhosis (p = 0.0085, 0.04, and 0.0283 for COL6A1, COL6A2, and COL6A3, respectively). In conclusion, our study shows the changes in the collagen gene cluster, particularly collagen type I and VI, expressions in patients with BA in a particular developing country. Our findings suggest the role of these collagen gene clusters in the liver fibrogenesis of BA.
Similar content being viewed by others
Introduction
Biliary atresia (BA) is the most common cause of cholestasis in infants under three months. It is characterized by microinflammation and fibrosis of intra- and extrahepatic bile ducts [1]. If therapy is not given, children suffering from BA will have progressive liver fibrosis and cirrhosis and usually will not survive more than two years of age [2].
Liver fibrosis in BA might manifest rapidly after Kasai surgery. Understanding liver fibrosis in BA might benefit patient prognosis, development of biomarkers for diagnosis, and targeted therapy of liver fibrosis in BA patients [3]. A recent study showed that the expression of the collagen gene cluster was strongly associated with liver fibrosis, including BA patients [4,5,6,7]. However, most studies were conducted in developed countries [5,6,7]. Therefore, our study investigated the role of COL6A1, COL6A2, COL6A3, and COL1A1 expressions on liver fibrosis in BA patients in Indonesia.
Material and methods
Patients
Twenty liver tissues of BA patients were acquired during Kasai surgery. The type of BA was determined using the Kasai classification system [8]. Cirrhosis was determined as bridging fibrosis with > 50% of portal tracts encompassed and nodular architecture [9]. At the same time, 18 control liver specimens were obtained from patients who underwent surgeries or biopsies for other diseases, including intrahepatic cholestasis (n = 7), choledochal cyst (5), internal bleeding (1), intraabdominal tumor (n = 2), gastric volvulus (n = 1), liver abscess (1), and Alagille syndrome (n = 1).
Total RNA isolation
Total RNA was isolated from 25 to 30 mg of liver tissue using the Quick-RNA Miniprep Kit (Zymo Research, Irvine, California, US). The RNA concentration was quantified by a NanoDrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) with the OD260/280 ratios ranging from 1.8 to 2.0 to ensure RNA purity. The samples were immediately stored at − 80 °C for future use.
Quantitative RT-PCR
One-step qPCR was performed using SensiFAST SYBR No-ROX Kit (Bioline, Tennessee, USA) and BioRad CFX Real-Time PCR System (California, USA) for collagen gene cluster (COL6A1, COL6A2, COL6A3, and COL1A1). A housekeeping gene, GAPDH, was used as a reference gene. The reaction mix contained 10 μL sensiFAST one-step mix, 0.8 μL of each primer, 0.2 μL Reverse Transcriptase, 0.4 μL RNase Inhibitor, RNAse free water was added to reach 16 μL. Lastly, the mRNA was added, resulting in the final volume of 20 μL. PCR cycling conditions were: 45 °C for 10 min followed by 1 cycle of 95 °C for 2 min and 40 cycles of 95 °C for 5 s (denaturation), 60 °C for 1 min (Annealing/Extension). The primers that were used for collagen gene cluster were as follows: COL6A1 5′—TAAAGGCTACCGAGGCGATG—3′ (forward) and 5′—GCCGTCTTCTCCCCTTTCAC—3′ (reverse); COL6A2: 5′—CTCCTCGGGACCAGGACTTC—3′ (forward) and 5′—CGGTCTTCTCTGGGCAGTTG—3′ (reverse); COL6A3: 5′—TTAGCCAGCACTCGCTATCC—3′ (forward) and 5′—TTACTGGGGCCGATGTTGAG—3′ (reverse); COL1A1: 5′—CAATGCTGCCCTTTCTGCTCCTTT—3′ (forward) and 5′—ATTGCCTTTGATTGCTGGGCAGAC—3′ (reverse) [4]; and GAPDH: 5′—GCACCGTCAAGGCTGAGAAC—3′ (forward) and 5′—TGGTGAAGACGCCAGTGGA—3′ (reverse) [10].
Statistical analysis
The expression of COL6A1, COL6A2, COL6A3, and COL1A1 were determined using the Livak method (2−ΔΔCT). The expression of COL6A1, COL6A2, COL6A3, and COL1A1 was presented as mean ± standard deviation (SD). The normality of the continuous variables was defined by the Kolmogorov–Smirnov test. The differences in the expressions of collagen gene clusters between BA patients and controls were analyzed using an independent t-test with a significance value of p < 0.05. All statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) version 21 (Chicago, USA).
Results
Baseline characteristics
We ascertained 20 BA patients and 18 controls. Most BA patients were female (60%) and type 3 (60%). The median age of patients who underwent the Kasai procedure was 124 (IQR, 95.5–174.5) days (Table 1).
Expression of the collagen gene cluster in BA patients
There was a significant down-regulated expression of COL6A1 (p = 0.0009), COL6A2 (p = 0.02), COL6A3 (p = 0.024), and COL1A1 (p = 0.0015) in liver BA patients compared to controls with the fold change of 12.53-, 5.58-, 21.35-, and 8.41-times, respectively (Additional file 1: Fig. S1; Table 2).
Association between collagen gene cluster expression and outcomes
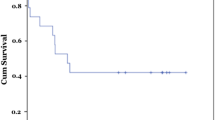
Interestingly, the collagen gene cluster expressions were significantly associated with the presence of cirrhosis (p = 0.0085, 0.04, and 0.0283 for COL6A1, COL6A2, and COL6A3, respectively) but not with the survival of BA patients and age at Kasai procedure (Table 3).
Discussion
Our study shows the aberrant expressions of collagen type VI genes (COL6A1, COL6A2, and COL6A3) in BA patients compared with controls. A previous study showed that the collagen gene cluster (COL6A1, COL6A2, COL6A3, and COL1A1) expressions were associated with the hepatic stellate cells (HSCs) activation [4]. Collagen VI (COL6A1, COL6A2, and COL6A3) genes, but not COL1A1, have been associated with liver fibrosis [11,12,13]. The accumulation of type VI collagen might cause the destruction of the liver's structure and function in liver fibrosis [11]. It is compatible with our findings that COL6A1, COL6A2, and COL6A3 expressions, not COL1A1, were strongly associated with the presence of cirrhosis (Table 3). Moreover, collagen type VI has been shown as a potent activating factor for HSCs and an inducing factor for fibrosis-associated gene expression changes, including αSMA and TGF-β [12].
In addition, CO6-MMP, a collagen type VI fragment, was elevated in bile-duct ligation (BDL) and the carbon tetrachloride (CCl4) induced fibrosis rat models. It suggests its role in liver fibrogenesis [4, 11]. However, previous studies investigated the association of collagen type VI genes with liver fibrosis in other diseases, including cholestatic liver diseases and acute liver injury, not BA, as the novelty of our study [4, 11,12,13]. In addition, previous studies showed the aberrant other collagen gene expressions (COL10A1, COL15A1, COL22A1, COL9A2 [5] and COL3A1, COL1A2 [7]), not COL6A1, COL6A2, COL6A3, and COL1A1 genes as our study, in patients with BA. These differences are another novelty of our findings.
Our study also shows the aberrant expressions of COL1A1 in BA patients compared to the control. A recent study demonstrated the involvement of COL1A1 in liver fibrosis through miRNA-29 signaling [14]. Moreover, another study showed a constantly high expression of 12 genes, including the collagen gene cluster, in BA patients one year after a successful Kasai surgery compared to at the time of the Kasai procedure [5]. This suggests that the fibrosis process evolved with time instead of as a one-time process. The genes involved may not be a consequence but a precursor of the fibrotic changes [5]. Moreover, Baiocchini et al. [15] also identified COL1A1 as one of the molecules associated with liver fibrosis in HCV-infected patients.
Interestingly, our findings show downregulated collagen gene cluster expressions in cirrhosis patients compared with non-cirrhosis patients (Table 3). However, a previous study revealed a significantly higher expression of the collagen gene cluster in the fibrosis group than in the inflammation group [16]. Our previous study showed that the expression of COL1A1 and COL6A1 were significantly downregulated in the hypospadias patients with more severe chordee than the milder one (Indonesian) [17]. In contrast, there is no difference in collagen intensity between hypospadias patients and controls (Caucasian) [18]. Moreover, some collagen genes have different molecular evolutionary characteristics among different populations [19].
Conclusions
Our study shows the changes in the collagen gene cluster, particularly collagen type I and VI genes, expressions in patients with BA in a particular developing country. Our findings suggest the role of this collagen gene cluster in the liver fibrogenesis of BA.
Limitations
Our study has several limitations, including a small sample size and one pediatric surgical center that might not reflect the Indonesian population. Moreover, we do not validate the protein levels of the collagen gene cluster in BA, i.e., lack of pathological images and collagen deposition staining of the liver tissues in patients with BA due to limited resources. Notably, the control liver specimens were patients with chronic liver and gallbladder diseases, not subjects with normal liver tissue. These facts should be considered during the interpretations of our findings. In this study, we focus on the effect of the collagen gene cluster expressions in liver fibrogenesis of BA, while the association between the prognostic factors, including histopathological findings, with the survival rate of BA patients after Kasai procedures have been reported in our previous studies [9, 20].
Availability of data and materials
All data generated or analyzed during this study are included in the submission. The raw data are available from the corresponding author upon reasonable request.
Abbreviations
- BA:
-
Biliary atresia
- IQR:
-
Interquartile range
References
Lakshminarayanan B, Davenport M. Biliary atresia: comprehensive review. J Autoimmun. 2016;73:1–9.
Hartley J, Harnden A, Kelly D. Biliary atresia. BMJ. 2010;340: c2383.
Haafiz AB. Liver fibrosis in biliary atresia. Expert Rev Gastroenterol Hepatol. 2010;4:335–43.
Chen W, Zhao W, Yang A, et al. Integrated analysis of microRNA and gene expression profiles reveals a functional regulatory module associated with liver fibrosis. Gene. 2017;636:87–95.
Kyrönlahti A, Godbole N, Akinrinade O, et al. Evolving Up-regulation of biliary fibrosis-related extracellular matrix molecules after successful portoenterostomy. Hepatol Commun. 2021;5:1036–50.
Jaramillo C, Guthery SL, Lowichik A, et al. Quantitative liver fibrosis using collagen hybridizing peptide to predict native liver survival in biliary atresia: a pilot study. J Pediatr Gastroenterol Nutr. 2020;70:87–92.
Li H, Cao L, Li H. COL3A1, CXCL8, VCAN, THBS2, and COL1A2 are correlated with the onset of biliary atresia. Medicine. 2023;102: e33299.
Wildhaber BE. Biliary atresia: 50 years after the first kasai. ISRN Surg. 2012;2012: 132089.
Gunadi SDN, Budiarti LR, et al. Histopathological findings for prediction of liver cirrhosis and survival in biliary atresia patients after Kasai procedure. Diagn Pathol. 2020;15:79.
Makhmudi A, Supanji R, Putra BP, et al. The effect of APTR, Fn14 and CD133 expressions on liver fibrosis in biliary atresia patients. Pediatr Surg Int. 2020;36:75–9.
Veidal SS, Karsdal MA, Vassiliadis E, et al. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis–identification and validation of a novel biochemical marker assay. PLoS ONE. 2011;6: e24753.
Freise C, Lee H, Chronowski C, et al. Alpha-single chains of collagen type VI inhibit the fibrogenic effects of triple helical collagen VI in hepatic stellate cells. PLoS ONE. 2021;16: e0254557.
Williams L, Layton T, Yang N, et al. Collagen VI as a driver and disease biomarker in human fibrosis. FEBS J. 2022;289:3603–29.
Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–18.
Baiocchini A, Montaldo C, Conigliaro A, et al. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS ONE. 2016;11: e0151736.
Moyer K, Kaimal V, Pacheco C, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med. 2010;2:33.
Yuri P, Gunadi LRP, et al. The impact of COL1A1 and COL6A1 expression on hypospadias and penile curvature severity. BMC Urol. 2020;20:189.
Erol A, Baskin LS, Li YW, et al. Anatomical studies of the urethral plate: why preservation of the urethral plate is important in hypospadias repair. BJU Int. 2000;85:728–34.
Chan TF, Poon A, Basu A, et al. Natural variation in four human collagen genes across an ethnically diverse population. Genomics. 2008;91:307–14.
Gunadi GTA, Widiyanto G, et al. Liver transplant score for prediction of biliary atresia patients’ survival following Kasai procedure. BMC Res Notes. 2018;11:381.
Acknowledgements
We thank the staff and nursing team involved in the patient's care.
Funding
This study received funding from our institution.
Author information
Authors and Affiliations
Contributions
G and AM conceived the study. DAP, KAV, FDTU, EMD, and FVH performed the RNA extraction and qPCR. G and KI analyzed the data. DAP, KAV, FDTU, EMD, and FVH drafted the manuscript, and G and KI critically revised the manuscript for important intellectual content. G and AM facilitated all project-related tasks. All authors have read and approved the manuscript and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital (KE/FK/0439/EC/2021). Written informed consent was signed by the parents or legal guardians.of BA patients and control before joining the study. The research has been performed following the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Box-plot graph of ΔCT value of the collagen gene cluster expressions in liver BA patients and controls. Box-plot graph of ΔCT value reveals the mean values as lines across the box.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gunadi, Puspitarani, D.A., Vujira, K.A. et al. Collagen gene cluster expression and liver fibrogenesis in patients with biliary atresia: a preliminary study. BMC Res Notes 16, 356 (2023). https://doi.org/10.1186/s13104-023-06636-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06636-0