Abstract
Objective
This work aimed to examine the leishmanicidal, cellular mechanisms and cytotoxicity effects of formononetin (FMN), a natural isoflavone, against Leishmania tropica. We used the MTT assay to determine the leishmanicidal effects of FMN against promastigotes and its cytotoxicity effects on J774-A1 macrophage cells. The Griess reaction assay and quantitative real-time PCR were used to determine the nitric oxide (NO) and the mRNA expression levels of IFN-γ and iNOS in infected J774-A1 macrophage cells.
Results
FMN significantly (P < 0.001) decreased the viability and number of promastigotes and amastigotes forms. The 50% inhibitory concentrations value for FMN and glucantime was 9.3 and 14.3 µM for promastigote and amastigote, respectively. We found that the macrophages exposed with FMN especially at concentrations of 1/2 IC50 and IC50 significantly activated the NO release and the mRNA expression levels of IFN-γ, iNOS. The findings of the current research showed the favorable antileishmanial effects formononetin, a natural isoflavone, against various stages of L. tropica through inhibition of infectivity rate of macrophage cells and triggering the NO production and cellular immunity. However, supplementary works are essential to evaluate the ability and safety of FMN in animal model before use in the clinical phase.
Similar content being viewed by others
Introduction
Leishmaniasis is a parasitic infection which found in various parts of the worlds as a neglected tropical disease (NTD). The disease is caused by Leishmania parasites, which are transmitted by the bite of phlebotomine sand flies [1]. Leishmaniasis can be clinically separated into four categories: cutaneous, muco-cutaneous, diffuse and kala-azar [2]. The cutaneous form as more frequent form is found in some countries such as Iran, Saudi Arabia, Syria, Iraq [3]. Because, there is no effective vaccine to prevent this disease, treatment of patients especially using synthetic drugs, is one of the most important ways to break the chain of transmission and prevent the disease [4, 5]. The most synthetic drugs available are the use of pentavalent antimony compounds [5]. Glucantime (meglumine antimoniate, MA) is the most common drug; whereas the use of this drug is linked with common complications such as anorexia, fever, chills, and joint pain usually in patients with liver and kidney problems [6]. In addition, the high cost and drug resistance to this drug results in the efforts for discovering the alternative therapies [7]. Todays, it has been proven that natural products are a main rich source of anti-infective agents [8]. Flavonoids as the main polyphenolic compounds are widely present in the herbs with a wide range of pharmacological and therapeutic properties [9]. Isoflavones with the chemical formula (C20H18O6) are a subset of flavonoid compounds with various beneficial effects on human health and in particular prevention of cancer and cardiovascular disease and reduction of the symptoms of menopause [10]. Formononetin (7-Hydroxy-4’-methoxy-isoflavone, C16H12O4) is a natural isoflavone found in low concentrations in many foods belonging to the Fabaceae family [11]. Formonontin (FMN) exhibited some properties in modern medicine, e.g., antimicrobial, antioxidant, anti-hyperlipidemic, anti-hyperlipidemic, anti-diabetic, anti-tumor, neuroprotective, and cardioprotective activity [12, 13]. With respect to various biological effects of FMN, this work intended to examine the in vitro anti-leishmanial effects and cytotoxicity effects of FMN on Leishmania tropica.
Materials and methods
Chemicals
Formononetin (purity > 99%), Griess reagent, Fetal Bovine Serum (FBS), 1640 RPMI medium, Eosin powder were prepared from Sigma-Aldrich, Germany. Giemsa powder and dimethyl sulfoxide (DMSO) were also prepared from Merck, Germany.
Cell and parasite
L. tropica (MHOM/AF/88/KK27) and macrophage cells (J774-A1 cell lines, Pasteur Institute, Iran) were cultured in 1640 RPMI medium with FBS (10%), penicillin/streptomycin (100 mL/IU) at 24 ± 1 and 37 °C, respectively.
Effect of FMN on promastigotes
Initially, promastigotes (1 × 106/cells) were treated with FMN (6.25–100 µg/mL) at 24 ° C for 48 h. After discarding the supernatant of the mixture in tested wells, 20 µL of MTT solution (0.5 mg/mL) was added and wells kept again 5% CO2 at 37 °C for 4 h. followed by adding DMSO (50 µL) the absorbance of solution was read at 570 nm by an ELISA plate reader [14]. Amphotericin B (AmB) and non-treated parasites were considered as the positive and control groups. The half maximal inhibitory concentration (IC50) values were calculated by Probit test in SPSS software ver. 26.0.
Effect of FMN on amastigotes
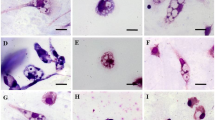
One hundred µL of promastigotes (1 × 106/mL) in stationary phase (10:1) were exposed with macrophage cells (1 × 105/mL) at 37 °C in 5% CO2 for 24 h. Then, macrophages were exposed with FMN (6.25–200 µM) and MA for 48 h. After preparing the slides and staining by Giemsa dye, the number of amastigotes were recorded [15].
Cytotoxic effects of FMN on macrophages
Cytotoxic effects of FMN on normal macrophage cells was performed similar to the effect of FMN on promastigotes based on the MTT assay. The selectivity index (SI) was recorded through the CC50 /IC50 for amastigotes [16].
Effect of FMN on the infectivity rate
Briefly, L. tropia promastigotes (1 × 106/mL) were pre-treated with FMN at ¼ (2.35 µM), 1/3 (3.1 µM), and ½ IC50 (4.65 µM) for 120 min at 21 °C. Then, parasites were exposed to macrophages cells for one day. After preparing the slides and staining by Giemsa dye, the number of infected macrophages were recorded [16].
Effect on nitric oxide (NO) generation
The macrophage cells (1 × 105/mL) were incubated with FMN at ¼ (3.5 µM), 1/3 (4.7 µM), and ½ IC50 (7.15 µM) for two days, then, the collected supernatants (100 µL) along with 50 µL of the Griess reagent A and B were added to the tested wells. The absorbance of microplates was recorded by an ELISA reader at 540 nm [17]. lipopolysaccharide (10 ng/ml) + IFN-γ (10 U/ml) were reflected as the positive control.
Genes expression of (iNOS and IFNγ) in infected macrophages
The mRNA expression levels of IFN-γ and iNOS in infected J774-A1 macrophage cells treated with FMN at ¼ (3.5 µM), 1/3 (4.7 µM), ½ IC50 (7.15 µM) and IC50 (14.3 µM) were examined by quantitative real-time PCR. At first total RNA was extracted based on the instructions of RNeasy tissue kit (Qiagen, Germany). Next, the complementary DNA (cDNA) was obtained through random primers according to the instructions of the commercial kit (Qiagen, Germany). Lastly, the obtained cDNA was considered for real-time PCR through SYBR green. The thermal cycle for this experiment was 92 °C for 6 min, 42 cycles of 92 °C for 10 s and 55 °C for 30 s, respectively. The iQTM5 optical system software (Bio-Rad, Hercules, CA) was applied for the ΔCt− 2 and the results were standardized by the value found from the β-actin mRNA. The oligonucleotide primers for real-time PCR was prepared based on the study conducted by Gharavi et al. [18].
Statistical analysis
To increase the validity of the results all tests were repeated in triplicate. The findings were analyzed by SPSS software (version 26.0). One-way analysis of variance (ANOVA) was also performed to data analysis. P < 0.05 revealed the significance level. All examinations were carried out for three times.
Results
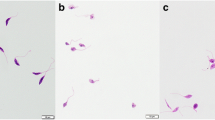
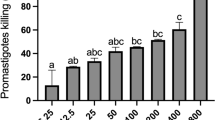
Based on the findings of the MTT assay (Fig. 1A), the FMN significantly (P < 0.001) reduced the viability of L. tropica promastigotes compared with the negative control. Furthermore, the IC50 levels for FMN and AmB were 9.3 µM and 2.31 µM, respectively. According to the results of the macrophage model (Fig. 1B), FMN represented the considerable leishmanicidal activity on amastigote forms as dose-dependent response. FMN markedly inhibited the mean number of intracellular amastigotes into macrophage cells as a dose dependent response in comparison to the non-treated control group (p < 0.001). The IC50 values for the FMN and MA were 14.3 and 26.2 µM, respectively. The cytotoxicity of various concentrations of the FMN and MA on mice macrophage cells was evaluated by MTT assay (Fig. 1C). The CC50 level of the FMN and MA was 159.3 and 874.6 µM, respectively. The findings of microscopic assay displayed (Table 1) that percent of infected macrophages by no pre-treated promastigotes was 81.6 ± 3.7; while, after pre-treatment of promastigotes with the FMN at ¼ IC50, 1/3 IC50, 1/2 IC50 indicated the rate of cell infection was declined by 8.9, 34.5, and 64.9%, respectively (p < 0.05). As displayed in Table 1, the macrophages exposed with FMN activated the NO release, but a significant (p < 0.001) rise was reported at 1/3 IC50 and ½ IC50 compared to the control group. As shown in Fig. 2, the mRNA expression levels of IFN-γ and iNOS in infected J774-A1 macrophage cells treated with AMCE especially at ½ IC50 and IC50 was significantly (p < 0.001) elevated in quantitative real-time PCR.
Discussion
Based on our findings, the FMN significantly (P < 0.001) declined number and viability of promastigotes and amastigotes forms of L. tropica compared with the negative control. In line with our results, previous study showed the effective antimicrobial activity of FMN against some pathogenic bacterial (Staphylococcus aureus, S. aureus, S. epidermides, and Pseudomonas aeruginosa) and fungal (Candida albicans, C. tropicalis, Cryptococcus neoformans) strains with values ranging from 25 to 200 µg/mL [19]. Yang Ying et al. (2008), showed the antibacterial effects of FMN against the gram-positive and the gram-negative bacteria [20]. Wang et al. (2015) indicated the antiviral effects of FMN on enterovirus-51, they fund that FMN fraction can dose-dependently reduce enterovirus-51 RNA and protein synthesis [21]. Lauwaet et al. (2010) revealed that FMN significantly inhibited not only attachment and flagellar motility but also the number of Giardia trophozoites in mice after 1.5 h treatment [22]. Mead and McNair (2006) have showed that among some tested flavonoids and isoflavones compounds (e.g., galangin, galangin, luteolin, sylibinin, quercetin, genistein, apigenin, and narigenin), quercetin, apigenin, and genistein revealed the highest in vitro antiparasitic effects against Cryptosporidium parvum and Encephalitozoon intestinalis with effective concentration (EC50) values ranging from 5.5 to 15 µM [23]. Recently, Faixová et al. (2021) have reported the antiparasitic effects of a number of Isoflavones (genistein, curcumin, silymarin, and quercetin) against some flatworms, e.g., Raillietina spp., Fasciola spp., Schistosoma spp., and Echinococcus spp through affecting the tegumental construction and disturbance in metabolism by interaction with enzymes or signaling molecules [24]. Although, the antimicrobial mechanism of action of isoflavonoids, such as formonontin, has not yet been reported; but, previous studies reported that isoflavones act mainly through the disrupting the cell permeability which subsequently caused the leakage of vital metabolites minerals, and contents, e.g., amino acids, ions, calcium [9,10,11,12, 25]. Kaur et al. (2021) showed the promising in vitro and in vivo effects of Bauhinia variegate, a flavonoid-rich plant, against L. donovani through cell cycle arrest at sub-G0/G1 phase, the improvement of disease-suppressing Th1 cytokines and inhibition of disease-progressing Th2 cytokines with no toxicities [26]. It has been proven that macrophage cells are the key immune cells to control and removing Leishmania parasites. Macrophage cells by the triggering NO synthesis and then release of NO lead to elimination and controlling of the Leishmania parasite [27]. NO is one of the most important effector molecules that rise due to IFN-gamma stimulation or intracellular infection in macrophages along with reactive oxygen species (ROS) production [27, 28]. At the level of cell signaling, SHP-1 phosphatases have been shown to have major biological role in controlling Leishmania in macrophages [29]. On the other hand, inhibition of contamination in macrophages cells is well-known as vital mechanisms targeted by means of drugs for controlling of Leishmania [18]. We found that the macrophages exposed with FMN especially at concentrations of 1/3 IC50 and ½ IC50 significantly activated the NO release. In addition, we reported that the mRNA expression levels of IFN-γ and iNOS in infected J774-A1 macrophage cells treated with AMCE especially at ½ IC50 and IC50 was significantly (p < 0.001) elevated in quantitative real-time PCR Our findings also displayed that pre-treatment of promastigotes with FMN markedly dropped the rate of infectivity by 60.3%. Considering the cytotoxicity effects of FMN, the cytotoxicity of various concentrations of the FMN and MA on mice macrophage cells was evaluated by MTT assay. The CC50 level of the FMN and MA was 159.3 and 874.6 µM, respectively. Consequently, the obtained SI of > 10 for FMN and MA represented their specificity to L. tropica amastigotes with lowest harmfulness on macrophage cells.
Conclusion
The findings of the current research showed the promising in vitro antileishmanial effects formononetin, a natural isoflavone, against promastigote and amastigote forms of L. tropica through inhibition of infectivity rate of macrophage cells and triggering the NO production. However, supplementary works are essential to evaluate the ability and safety of FMN in animal model before use in the clinical phase.
Limitation
The main limitations of this study are the lake of in vivo study and others cellular mechanis of action of FMN.
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- NO:
-
nitric oxide
- ANOVA:
-
One-way analysis of variance (ANOVA)
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl
- MA:
-
meglumine antimoniate
- DMSO:
-
dimethyl sulfoxide
- SI:
-
the selectivity index
- IC50 :
-
The half maximal inhibitory concentration
References
Monzote L. Current treatment of leishmaniasis: a review. Open Antimicrob Agents J. 2009 Aug 31;1(1).
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Research. 2017;6.
Shirzadi MR, Esfahania SB, Mohebalia M, Ershadia MR, Gharachorlo F, Razavia MR, et al. Epidemiological status of leishmaniasis in the Islamic Republic of Iran, 1983–2012. East Mediterr Health J. 2015;21(10):736–42.
Arana B, Rizzo N, Diaz A. Chemotherapy of cutaneous leishmaniasis: A review. Med Microbiol Immunol 2001; 190(1–2): 93 – 5.
Brito NC, Rabello A, Cota GF. Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: a systematic review. PLoS ONE. 2017;12(9):e0184777.
Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, Andrade CA. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta tropica. 2011 May 1;118(2):87–96.
Santos DO, Coutinho CE, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR. Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res. 2008 Jun;103(1):1–0.
Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12(6–7):514–35.
Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005 Nov;26(5):343 – 56. doi: 10.1016/j.ijantimicag.2005.09.002. Erratum in: Int J Antimicrob Agents. 2006 Feb;27(2):181. PMID: 16323269; PMCID: PMC7127073.
Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit reviews food Sci Nutr 2017 Apr 13;57(6):1280–93.
Dutra JM, Espitia PJ, Batista RA. Formononetin: Biological effects and uses–A review. Food Chem. 2021 Oct;15:359:129975.
Machado Dutra J, Espitia PJ, Andrade Batista R. Formononetin: Biological effects and uses–A review.
Tay KC, Tan LT, Chan CK, Hong SL, Chan KG, Yap WH, Pusparajah P, Lee LH, Goh BH. Formononetin: a review of its anticancer potentials and mechanisms. Frontiers in pharmacology. 2019 Jul 26;10:820.
Ezatpour B, Saedi Dezaki E, Mahmoudvand H, Azadpour M, Ezzatkhah F. In Vitro and In Vivo Antileishmanial Effects of Pistacia khinjuk against Leishmania tropica and Leishmania major. Evid Based Complement Alternat Med. 2015; 2015: 149707.
Mahmoudvand H, Ezzatkhah F, Sharififar F, Sharifi I, Dezaki ES. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of Myrtus communis L. The korean journal of parasitology. 2015 Feb;53(1):21.
Mahmoudvand H, Ezzatkhah F, Sharififar F, Sharifi I, Dezaki ES. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of Myrtus communis L. The korean journal of parasitology. 2015 Feb;53(1):21.
Albalawi AE, Abdel-Shafy S, Khudair Khalaf A, Alanazi AD, Baharvand P, Ebrahimi K, Mahmoudvand H. Therapeutic Potential of Green Synthesized Copper Nanoparticles Alone or Combined with Meglumine Antimoniate (Glucantime®) in Cutaneous Leishmaniasis. Nanomaterials (Basel). 2021 Mar 31;11(4):891. doi: https://doi.org/10.3390/nano11040891. PMID: 33807273; PMCID: PMC8065924.
Gharavi M, Nobakht M, Khademvatan Sh, Bandani E, Bakhshayesh M, Roozbehani M. The Effect of Garlic Extract on expression of INFγ and Inos genes in Macrophages infected with Leishmania major. Iran J Parasitol. 2011 Aug;6(3):74–81. PMID: 22347300; PMCID: PMC3279889.
das Neves MV, da Silva TM, Lima Ede O, da Cunha EV, Oliveira Ede J. Isoflavone formononetin from red propolis acts as a fungicide against Candida sp. Braz J Microbiol 2016 Jan-Mar;47(1):159–66. doi: 10.1016/j.bjm.2015.11.009. Epub 2016 Jan 27. PMID: 26887239; PMCID: PMC4822756.
Yang Y, Mao WJ, Li HQ, Zhu TT, Shi L, Lv PC, Zhu HL. Synthesis and biological evaluation of 7-O-modified formononetin derivatives. Res Lett Org Chem 2008 Dec 18;2008.
Wang H, Zhang D, Ge M, Li Z, Jiang J, Li Y. Formononetin inhibits enterovirus 71 replication by regulating COX- 2/PGEâ expression. Virol J. 2015 Mar 1;12:35.
Lauwaet T, Andersen Y, Van de Ven L, Eckmann L, Gillin F. Rapid detachment of Giardia lamblia trophozoites as a mechanism of antimicrobial action of the isoflavone formononetin. J Antimicrob Chemother. 2010;65:531–4.
Mead J, McNair N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol Lett. 2006 Jun;259(1):153-7. doi: https://doi.org/10.1111/j.1574-6968.2006.00263.x. PMID: 16684116.
Faixová D, Hrčková G, Mačák Kubašková T, Mudroňová D. Antiparasitic Effects of Selected Isoflavones on Flatworms. Helminthologia. 2021 Feb 10;58(1):1–16. doi: https://doi.org/10.2478/helm-2021-0004. PMID: 33664614; PMCID: PMC7912234.
Mahmoudvand H, Pakravanan M, Kheirandish F, Jahanbakhsh S, Sepahvand M, Niazi M, Rouientan A, Aflatoonian MR. Efficacy and safety Curcuma zadoaria L. to inactivate the hydatid cyst protoscoleces. Current Clinical Pharmacology. 2020 Apr 1;15(1):64–71.
Kaur G, Chauhan K, Anand N, Kaur S. Evaluation of in vitro and in vivo protective efficacy of Bauhinia variegata against Leishmania donovani in Murine Model. Acta Parasitol. 2021 Sep;66(3):812–26. https://doi.org/10.1007/s11686-020-00326-8. Epub 2021 Feb 2. PMID: 33528770.
Anand N, Lutshumba J, Whitlow M, Abdelaziz MH, Mani R, Suzuki Y. Deficiency in indoleamine-2, 3-dioxygenase induces upregulation of guanylate binding protein 1 and inducible nitric oxide synthase expression in the brain during cerebral infection with Toxoplasma gondii in genetically resistant BALB/c mice but not in genetically susceptible C57BL/6 mice. Microbes Infect. 2022 Apr-May;24(3):104908. doi: 10.1016/j.micinf.2021.104908. Epub 2021 Nov 13. PMID: 34781010; PMCID: PMC9081123.
Anand N, Kanwar RK, Dubey ML, Vahishta RK, Sehgal R, Verma AK, Kanwar JR. Effect of lactoferrin protein on red blood cells and macrophages: mechanism of parasite-host interaction. Drug Des Devel Ther. 2015 Jul 27;9:3821-35. doi: 10.2147/DDDT.S77860. Erratum in: Drug Des Devel Ther. 2020 May 28;14:2123–2124. PMID: 26251568; PMCID: PMC4524381.
Khan TH, Srivastava N, Srivastava A, Sareen A, Mathur RK, Chande AG, Musti KV, Roy S, Mukhopadhyaya R, Saha B. SHP-1 plays a crucial role in CD40 signaling reciprocity. J Immunol. 2014 Oct 1;193(7):3644-53. doi: https://doi.org/10.4049/jimmunol.1400620. Epub 2014 Sep 3. PMID: 25187664.
Acknowledgements
We would like to thank Ms. Massumeh Niazi for critial revision of the final copy of the English manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
J.G.Y. and H.M. designed the study, A.K.K., P.Z.R. and N.K. collected sample and prepared for experiment, P.Z.R, and N.K. conducted experiments, AK.K. and J.G.Y. analysed and interpreted data, A.K.K. and H.M. wrote the main manuscript text. All authors contributed to helpful discussions, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the ethics committee of Lorestan University of Medical Sciences, Khorramabad, Iran, with the ethics number of IR.LUMS.REC.1401.189.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahmoudvand, H., Khalaf, A.K., Rajabi, P.Z. et al. Leishmanicidal and immunomodulatory activities of the formononetin (a natural isoflavone) against Leishmania tropica. BMC Res Notes 16, 120 (2023). https://doi.org/10.1186/s13104-023-06403-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-023-06403-1