Abstract
Background
The sandfly, Phlebotomus duboscqi is a vector of zoonotic cutaneous leishmaniasis (ZCL) that is an important public health problem in Eastern Africa. Repellents have been used for protection of humans against vectors of ZCL and other vectors that transmit killer diseases including malaria, Rift Valley fever, dengue, and yellow fever. The repellent effects of different doses of the essential oils from the lemon grass, Cymbopogon citratus and Mexican marigold, Tagetes minuta were evaluated in a two-chamber bioassay against 3- to 7-day-old unfed females of P. duboscqi in the laboratory. The results were compared with those that were obtained when test animals were treated with an equivalent dose of diethyl-3-methylbenzamide, which is a repellent that is commonly used as a positive control.
Results
Overall, percentage repellency increased with increasing doses of the essential oils while biting rates decreased with increasing concentrations of the oils. Further, the oil of C. citratus was more potent than that of T. minuta with regard to protection time and biting deterrence. The effective doses at 50% (ED50) and at 90% (ED90) for the oil of C. citratus, were 0.04 and 0.79 mg/ml, respectively. Those of the oil of T. minuta were 0.10 and 12.58 mg/ml. In addition, the percentage repellency of 1 mg/ml of the essential oils of C. citratus and T. minuta against sandflies was 100% and 88.89%, respectively. A lower dose of 0.5 mg/ml of the oils, elicited 89.13% repellency for C. citratus and 52.22% for T. minuta.
Conclusion
The laboratory tests showed that the essential oils of the two plants were highly repellent to adult sand flies, P. duboscqi. Thus, the two essential oils are candidate natural repellents that can be used against P. duboscqi due to their high efficacy at very low doses, hence, the envisaged safety in their use over chemical repellents. It remains to carry out clinical studies on human subjects with appropriate formulations of the oils prior to recommending their adoption for use against the sandflies.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Phlebotomine sandflies transmit leishmaniases which is a group of diseases that to date puts at risk of disease 350 million people in 88 countries worldwide [1]. The World Health Organization [2] estimates that over 2.3 million new cases of leishmaniasis occur each year and that at least 12 million people are presently infected worldwide. In some countries, sandflies also carry and transmit other zoonoses such as bartonellosis [3], phleboviruses [4, 5], certain flaviviruses, orbiviruses and vesiculoviruses [6, 7], that cause health problems for humans and domestic animals.
In Kenya, phlebotomine sandflies transmit visceral and cutaneous leishmaniases. Visceral leishmaniasis (VL), caused by Leishmania donovani is transmitted by Phlebotomus martini (Diptera: Psychodidae) [8, 9]. On the other hand, P. duboscqi sandflies transmit L. major, one of the causative agents of zoonotic cutaneous leishmaniasis (ZCL) [5, 10]. The current management strategy for leishmaniasis is mainly based on chemotherapy for treatment of infected cases and use of insecticides in vector control to reduce transmission [11, 12]. However, usage of highly persistent and toxic synthetic insecticides has led to development of resistance in vector populations. In addition, environmental pollution due to the repeated applications is a challenge. Thus, the harmful side effects of these chemicals on both animals and humans have progressively limited their usage and have led to increased interest in alternative new natural chemicals that are environmentally safe, affordable and effective in management of leishmaniases. In this context, screening of natural products has received the attention of researchers around the world. Since many diseases that are transmitted by insects such as malaria, dengue, yellow fever, leishmaniasis and Chaga’s disease are endemic in developing countries, the search for insecticides and repellents of botanical origin has been driven by the need to find new products that are effective, but also safer and more affordable than currently available products [13]. In recent decades, research on the interactions between plants and insects has revealed the potential use of plant metabolites or allelochemicals for this purpose [14]. Some chemical constituents of essential oils from various plants have insecticidal properties [15]. Specific compounds isolated from plant extracts or essential oils have been tested for fumigation purposes [16].
Essential oils of an appreciable number of plants have been shown to be repellent against various haematophagous arthropods [17,18,19]. Lemongrass, Cymbopogon spp. produce the most used natural repellents in the world [20]. For example, essential oils from Cymbopogon martinii elicited 100% repellency against Anopheles sp. mosquitoes in field tests for 12 h [21]. Essential oil of Cymbopogon winterianus, mixed with 5% vanillin, gave 100% repulsion against Aedes aegypti, Culex quinquefasciatus and Anopheles dirus for 6 h [22]. Lemongrass, C. citratus essential oil is obtained from the aerial parts of the plant. The plant has been widely recognized for its enthnobotanical and medicinal usefulness [23]. Other documented effects of essential oils of plants include insecticidal [24,25,26,27,28,29] antifungal [30], antimicrobial [31, 32], and the therapeutic properties [23]. However, there are relatively few studies that have been carried out to determine the efficacy of essential oils from citronella as arthropod repellents [33] and specifically against sandflies.
On the other hand, the essential oil of the Mexican marigold, T. minuta, has been shown to have both larvicidal and adulticidal effects on mosquitoes [34,35,36]. The active components were isolated from different parts of the plant. Green et al. [34], reported mosquito larvicidal activity in the extract of Tagetes minuta flowers. Perich et al. [36] compared biocidal effects of the whole-plant extracts of three Tagetes spp. and showed that T. minuta had the greatest biocidal effect on the larvae and adults of Ae. aegypti (L.) and Anopheles stephensi (L). Bioassays carried out with simultaneous steam distillates of T. minuta flowers showed 90% larval mortality at lethal concentrations (LC90) of 4 and 8 ppm and against the adult at 0.4 and 0.45% against Aedes aegypti and Anopheles stephensi, respectively [36]. Recently, Ireri et al. [37] demonstrated that methanol and ethyl acetate crude extracts of T. minuta derived from the aerial parts had significant mortality against both male and female P. duboscqi, Neveu Lemaire (Diptera: Psychodidae). Further, Mong’are et al. [38] found that similar crude extracts reduced the fecundity of P. duboscqi by 53%.
The present study sought to evaluate the repellent and insecticidal effects of the essential oils of the lemon grass, C. citratus and T. minuta against adult sandflies, P. duboscqi.
Methods
Extraction of essential oils
Extraction of essential oils of T. minuta and lemon grass, C. citratus and gas chromatography–mass spectrometric (GC–MS) analysis were done in the Behavioural and Chemical Ecology Department (BCED) laboratory at the International Centre for Insect Physiology and Ecology (icipe), Kasarani, Nairobi, Kenya. Repellency experiments were conducted at the Centre for Biotechnology Research and Development (CBRD) of the Kenya Medical Research Institute (KEMRI), Nairobi in the Leishmaniasis laboratory. The institute has a viable sandfly colony and the requisite facilities that facilitated the studies to be undertaken.
Collection of plant materials
Fresh leaves of the lemon grass, Cymbopogon citratus were collected from the equatorial rainforest in Kakamega, Kenya. A voucher specimen reference number CBRD/CC/001/2015 was deposited at KEMRI’s Center for Biotechnology Research and Development (CBRD) for future reference. The leaves were screened and dry and/or damaged ones were discarded. The remaining good leaves were used for extraction while still fresh. On the other hand, floral and foliar parts of T. minuta plants were collected from Marigat division of Baringo district, Rift Valley province, Kenya. The plants’ identities were confirmed by a taxonomist at the University of Nairobi. These were packed in a cold box and transported to the International Centre for Insect Physiology and Ecology (icipe), Kasarani, Nairobi, Kenya where extraction of the essential oils was done. A voucher specimen reference number CBRD/TM/001/2015 was deposited at KEMRI’s Center for Biotechnology Research and Development (CBRD) for future reference.
Extraction of essential oils of Tagetes minuta and Cymbopogon citratus
Extraction of the essential oil of the lemon grass c. citratus was done as described by Adeniran and Fabiyi [39]. The fresh leaves were immersed in distilled water after which they were subjected to steam distillation. The mixture of steam and the volatile oil generated was passed through a condenser and collected in a flask. Then, a separating funnel was used to separate the oil from water. The recovered oil was dried using anhydrous sodium sulphate and kept in a refrigerator at 4 °C for subsequent use [39].
For the extraction of the essential oil from T. minuta, fresh plant material was sliced and hydro-distilled by using a Clevenger-type apparatus [40], with slight modifications [41]. Heat was provided by a heating-mantle equipped with a thermostat and the temperature maintained at 90 °C. The plant material was immersed in distilled water then placed into a 2 litre round-bottomed flask and hydro-distilled for 2 h. The distillate was collected as the essential oil band above the water [42].
Sand fly colony
Sandflies were obtained from a colony of P. duboscqi Neveu Lemaire that originated from Marigat Division, Baringo district, Rift Valley, and were maintained at the Centre for Biotechnology Research and Development (CBRD) insectaries in Kenya Medical Research Institute, Nairobi. The colony of P. duboscqi was established using field-captured females that were held in cages and maintained according to the methods of Beach et al. [43] with some modifications. Briefly, female sandflies were fed on blood using Syrian golden hamsters that had been anaesthetized with sodium pentobarbitone (Sagatal®). The hamsters’ underbellies were usually shaven using an electric shaver for easy access for feeding by sandfly. The sandflies were reared at 28 ± 1 °C, and an average RH of 85–95% and 12:12 h (light: dark) photoperiod in Perspex® insect rearing cages. Sandflies were fed ad libitum on slices of apple that were supplied daily as a source of carbohydrates.
Preparation of oil extracts for repellent tests
Test samples of the essential oils of T. minuta and C. citratus were prepared by reconstituting measured amounts of the essential oils in olive oil to have a series of concentrations of 0.125; 0.250; 0.500; 0.750 and 1 mg/ml. Separate experiments using different cages were done in triplicates and hamsters were treated with the above preparations of the essential oils To prevent any cross-over effects between treatments with the different concentrations, each test with a given dose of each oil was applied to one hamster per cage. The olive oil and a standard repellent, N,N-diethyl-3-methylbenzamide (DEET) were used as negative and positive controls, respectively.
Assessing repellent effects of essential oils
Sandflies, Phlebotomus duboscqi were obtained from a colony which was maintained at the CBRD insectary. The basic design of this experiment was a modification of the World Health Organization WHO Pesticide Evaluation Scheme (WHOPES) [44]. Experiments were carried out in the laboratory within tunnels constructed from glass cages with plaster of Paris on their bases. Two such cages, each measuring 25 cm (width) × 25 cm (height) x 40 cm (length), were joined on their open ends with an adhesive tape to form a tunnel measuring 25 × 25 × 80 cm. Before joining the two cages with tapping material, a removable cardboard frame of 1 cm thick that had holes (of 20 mm diameter) drilled through was fitted in between the cages.
The repellency tests were conducted as previously described by Kasili et al. [45], with some modifications. In the shorter section of the tunnel, a restrained hamster, anesthetized with sodium pentobarbitone (Sagatal®), and acting as a bait (host) was placed. Separate experiments using different cages were conducted in which hamsters were treated by smearing their legs, tail, and mouth parts with 0.1 ml of the various serial concentrations of 0.125; 0.250; 0.500; 0.750 and 1 mg/ml of T. minuta and C. citratus extracts.
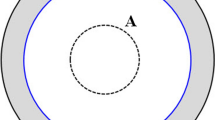
On one side of the flight tunnel were 100 sandflies that were held in Perspex cage while on the other, a hamster that had been smeared with the oil preparation on the legs, tail, and mouth parts was placed. Sand flies that were pre-starved for 4 h or more prior to testing were used for the experiment. Different concentrations of the oils were tested in different cages and each was replicated three times. Each test cage contained 100 flies, thus for a given dose, 300 flies were used. In addition, for each dose, only one hamster was used. The bioassay set up was such that flies flew freely in in the tunnel but had to make contact with the removable cardboard and pass through the holes to reach the bait (hamster) (Figs. 1, 2).
Perspex cage measuring 25 X 25 x 80 cm (length) separated by a removable cardboard frame (P). The restrained hamster smeared with the oil preparation on the legs, tail, and mouth parts is on the test chamber B. Sand flies were held in chamber A and had to make contact with the removable cardboard and pass through the perforated partition (P) to reach the bait (hamster) in chamber B. Repellency was determined by counting the number of sand flies that landed on the hamsters’ legs, tail, and mouth parts for a period of five minutes each at intervals of 30 min
Following the release of flies in the tunnel, landing counts (the number of sand flies that landed on the hamsters’ legs, tail, and mouth parts that had been smeared with oil preparations) was done for five minutes each at intervals of 30 min between 08:00 and 11:00 h. Mean percent repellency for each concentration was calculated based on the data of the three replicates at the given times of observation. Percent repellency for the test oils and DEET was calculated using the formula:
where, N = number of flies landing on the negative control side; R = number of flies landing on side treated with test oil or DEET. Thus, efficacy of the candidate repellent could be assessed relative to DEET.
During tests, the bioassay room was maintained at 27 °C and 80–95% RH. To obtain an acceptable estimate of effective dose (ED), ED50 and ED90, the treated areas on the hamster were swabbed with isopropanol pads.
Estimation of Protection time
To determine protection time, a modified screened two-cage arena [46] was used. For the tests, 100 nulliparous, 5–7 day old sandflies, P. duboscqi were released into the holding cage. The sandflies were free to fly in the tunnel, make contact with the piece of removable perforated cardboard partition, pass through the holes and locate the restrained hamster in the adjacent cage. As in the other bioassays, the hamsters were treated by smearing their legs, tail, and mouth parts with 0.1 ml of concentrations of 0.125; 0.250; 0.500; 0.750 and 1 mg/ml of T. minuta and C. citratus extracts. Following the release of flies into the tunnel, their biting ability was monitored between 08:00 to 11:00 h at intervals of 30 min. Observations were done for three minutes within each half hour and the total number of sandflies biting on the treated and control areas recorded. If no observations were made for the first 3 min of every half an hour exposure, the experiment was discontinued until the next half hour. The test was continued until at least two bites occurred and were followed by a confirmatory bite (second bite) in the subsequent exposure period. The time between application of the test oil and the second successive bite was recorded as the protection time.
Data analysis
All experiments were replicated three times. Data on repellency, protection time, and biting rates were recorded using the Microsoft Excel programme. Control groups in the experimental bioassays with >20% repellency were repeated. Where repellency in the control groups fell between 5 and 20%, the observed percentage repellency was corrected using Abbott’s formula [47]. The dose-repellency data was analysed by log-probit method of Finney [48] and effective concentrations for 50% (ED50) and 90% (ED90) repellency determined. Statistical significance of the recorded repellency of the various test concentrations and the controls were analyzed using one-way analysis of variance (ANOVA) at P < 0.05.
Results
This study sought to determine the repellent activity of two essential oils of the lemon grass, C. citratus and T. minuta. The results of GC–MS of C. citratus and T. minuta demonstrated that the oils were dominated by monoterpene hydrocarbons. The monoterpene fraction for C. citratus was characterized by a high percentage of geranial (20.45%), myrcene (14.24%), neral (11.57%), and verbenene (9.26%) while that of T. minuta composed of dihydro-tagetone (21.15%), (E)-tagetone (16.21%), (Z)-tagetone (14.99%), (Z)-β-ocimene (9.84%), limonene (7.40%), allo-ocimene (6.69%) and (Z)-ocimenone (4.12%). In the dose–response study for determining effective dose, the results on ED50 and ED90 values are shown in Table 1. The ED50 and ED90 values of essential oil of lemon grass, C. citratus were determined to be 0.04 and 0.79 mg/ml, respectively, while those for the oil of T. minuta were 0.1 and 12.58 mg/ml, respectively. In addition, the percentage repellency of the two essential oils against P. duboscqi is presented in Table 2. The essential oil of C. citratus at three concentrations (1, 0.75 and 0.5 mg/ml) provided the highest repellency with 100%, 87.67 and 89.13 resp1ectively at 180 min. On the other hand, the repellency of T. minuta essential oil at similar concentrations of 1, 0.75 and 0.5 mg/ml was relatively lower than that of C. citratus at 88.89%, 79.56 and 52.2 respectively at 180 min. The most potent oil was that of C. citratus at 1 mg/ml that elicited an average repellency of 99.8% (range 99.8–100%; ED50 0.04) and a mean biting rate of 0.8 at various concentrations (Table 3).
In general, the percentage repellency of the two essential oils increased when the concentration of these essential oils increased, in contrast, biting rates decreased when the concentration increased. The results showed significant differences in both the percentage of repellency and the number of sand flies biting (P < 0.05).
Data on the protection time conferred by different concentrations of the two essential oils are shown in Table 4. The data shows that 1 mg/ml and 0.75 of essential oil of C. citratus provided 100% protection for up to 3 h. On the other hand, 1 mg/ml of the oil of T. minuta conferred lesser protection as compared to that of C. citratus for up to 150 min.
Discussion
The essential oils of C. citratus and T. minuta have not previously been tested against the sandfly P. duboscqi. However, most of the previous studies on repellency by essential oil of the lemon grass were carried out with mosquitoes. Other plant-derived compounds that have been shown to reduce mosquito and/or sandfly trap catches include geraniol, linalool, and citronella [49]. The results of this study demonstrate high repellent effects of these essential oils on the adults of the sandfly, P. duboscqi. Grasses of Cymbopogon spp. have been traditionally used for repelling mosquitoes in jungle regions such as the Bolivian Amazon [50]. Plants of this genus produce the most used natural repellents in the world [51]. A wide range of extracts and essential oils isolated from these plants have been tested against a broad range of species of arthropods. In particular, formulations of the oil of C. citratus in paraffin oil have been successfully utilized [52]. However, the essential oil of Cymbopogon nardus oil that was evaluated against Cydia pomonella (Lepidoptera: Tortricidae) was inactive [53]. Further, oils of C. nardus and Cymbopogon flexuosus were ineffective on the cigarette beetle, Lasioderma serricorne (Coleoptera: Anobiidae) [52].
In one particular study, in which the ED50 was closest to what was obtained for this study, Phasomkusolsil and Soonwera [27] demonstrated that the essential oils of various species of plants including C. citratus, Cymbopogon nardus, Syzygium aromaticum and Ocimum basilicum exhibited high repellency against Ae. aegypti with the ED50 at less than 0.045 mg/cm2 of the substrate. In the same study, oils of C. citratus, C. nardus and S. aromaticum showed repellency against An. dirus with ED50 at less than 0.07 mg/cm2. On the other hand, the essential oils of C. citratus, C. nardus, S. aromaticum, O. basilicum and Cananga odorata gave strong effective dose (ED50) values of less than 0.003 mg/cm2 of substrate when tested against Culex quinquefasciatus [27]. Similar findings have been documented by Soonwera and Sinthusiri [54] who obtained 87.9% effective repellency of the essential oil of C. citratus among other oils tested on the house fly, Musca domestica.
Dried plants of T. minuta are usually indoors to repel a broad range of insect species [37, 55]. Repellent activity of Tagetes species have been reported against Anopheles gambiae, the vector of malaria [55]. Tagetes species have also showed insecticidal activity against stored product pests [56]. The efficacy of 100 ppm of T. minuta essential oil against head lice Pediculus humanus capitis (Phthiraptera: Pediculidae) was evaluated and it was found to be toxic to the insects [56]. The toxic effect of the oil of T. minuta to dipterans was attributed to the presence of terpenes [35]. In addition, the GC–MS results obtained in this study indicated that the T. minuta essential oil prepared in that study included β-ocimene that has been shown to be a tick repellent [57]. The soft tick, Hyalomma rufipes adults showed a significant dose-repellent response to the essential oil T. minuta [42].
With regard to protection time of C. citratus essential oil, a similar study [27] using the method of inserting a human arm treated with 0.21 mg/cm2 of essential oil into a cage with mosquitoes. They found that the oil gave the longest protection periods against three mosquito species; 72 min for Ae. aegypti, 132 min for An. dirus and 84 min for Cx. quinquefasciatus. However, the essential oils C. nardus and Syzygium aromaticum, exhibited moderate repellency against Ae. aegypti, An. dirus and Cx. quinquefasciatus.
In a study carried out in Ethiopia, Phlebotomus bergeroti Parrot adults that are vectors of visceral leishmaniasis were repelled by neem (Azadirachta indica) and chinaberry (Melea azedarach) oils at 2 and 5% formulations in coconut oil. These oil formulations provided protection of up to 98.3% protection for up to 9 h at the higher concentration, under laboratory conditions. In tests against field populations of Phlebotomus orientalis Parrot and P. bergeroti, 2 and 5% neem oil in coconut oil mixtures and DEET, the essential oil and DEET were not effective. Other essential oils that have been tested include garlic clove (Allium sativum) oil for which 1% preparation elicited a repellency of 97.0% mature female sandflies, Phlebotomus papatasi Scopoli [58].
Over the years, researchers have demonstrated that effectiveness of repellents over several hours can be improved by synergizing the repellent with a base or fixative materials such as vanillin, salicylic acid and mustard and coconut oils, among others [22, 59, 60]. However, the effectiveness of the repellents depends on multiple factors including the type of repellents (active ingredients), formulation, mode of application, environmental factors (temperature, humidity, and wind), the attractiveness of individual people to insects, loss due to removal by perspiration and abrasion, the sensitivity of the insects to repellents, and the biting density [61,62,63,64,65,66,67].
Conclusion
In conclusion, the results of this study show that the essential oils of C. citratus and T. minuta at relatively physiological doses have strong repellency against adult sandflies, P. duboscqi. It remains to carry out chemical analytical studies to characterize the candidate repellent compounds by testing them in bioassays individually and in blends.
Abbreviations
- ANOVA:
-
analysis of variance
- CBRD:
-
Centre for Biotechnology Research and Development
- DEET:
-
diethyl-3-methylbenzamid
- ED50 :
-
effective dose 50
- ED90 :
-
effective dose 90
- GC–MS:
-
gas chromatography–mass spectrometry
- KEMRI:
-
Kenya Medical Research Institute
- VL:
-
visceral leishmaniasis
- ZCL:
-
zoonotic cutaneous leishmaniasis
References
Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–18.
Leishmaniasis Burden and Distribution. World Health Organization Leishmaniasis page. http://www.who.int/leishmaniasis/burden/en. Accessed on 10 Aug 2015.
Birtles RJ. Carrion’s disease. In: Service MW, editor. The encyclopedia of arthropod-transmitted infections. Wallingford: CABI Publishing; 2001. p. 104–6.
Xu F, Liu D, Nunes MR, Rosa AP, Tesh RB, Xiao SY. Antigenic and genetic relationships among Rift Valley fever virus and other selected members of the genus Phlebovirus (Bunyaviridae). Am J Trop Med Hyg. 2007;76:1194–200.
Ready PD. Biology of phlebotomine sand flies as vectors of disease Agents. Annu Rev Entomol. 2013;58:227–50.
Comer JA, Tesh RB. Phlebotomine sand flies as vectors of vesiculovirus: a review. Parassitologia. 1991;33:143–50.
Ashford RW. Phlebotomus fevers. In: The encyclopedia of arthropod-transmitted infections. MW Service Editors. Wallingford: CABI Publishing; 2001. p. 397–401.
Heisch RB, Wijers DJ, Minter DM. In pursuit of the vector of kala-azar in Kenya. Br Med J. 1962;1:1456–8.
Tonui WK. Situational analysis of leishmaniasis research in Kenya. Afr J Health Sci. 2006;13:7–21.
Beach R, Kiilu G, Hendricks L, Oster C, Leeuwenburg J. Cutaneous leishmaniasis in Kenya. Transmission of Leishmania major to man by the bite of a naturally infected Phlebotomus duboscqi. Trans R Soc Trop Med Hyg. 1984;78:747–51.
Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ. 2003;326:377–82.
Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–82.
De Paula JP, Farago PV, Checchia LEM, Hirose KM, Ribas JLC. Atividade repelente do o´ leo essencial de Ocimum selloi Benth (variedade eugenol) contra o Anopheles braziliensis Chagas. Acta Farm Bonaerense. 2004;23:376–8.
Pavela R. Insecticidal activity of certain medicinal plants. Fitoterapia. 2004;75:745–9.
Spitzer CMOSV. O´leos vola´ teis. In: Simo˜es, CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, editors. Farmacognosia: da planta ao medicamento. Porto Alegre; 2004. p. 467–95.
Rajendran S, Sriranjini V. Plant products as fumigants for stored product insect control. J Stored Prod Res. 2008;44:126–35.
Adorjan B, Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragr J. 2010;25:407–26.
Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372–8.
Regnault-roger C. The potential of botanical essential oils for insect pest control. Integr Pest Manag Rev. 1997;2:25–34.
Olivero-Verbel J, Nerio LS, Stashenko EE. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag Sci. 2010;66:664–8.
Ansari MA, Razdan RK. Repellent action of Cymbopogon martini martini Stapf. var. Sofia against mosquitoes. Indian J Malariol. 1994;31:95–102.
Tawatsin A, Wratten SD, Scott RR, Thavara U, Techadamrongsin Y. Repellency of volatile oils from plants against three mosquito vectors. J Vector Ecol. 2001;26:76–82.
Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann AS. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J Adv Pharm Technol Res. 2011;2:3–8.
Rojas de Arias A, Schmeda-Hirschmann G, Falcao A. Feeding deterrency and insecticidal effects of plant extracts on Lutzomyia longipalpis. Phytother Res. 1992;6:64–7.
Aziz EE, Abbas MH. Chemical composition and efficiency of five essential oils against the Pulse beetle Callosobruchus maculates F on Vigna radiata seeds. American-Eurasian J Agric Environ Sci. 2010;8:411–9.
Kabera J, Gasogo A, Uwamariya A, Ugirinshuti V, Nyetera P. Insecticidal effects of essential oils of Pelargonium raveolens and Cymbopogon citratus on Sitophilus zeamais (Motsch). Afr J Food Sci. 2011;5:366–75.
Phasomkusolsil S, Soonwera M. Comparative mosquito repellency of essential oils against Aedes aegypti (Linn.), Anopheles dirus (Peyton and Harrison) and Culex quinquefasciatus (Say) Asian Pac. J Trop Biomed. 2011;1:S113–8.
Pushpanathan T, Jebanesan A, Govindarajan M. Larvicidal, ovicidal and repellent activities of Cymbopogan citratus Stapf (Graminae) essential oil against the filarial mosquito Culex quinquefasciatus (Say) (Diptera: Culicidae). Trop Biomed. 2006;23:208–12.
Hindumathy CK. In vitro study of antibacterial activity of Cymbopogon Citratus. World Acad Sci Eng Technol. 2011;74:193–7.
Matasyoh JC, Wagara IN, Nakavuma JL, Kiburai AM. Chemical composition of Cymbopogon citratus essential oil and its effect on mycotoxigenic Aspergillus species. Afr J Food Sci. 2011;5:138–42.
Syed M, Qamar S, Riaz M, Chaudhary FM. Essential oils of the family Gramineae with antibacterial activity. Part 2: the antibacterial activity of a local variety of Cymbopogon citratus oil and its dependence on the duration of storage. Pak J Sci Ind Res. 1995;38:146–8.
Akin-Osanaiye BC, Agbaji AS, Dakare MA. Antimicrobial activity of oils and extracts of Cymbopogon citratus (Lemon Grass), Eucalyptus citriodora and Eucalyptus camaldulensis. J Med Sci. 2007;7:694–7.
Maia MF, Sarah J, Moore SJ. Plant-based insect repellents: a review of their efficacy, development and testing. Malar J. 2011; 10: (Suppl 1), S1.
Green MM, Singer JM, Sutherland DJ, Hibben CR. Larvicidal activity of Tagetes minuta (marigold) toward Aedes aegypti. J Am Mosq Control Assoc. 1991;7:82–286.
Perich MJ, Hoch AL, Rizzo N, Rowton ED. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485–8.
Macedo ME, Consoli RA, Grandi TS, dos Anjos AM, de Oliveira AB, Mendes NM, Queiróz RO, Zani CL. Screening of Asteraceae (Compositae) plant extracts for larvicidal activity against Aedes fluviatilis (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 1997;92:565–70.
Ireri LN, Kongoro J, Ngure P, Mutai C, Langat B, Tonui W, Kimutai A, Mucheru O. The potential of the extracts of Tagetes minuta Linnaeus (Asteraceae), Acalypha fruticosa Forssk (Euphorbiaceae) and Tarchonanthus camphoratus L. (Compositae) against Phlebotomus duboscqi Neveu Lemaire (Diptera: Psychodidae), the vector for Leishmania major Yakimoff and Schokhor. J Vector Borne Dis. 2010;3:168–74.
Mongáre S, Ngángá Z, Maranga R, Osiemo Z, Ngure P, Ngumbi P, Tonui W. Effect of leaf crude extracts of Tarchonanthus Camphoratus (Asteraceae), Acalypha Fruticosa (Fabacea) and Tagetes Minuta (Asteraceae) on fecundity of Phlebotomus Duboscqi. Am Int J Contemp Res. 2012;2:194–200.
Adeniran OI, Fabiyi E. A cream formulation of an effective mosquito repellent: a topical product from lemongrass oil (Cymbopogon citratus) Stapf. J Nat Prod Plant Resour. 2012;2:322–7.
Clevenger JF. Apparatus for the determination of volatile oil. J Am Pharm Assoc. 1928;1928(17):345–9.
Evans WC. Trease and Evans’ pharmacognosy. 13th ed. London: Oxford University Press; 1989.
Nchu F, Magano SR, Eloff JN. In vitro anti-tick properties of the essential oil of Tagetes minuta L. (Asteraceae) on Hyalomma rufipes (Acari: Ixodidae). Onderstepoort J Vet Res. 2012;79:E1–5.
Beach R, Young DG, Kiilu G. New phlebotomine sandfly colonies II. Laboratory colonization of Phlebotomus duboscqi (Diptera: Psychodidae). J Med Entomol. 1986;23:114–5.
World Health Organization. Guidelines for laboratory and field testing of long-lasting insecticidal mosquito nets. Geneva: WHO Pesticide Evaluation Scheme. 2005. http://whqlibdoc.who.int/hq/2005/WHOCDSWHOPES_ GCDPP2005.11.pdf. Accessed 10 Aug 2015.
Kasili S, Kutima H, Mwandawiro C, Ngumbi PM, Anjili CO, Enayati AA. Laboratory and semi-field evaluation of long-lasting insecticidal nets against leishmaniasis vector, Phlebotomus (Phlebotomus) duboscqi in Kenya. J Vector Borne Dis. 2010;47:1–10.
Barnard DR. Biological assay methods for mosquito repellents. J Am Mosq Control Assoc. 2005;2:12–6.
Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–7.
Finney DJ. Probit analysis. 3rd ed. London: Cambridge University Press; 1971.
Muller GC, Junnila A, Kravchenko VD, Revay EE, Butler J, Orlova OB, Weiss RW, Schlein Y. Ability of essential oil candles to repel biting insects in high and low biting pressure environments. J Am Mosq Control Assoc. 2008;24:154–60.
Moore SJ, Hill N, Ruiz C, Cameron MM. Field evaluation of traditionally used plant-based repellents and fumigants against the malaria vector Anopheles darlingi in Riberalta, Bolivian Amazon. J Med Entomol. 2007;44:624–30.
Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C. Comparative repellency of 38 essential oils against mosquito bites. Phytotherap Res. 2005;19:303–9.
Oyedele AO, Gbolade AA, Sosan MB, Adewoyin FB, Soyelu OL, Orafidiya OO. Formulation of an effective mosquito-repellent topical product from lemongrass oil. Phytomedicine. 2002;9:259–62.
Landolt PJ, Hofstetter RW, Biddick LL. Plant essential oils as arrestants and repellents for neonate larvae of the codling moth (Lepidoptera: Tortricidae). Environ Entomol. 1999;28:954–60.
Soonwera M, Sinthusiri J. Thai Essential oils as botanical insecticide against house fly (Musca domestica L.). International Conference on Agricultural, Ecological and Medical Sciences. 6–7, Bali (Indonesia), 2014.
Seyoun A, Kabirru EW, Lwande W, Killen DF, Hassanali A, Knols BG. Repellency of live potted plants against Anopheles gambiae from human baits in semi-field experimental huts. Am J Trop Med Hyg. 2002;67:191–5.
Cestari IM, Sarti SJ, Cláudia M, Waib CM, Branco AC Jr. Evaluation of the potential insecticide activity of Tagetes minuta (Asteraceae) essential oil against the head lice Pediculus humanus capitis (Phthiraptera: Pediculidae). Neotrop Entomol. 2004;33:805–7.
Lwande W, Ndakala AJ, Hassanali A, Moreka L, Nyandat E, Ndungu M, et al. Gynandropsis gynandra essential oil and its constituents as tick (Rhipicephalus appendiculatus) repellents. Phytochemistry. 1999;50:401–5.
Valerio L, Maroli M. Evaluation of repellent and anti-feeding effect of garlic oil (Allium sativum) against the bite of phlebotomine sandflies (Diptera: Psychodidae). Annali dellʼIstituto Superiore di Sanita. 2005;41:253–6.
Stuart AE, Brooks CJ, Prescott RJ, Blackwell A. Repellent and antifeedant activity of salicylic acid and related compounds against the biting midge, Culicoides impunctatus (Diptera: Ceratopogonidae). J Med Entomol. 2000;37:222–7.
Das NG, Baruah I, Talukdar PK, Das SC. Evaluation of botanicals as repellents against mosquitoes. J Vector Borne Dis. 2003;40:49–53.
Rozendaal JA. Vector control. Methods for use by individuals and communities. Geneva: World Health Organization; 1997. p. 7–177.
Barnard DR. Repellents and toxicants for personal protection. Geneva: World Health Organization; 2000. p. 23–7.
Hossain E, Rawani A, Chandra G, Mandal SC, Kumar JG. Larvicidal activity of Dregea volubilis and Bombax malabaricum leaf extracts against the filarial vector Culex quinquefasciatus. Asian Pac J Trop Med. 2011;4:436–41.
Wabo PJ, Ngankam NJD, Bilong BCF, Mpoame M. A comparative study of the ovicidal and larvicidal activities of aqueous and ethanolic extracts of pawpaw seeds Carica papaya (Caricaceae) on Heligmosomoides bakeri. Asian Pac J Trop Med. 2011;2011(4):447–50.
Singha S, Chandra G. Mosquito larvicidal activity of some common spices and vegetable waste on Culex quinquefasciatus and Anopheles stephensi. Asian Pac J Trop Med. 2011;4:288–93.
Ahmad N, Fazal H, Abbasi BH, Iqbal M. In vitro larvicidal potential against Anopheles stephensi and antioxidative enzyme activities of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous. Asian Pac J Trop Med. 2012;4:169–75.
Govindarajan M, Sivakumar R, Amsath A, Niraimathi S. Mosquito larvicidal properties of Ficus benghalensis L. (Family: Moraceae) against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med. 2011;4:505–9.
Authors’ contributions
AI, MM and MN conceived the study. A.I., PGNN, PN and JI performed GC–MS analyses and the bioassay experiments. LN performed the statistical analyses and helped in their interpretation. The manuscript was written by AI critically reviewed by PGNN, CO and MN.
Authors’ information
Albert Kimutai is a Zoology Lecturer at the Department of Biological Sciences in the University of Kabianga, Kericho, Kenya. Cyprian Ombati is a Biochemistry Lecturer at the Department of Biological Sciences in the University of Kabianga, Kericho, Kenya. Moses Ngeiywa is a Senior Lecturer at the Department of Biologicals, University of Eldoret, Kenya. Margaret Mulaa is currently a training officer with the Centre for Agriculture and Biosciences International (CABI) plantwise programme. Lydia Nyamwamu is a lecturer at the Center for Teacher Education at Moi University, Eldoret, Kenya. Peter Njagi is a Professor of entomology at the University of Kabianga, Kericho, Kenya. Philip Ngumbi is a Senior Researcher and head of Leishmaniasis Laboratory at the Center for Biotechnology Research and Development of the Kenya Medical Research Institute, Nairobi, Kenya. Johnstone Ingonga is a Research Technologist at the Center for Biotechnology Research and Development of the Kenya Medical Research Institute, Nairobi, Kenya.
Acknowledgements
Immense appreciation goes to Mr. Hillary Kirwa, Onesmus Kaye Wanyama and Prof. Baldwyn Torto of the International Centre of Insect Physiology & Ecology (Icipe) for guidance and on the hydro distillation of the essential oils.
Availability of data and materials
All materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, provided this will not breach confidentiality.
Competing interests
The authors declare that there are no competing interests.
Ethics approval and consent to participate
Approval for the study was sought from Kenya Medical Research Institute’s ethical review committee (IREC) and the Board of Postgraduate Studies of the University of Eldoret. The experiments were done in compliance with KEMRI’s Animal Care and Use Committee (ACUC) guidelines and in conformity with Good Laboratory Practices (GLP).
Funding
This work was supported by the University of Kabianga Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kimutai, A., Ngeiywa, M., Mulaa, M. et al. Repellent effects of the essential oils of Cymbopogon citratus and Tagetes minuta on the sandfly, Phlebotomus duboscqi . BMC Res Notes 10, 98 (2017). https://doi.org/10.1186/s13104-017-2396-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-017-2396-0