Abstract
Background
As part of malaria characterization study in the South-Tongu district of Ghana, the current study was conducted to explore relationships between malaria, schistosomiasis, soil transmitted helminths and malnutrition in riparian community settings that had hitherto encountered episodes of mass deworming exercises.
Methods
School-age children were enrolled in a cross-sectional study from April through July 2012. Stool and urine samples were examined respectively for helminths and Schistosoma haematobium. Blood samples were analyzed for malaria parasites and haemoglobin (Hb) concentrations, respectively. Anthropometric indices were measured. Relationships were determined using generalized linear models.
Results
The results show low numbers of asymptomatic Plasmodium falciparum (9.2 %, n = 37/404) and S. haematobium (2.5 %, n = 10/404) infections. The associations between significance terms in the multivariate analysis for P. falciparum infections were further assessed to test the significance of the product terms directly i.e., age in years [adjusted odds ratio (AOR), 3.1; 95 % confidence interval (CI) 1.1–5.6], Hb concentration (AOR = 0.71; 95 % CI 0.42–2.3), and stunted malnutrition (AOR, 8.72; 95 % CI 4.8–25.1). The P. falciparum-associated decrease in mean Hb concentration was 2.82 g/dl (95 % CI 1.63–4.1 g/dl; P = 0.001) in stunted children, and 0.75 g/dl (95 % CI 1.59–0.085 g/dl; P = 0.076) in the non-stunted cohort. The anaemia-associated decrease in mean parasitaemia in stunted children was 3500 parasites/µl of blood (95 % CI 262.46–6737.54 parasites/µl of blood; P = 0.036), and in non-stunted children 2127 parasites/µl of blood (95 % CI −0.27 to 4.53; P = 0.085). Stunted malnutrition was the strongest predictor of S. haematobium infection (AOR = 11; 95 % CI 3.1–33.6) but significant associations as described for P. falciparum infections were absent. The population attributable risk of anaemia due to P. falciparum was 6.3 % (95 % CI 2.5–9.3), 0.9 % (95 % CI 0.4–2.3) for S. haematobium, and 12.5 % (95 % CI 9.11–19.52) for stunted malnutrition.
Conclusion
Plasmodium falciparum, S. haematobium, intestinal helminths and their co-infections were uncommon in our school-age children. Stunting exacerbated the extent to which malaria was associated with loss in Hb concentration.
Similar content being viewed by others
Background
The South Tongu District is one of the 25 districts of the Volta region of Ghana drained by the Volta river and its tributaries [1]. In recent times, comprehensive initiatives with health education, personal hygiene instructions, and mass chemotherapy treatment in school-age children and high-risk adults have been emphasized in the Volta river basin [2–4]. There is the schistosomiasis control initiative Ghana (SCIG) which aims to implement an integrated national plan for sustainable control of schistosomiasis in Ghana [5]. There is also the West African international parasite control (WACIPAC) whose initiatives have included the institution of preventive measures on schistosomiasis and soil transmitted helminths [6]. Malaria is endemic in Ghana and represents a major cause of morbidity and mortality in children of school-going age. An accurate information on burden of malaria at the district level is requisite both to plan local parasitic control efforts and to measure the impact of such efforts especially in riparian communities [7–9]. But targeting the correct set of interventions to these major parasitic groups may not be done effectively without regard for nutritional status [2, 10]. Nutrition plays a major role in maintaining health; and malnutrition appears to generate vulnerability to a wide variety of diseases and general ill health. Whereas animal studies suggest that improved nutritional status is protective against malaria, consensus has yet to be reached regarding its effects in human populations [11]. More recent studies indicate either no evidence of benefit or some benefits resulting from nutritional adequacy [2, 10]. In this paper, we describe as our primary outcome, data from a district survey on school-age children (SAC) (6–13 years) regarding the prevalence of malaria, schistosomiasis, and intestinal helminths in riparian community settings that had hitherto encountered several episodes of preventive chemotherapy including mass deworming exercises; and explore relationships between such infections and nutritional inadequacies.
Methods
Study area and population
The study was conducted during the first annual rainy season (April through July) of 2012 in the South-Tongu district, an area with seasonal malaria in Volta region, Ghana [4, 12]. The study area comprises 594.75 km2 located on Latitude 5°58′37.2′′ and Longitude 0°38′49.2′′. Rainfall is bimodal with latter rains in September to November [13]. The main river draining the district is the Volta, which runs along its western border, but it is also drained by lakes, streams and lagoons in the southern sector of the district. Preliminary surveillance along the river bodies revealed lacustrine conditions favourable to mosquito breeding, growth of aquatic weeds and breeding of snails. Malaria, schistosomiasis, soil-transmitted helminths and onchocerciasis control programmes are active in this area [14, 15]. The district has the Comboni hospital which offers primary health care and diagnostic services. According to hospital records, malaria remains the number one cause of hospital admissions and child morbidity and mortality in the district, with the predominant parasite being Plasmodium falciparum. Malaria transmission occurs throughout the year with peaks during the two rainy seasons.
Study design
The target population comprised school-age children (ages 6–13 years) without manifestations compatible with malaria in the past 14 days. Children on nutritional supplements were excluded. Four primary schools across the district namely Dabala Evangelical, Tefle Presbyterian Church of Ghana, Sogakope Cuniberto, and the Tsavanya district assembly basic in Agbakope were selected (Fig. 1). Children were selected using a 2-stage cluster sampling procedure. At the first sampling stage, four of seven district circuits in the study area were sampled systematically, with a sampling probability proportional to size, based on Ghana statistical service census data of 2009, as measured by the number of primary schools. At the second sampling stage, one primary school was randomly selected within each circuit. Since malaria and helminth infections detected by blood, urine and stool analysis were our focus of interest, this study was confined to children who could provide all study samples.
Data collection
Fieldwork took place from April to June of 2012. Parents and guardians were requested for assistance in provision of information. Interviews were conducted in the local dialect, Ewe, spoken by study participants. Data were abstracted through interviews using standardized questionnaires with the help of trained field workers (Additional file 1). Data was collected regarding the following: demographics (gender and age), malnutrition status (stunted, underweight, wasted), child’s knowledge of causes of malaria, helminth infections and schistosomiasis, deworming, availability of toileting facilities in household, sources of household water, child’s activity in any river and parent/guardian educational level. We also determined the household socio-economic status using proxy measures based on the World Bank asset scores for Ghana [16]. For each enrolee, anthropometric measurements including height and weight were determined. The anthropometric indices height-for-age (HA), weight-for-age (WA), weight-for-height (WH) were expressed as Z-scores using the WHO child growth standards [17, 18]. For all children, axillary temperature was measured, and inquiry was made if they had experienced fever in the previous 14 days.
Sample collection and examinations
Children were provided with plastic containers and requested to bring about 3 g of stool and 20 ml of urine at 10:00 a.m. in the morning. Stool samples were processed within 6 h of collection and examined microscopically within 1 h of preparation. They were examined for Schistosoma mansoni and intestinal helminths by wet mount for ova and larvae. The formol-ether concentration technique was used to confirm the presence of parasites in stool. Intensity of infection was assessed by egg count and expressed as mean eggs per gram of faeces (EPG). Ten millilitres of urine samples were examined for S. haematobium eggs using the nucleopore filtration method. The intensity of S. haematobium infections was calculated as the number of ova per 10 ml of urine. For haemoglobin (Hb) measurements and malaria testing, approximately 3 ml of blood samples were collected from each pupil. Thick blood smears were prepared, stained with Giemsa and examined microscopically for detection, identification and quantification of malaria parasites. Parasite densities were determined with absolute white blood cells (WBC) counts as a ratio of P. falciparum counts relative to 200 WBC in thick films. Hb concentrations were determined using the cyanmethaemoglobin method. Briefly, blood aliquots of 0.02mls were put into five milliliters of Drabkin’s solution to make a 1 in 250 dilution. The solutions were well mixed and incubated in the dark for 10 min before Hb estimations (g/dl) in a colorimeter at 540 nm wavelength. Quality control was performed by randomly selecting and microscopically re-examining 10 % of blood, urine and stool preparations by an experienced independent technician blinded to all previous results.
Data analyses
Data from interviews and parasitological investigations were captured into Microsoft Excel database, and exported into Statistical Package for Social Sciences (SPSS, version 16) for editing and statistical analyses. Missing data were excluded from analysis. Any anaemia was defined as Hb <12 g/dl; severe anaemia, Hb <7 g/dl; moderate anaemia, Hb 7 to <10 g/dl; and mild anaemia, Hb 10–11 g/dl. Based on positive samples only, infection intensities were calculated as parasites per microlitre of blood for P. falciparum, EPG of faeces for intestinal helminths, and eggs per 10 ml of urine for S. haematobium. Children were classified as stunted, underweight or wasted when their HA, WA or WH Z-scores were <−2 below the reference mean, respectively. A child was identified as being malnourished with a score of <−2 in 1 of the HA, WA, or WH assessment [17, 18]. Point estimates of statistical significance are indicated with two tailed P < 0.05. The proportion of anaemia cases attributable to malnutrition or to specific parasitic infections was estimated as population attributable risk percentages (PAR%) [19, 20]. Descriptive statistics (frequencies and cross-tabulations) were used to determine the prevalence of parasitic infections. For continuous variables, standard weighted-mean statistics using Student’s t test or one-way analysis of variance (ANOVA) were used to respectively estimate differences in two or more population means. Categorical data were compared across study parameters using Chi square or the Fisher’s exact test where appropriate. The Mantel–Haenszel Chi square was used to test for trends in linearity. Correlations were assessed, where appropriate, with Pearson coefficient (r) or Spearman’s rho (rs) and their coefficient of determination (r2 or r 2s ). Univariate comparisons between study outcomes and covariates were computed with Chi square tests and unadjusted odds ratios (OR) at 95 % confidence interval (CI). From univariate analyses, variable with a P < 0.05 were analyzed in multivariate logistic regression models to identify independent risk factors. Predictive accuracy of the models was assessed by Hosmer and Lemeshow goodness-of-fit test with P > 0.05 indicating that the model predicts accurately on average. The area under the receiver operating characteristic (ROC) curve >0.7 was used to evaluate the discriminatory capability of models. Multiple linear regression was used to compare stunted and non-stunted children regarding their associations between malaria, hemoglobin concentration, anaemia, P. falciparum parasitaemia, and S. haematobium egg count. Effect size determination and possible interaction of the variables were computed with analysis of covariance (ANCOVA).
Results
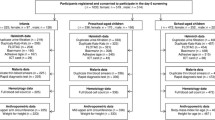
Overall 591 SAC were available for sampling based on parents/guardians informed consent. One-hundred and eighty seven of these were excluded from the study. Reasons for exclusion included absence from school (n = 51), unwillingness to provide blood, urine and stool samples (n = 46) and the refusal of children to assent (n = 74). A total of 404 children including 198 girls and 206 boys in the south-Tongu district of Ghana were enrolled. The mean age of SAC was 8.2 years (range 6–13 years); and about 94 % had received antihelminthic drugs in the previous 3 months.
Prevalence of infection, malnutrition and anaemia
Table 1 summarizes the baseline characteristics of our study population. Of the total 404 children, the prevalence of asymptomatic malaria was 9.2 % (n = 37/404; 95 % CI 6.6–12.5)—P. falciparum accounted for all infections. Ten children (2.5 %; 95 % CI 1.2–5.4) harboured S. haematobium ova. None of the children was identified with intestinal helminths nor suffered from co-infections with multiple species or different parasites. About 22 % of the children were stunted with a mean HA Z score of −1.90 ± 1.23; whilst 21.5 % were underweight with a mean WH Z score of −0.88 ± 0.57. The prevalence of wasted children was 8.4 % with a mean WA Z score of −2.04 ± 0.9. About 295 (73.0 %) of the children suffered from anaemia—5.7 % (n = 17) of which were severe.
Intensity of infections
Among P. falciparum infected children, the mean parasitaemia was 4641 parasites/µl (95 % CI 1812–8973) of blood. About 24.3 % (n = 9) of the children had low P. falciparum parasitaemia <500 parasites/µl of blood and more than half (n = 22; 62 %) had moderate parasitaemia ranging between 500 and 10,000 parasites/µl of blood. A few participants (n = 5/37, 13.5 %) had high parasite count >10,000 parasites/µl of blood. Of the ten infected by S. haematobium, six had light infections (<50 ova per 10 ml urine) with a mean of 37 ± 12. Four children suffered heavy S. haematobium infections (>50 ova per 10 ml urine) with egg intensity of 103 ± 21. The mean ova count for S. haematobium infections was 63 ± 24.
Univariate and multivariate analysis
From Table 2, five factors were identified to be associated with P. falciparum infection: older age (11–13 years), being malnourished and stunted, use of repellents as protection against malaria, use of river as source of household water, child’s activity in river, travel in past 4 weeks, and high parent/guardian education. The same variables were significantly associated with S. haematobium infection, with the exception of recent travel. However, no anti-helminthic drug use in past 3 months was also significantly associated with S. haemtobium infection.
In adjusted models (Table 3), older age 11–13 years [adjusted odds ratio (AOR), 3.1; 95 % CI 1.1–5.6] and stunted malnutrition (AOR, 8.72; 95 % CI 4.8–25.1) were identified as independent risk factors P. falciparum infection. Also, a unit increase in Hb concentration was determined to be putatively protective against P. falciparum infection (AOR = 0.71; 95 % CI 0.42–2.3). Stunted malnutrition was the strongest predictor of S. haematobium infection (AOR = 11; 95 % CI 3.1–33.6). The frequency of a child’s activities per week in river was identified as an independent risk factor (AOR = 1.68 per one activity increase) for S. haematobium infection—with infected children engaging in river activities almost twice as frequent as those uninfected. Reported use of anti-helminthic drug in the past 3 months was found to be 87 % protective against S. haematobium infection.
Trends in P. falciparum infections
The associations between significant terms in the multivariate regression analysis for P. falciparum infections were further assessed to test the significance of the product terms directly i.e., age, Hb concentration and stunted malnutrition. Figure 2 shows the age-specific distribution of P. falciparum infections among all study children—the trend of infections declined with increasing age (X2 for trend, P = 0.036). When data was adjusted for stunting, the frequency of infections showed no tendency to increase or decrease with age (X2 for trend, P = 0.196). Meanwhile, a significant drift towards lower parasitaemia with increasing age was observed in non-stunted children (r = −0.816, r2 = 0.666, P = 0.014) (Fig. 2). The mean parasite density of P. falciparum also remained relatively unchanged with increasing age (r = −0.870, P = 0.837). Among the infected children, the stunted group showed higher (P < 0.001) mean parasitaemia level (3218 ± 1184 parasites/µl) than their non-stunted counterparts (2064 ± 1287 parasites/µl). We observed within the stunted cohort a significant correlation between Hb concentration and parasite density (r = −0.527; r2 = 0.278; P = 0.013); such association was also present in the non-stunted cohort albeit at a slower rate (r = −0.375; r2 = 0.128; P = 0.035) (Fig. 3).
Age-specific distribution of P. falciparum infections in children with stunted and non-stunted malnutrition compared to mean parasitaemia. Pf, Plasmodium falciparum. For P. falciparum infections among all children, the trend of infections declines with increasing age (X2 for trend, P = 0.036); among non-stunted children, the trend of infections declines with increasing age (X2 for trend, P = 0.04); among stunted children, a zero linear trend observed with increasing age (X2 for trend, P = 0.196). For mean parasitaemia among all children, non-significant negative correlation with age (r = −0.650, r2 = 0.423, P = 0.081); among non-stunted children, significant drift towards lower parasitaemia of P. falciparum with increasing age (r = −0.816, r2 = 0.666, P = 0.014); among stunted children, P. falciparum parasitaemia relatively remained similar with increasing age (r = −0.087, P = 0.837)
Haemoglobin versus P. falciparum parasitaemia in stunted and non-stunted children. Within the stunted cohort a significant correlation between haemoglobin concentration and parasite density (r = −0.527; r2 = 0.278; P = 0.013) was observed. A similar association was present in the non-stunted group albeit at a slower rate (r = −0.375; r2 = 0.128; P = 0.035)
Associated effects of P. falciparum, Hb concentration and stunting
Figure 4a compares stunted and non-stunted children regarding their association between malaria and Hb. The P. falciparum-associated decrease in mean Hb concentration was 2.82 g/dl (95 % CI 1.63–4.1 g/dl; P = 0.001) in stunted children, and 0.75 g/dl (95 % CI 1.59–0.085 g/dl; P = 0.076) in the non-stunted cohort. Of the P. falciparum infected children, 93.7 % (n = 15/16) of those stunted were anaemic whiles 90.4 % (n = 19/21) of the group without stunting suffered from anaemia. Among the stunted and non-stunted children, the anaemia-associated decrease in mean parasitaemia were 3500 parasites/µl of blood (95 % CI 262.46–6737.54 parasites/µl of blood; P = 0.0358) and 2127 parasites/µl of blood (95 % CI −0.27 to 4.53; P = 0.085) respectively (Fig. 4b). The PAR % of anaemia attributable to P. falciparum infection was 6.3 % (95 % CI 2.5–9.3), and 12.5 % (95 % CI 9.11–19.52) for stunted malnutrition.
Comparisons of stunted and non-stunted children regarding the associated effect of a Plasmodium falciparum (P.f) on mean haemoglobin concentration. The P. falciparum-associated decrease in mean haemoglobin concentration was significant in stunted children; b anaemia on mean P. falciparum parasitaemia. Among stunted and non-stunted children, the anaemia-associated decrease in mean parasitaemia were respectively non-significant; c Schistosoma haematobium (S.h) on mean haemoglobin concentration. The S. haematobium-associated decrease in mean haemoglobin concentration was not significant in stunted and non-stunted children; d anaemia on mean S. haematobium infection intensity. There were no significant anaemia-associated increases in S. haematobium egg intensity in stunted or non-stunted children
S. haematobium infections and associated effects
The PAR % of anaemia attributable to S. haematobium infection was 0.9 % (95 % CI 0.4–2.3). When we compared the associated effect of S. haematobium infection and Hb concentration between the two cohorts, S. haematobium-associated decrease in mean Hb concentration was 0.6 g/l (95 % CI −158–1.358 g/l; P = 0.111) in stunted children; and 1.2 g/l (95 % CI −0.519–2.92 g/l; P = 0.170) in those without stunting (Fig. 4c). Similarly, there was no significant anaemia-associated increases in S. haematobium egg intensity in stunted or non-stunted children (Fig. 4d). Also no correlation was observed between S. haematobium egg intensity and Hb concentration (r = 0.2784; P = 0.117).
Discussion
The study showed low numbers of asymptomatic P. falciparum infections with largely low to moderate level parasitaemia. About half of the affected suffered from stunted malnutrition with a high proportion of moderate to severe anaemia. The prevalence of urinary schistosomiasis was 2.5 %, with low to high ova counts for S. haematobium—the principal manifestations were stunted malnutrition and moderate anaemia. For two reasons, our findings of low malaria levels deserve particular attention. First, P. falciparum accounted for all malaria cases reported here, and the level of infection using comparative methodology was lower than that reported for other malaria-affected children in Ghana [12, 21, 22], Tanzania [23] and in many other reviews spanning several endemic regions in Africa [24, 25]. Second, the parasite rates from our study was obtained through primary school survey and represent aggregated rates for communities in each school’s catchment area. Also, similar low levels of P. falciparum infections were observed between the various community schools. Taken together, the findings should not be considered as an artefact of a single study period but as results which perhaps points to a temporally stable infection rate among SAC in the South-Tongu district. The results is also in keeping with previous mass deworming, malarial prevention and schistosomiasis control exercises in this region [2–5]. Our data however suggest that despite the improved control efforts, there remain many inhabitants of riparian communities that suffer from urinary schistosomiasis [26]. Although we are not privy to the full repertoire of drugs admitted during these sessions, children had mostly been on praziquantel in various combinations with other drugs including antimalarial.
In multivariate analysis, the reported usage of antihelminthic by study respondents was determined to be protective against urinary schistosomiasis. The study also showed that stunted malnutrition was independently associated with increased odds of P. falciparum infections. Stunted malnutrition was also present after assessing data separately for S. haematobium infections. Stunting is a manifestation of recent and acute under nutrition. Children who are stunted are thought to have increased predisposition to malaria and other parasitic infection for a variety of reasons, most notably through a reduction in the function of T-lymphocytes, deficiencies in antibody formation, diminished complement formation, and degeneration of thymus and other lymphoid tissues [2, 10, 22, 27]. From our study, an increase of one unit of Hb was protective against P. falciparum infection and corresponded to an AOR of 0.71 (95 % CI, 0.42–2.3, P = 0.002). Meanwhile, children with stunted malnutrition showed higher parasitaemia levels that negatively correlated with lower Hb concentration. The PAR % of anaemia attributable to P. falciparum remained about twofold lower than that of stunted children who accounted for approximately one-eighth of anaemia cases. The data corroborates with our other results regarding stunted-malnutrition-associated decrease in Hb concentration among malaria affected children (Fig. 4). In the low malaria settings of South-Tongu, stunted malnutrition could be contributing to lower Hb concentrations in P. falciparum affected children. It is noteworthy that although these observations with stunting were not apparent in schistosomiasis affected children, a clear evidence that stunting is associated with increased prevalence in malarial infection is beyond the scope of this study.
It is conceivable that malaria may cause stunting. Yet given that stunting becomes manifest only after a prolonged period of nutritional insufficiencies, and that malaria infection is a transitory state, we consider it more likely that host immunity impairment which results from conditions such as Hb deficiency exacerbate host vulnerability to malaria [2, 22, 27]. A diagram describing the relationship between infections with P. falciparum, S. haematobium, Hb concentration and stunted malnutrition is considered in Fig. 5.
There are some potential limitations of this study that must be discussed briefly, and for which our data must be interpreted carefully. First, the studies for helminths used only one stool and urine sample from each child; hence, the proportion of children with low-intensity infections could have been misclassified as uninfected [23, 28]. The prevalence of helminth infections is therefore likely to be underestimated in this study. Second, another issue worth mentioning is the limited sampling size and the concomitant low levels of urinary schistosomiasis and other helminth infections. Whereas this may reflect the relative incidence of organisms in a riparian community with active preventive and curative programmes against parasitic infections, a more large-scale survey is likely to be with little bias for P. falciparum and S. haematobium. Last, other haematological markers such as serum C-reactive protein and soluble transferrin receptor concentrations were not determined [27]. The effect estimates of such parameters would present a more holistic discussion on our data. Despite the shortcomings, our study demonstrate that malaria, urinary schistosomiasis, soil-transmitted helminths, and co-infections with these parasites are uncommon among children in South-Tongu district; and highlights the success of parasite control programmes in our study area.
Conclusions
The silver lining to this study is the fact that our data shows malnourishment that cause stunting may also exacerbate the extent to which malaria is associated with deficiency in Hb concentration [2, 10, 22, 27]. We suggest further studies on whether nutrition intervention therapy targeted in stunted children may help reduce the anaemia burden of P. falciparum infections.
Abbreviations
- SAC:
-
school-age children
- S. haematobium :
-
Schistosoma haematobium
- P. falciparum :
-
Plasmodium falciparum
- AOR:
-
adjusted odds ratio
- CI:
-
confidence interval
- SCIG:
-
schistosomiasis control initiative Ghana
- WACIPAC:
-
West African international parasite control
- HA:
-
height-for-age
- WH:
-
weight-for-height
- WA:
-
weight-for-age
- EPG:
-
eggs per gram
- WBC:
-
white blood cells
- Hb:
-
haemoglobin
- r:
-
Pearson coefficient
- rs :
-
Spearman’s rho
- ROC:
-
receiver operating characteristic
- ANCOVA:
-
analysis of covariance
- PAR:
-
population attributable risk
References
Johnston R, McCartney M. Inventory of water storage types in the Blue Nile and Volta river basins. IWMI Work Pap. 2010;1:1–48.
Magalhães RJS, Clements AC. Mapping the risk of anaemia in preschool-age children: the contribution of malnutrition, malaria, and helminth infections in West Africa. PLoS Med. 2011;8:e1000438.
Kweku M, Webster J, Taylor I, Burns S, Dedzo M. Public–private delivery of insecticide-treated nets: a voucher scheme in Volta region, Ghana. Malar J. 2007;6:14.
Mba CJ, Aboh IK. Prevalence and management of malaria in Ghana: a case study of Volta region. Etude la Popul Africaine. 2007;22:137–65.
Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements ACA, Kabatereine NB, Toure S, Dembele R, Nyandindi U, Mwansa J, Koukounari A. The schistosomiasis control initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136:1719–30.
WACIPAC. The West African international parasite control (WACIPAC). Proj. Doc. 2008. p. 1–78.
Odai I. Integrated family planning, nutrition, and parasite-control project in Ghana: a baseline survey report. Integration. 1990;25:25–36.
Ayi I, Nonaka D, Adjovu JK, Hanafusa S, Jimba M, Bosompem KM, Mizoue T, Takeuchi T, Boakye DA, Kobayashi J. School-based participatory health education for malaria control in Ghana: engaging children as health messengers. Malar J. 2010;9:98.
Menaca A, Pell C, Manda-Taylor L, Chatio S, Afrah NA, Were F, Hodgson A, Ouma P, Kalilani L, Tagbor H, Pool R. Local illness concepts and their relevance for the prevention and control of malaria during pregnancy in Ghana, Kenya and Malawi: findings from a comparative qualitative study. Malar J. 2013;12:257.
Suchdev PS, Davis SM, Bartoces M, Ruth LJ, Worrell CM, Kanyi H, Odero K, Wiegand RE, Njenga SM, Montgomery JM, Fox LM. Soil-transmitted helminth infection and nutritional status among urban slum children in Kenya. Am J Trop Med Hyg. 2014;90:299–305.
Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. Am J Trop Med Hyg. 2004;71(Suppl 2):55–63.
Egbi G, Steiner-Asiedu M, Kwesi FS, Ayi I, Ofosu W, Setorglo J, Klobodu SS, Armar-Klemesu M. Anaemia among school children older than five years in the Volta region of Ghana. Pan Afr Med J. 2014;17(Suppl 1):10.
Koku JE, Gustafsson JE. Local institutions and natural resource management in the South Tongu district of Ghana: a case study. Sustain Dev. 2003;11:17–35.
Plan, Ghana: report on schistosomiasis, soil transmitted helminthes and onchocercaisis in upper Manya Krobo, South Tongu district and Hohoe. 2011.
IDEC. Ghana: phase 1 & 2 Bilharzia and soil transmitted helminthiasis (BOS) control project in schools and communities in the South Tongu district of Ghana. 2011.
Gwatkin RD, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-economic differences in health, nutrition, and population, Ghana. Washington, DC: HNP/Poverty Thematatic Group/World Bank; 2003.
de Onis M. The World Health Organization global database on child growth and malnutrition: methodology and applications. Int J Epidemiol. 2003;32:518–26.
van DenBroeck J, Willie D, Younger N. The World Health Organization child growth standards: expected implications for clinical and epidemiological research. Eur J Pediatr. 2009;168(2):247–51.
Walter SD. Calculation of attributable risk from epidemiologic data. Int J Epidemiol. 1978;7:175–82.
Walter SD. Local estimates of population attributable risk. J Clin Epidemiol. 2010;63:85–93.
Oduro AR, Koram KA, Rogers W, Atuguba F, Ansah P, Anyorigiya T, Ansah A, Anto F, Mensah N, Hodgson A, Nkrumah F. Severe falciparum malaria in young children of the Kassena-Nankana district of northern Ghana. Malar J. 2007;6:96.
Ronald LA, Kenny SL, Klinkenberg E, Akoto AO, Boakye I, Barnish G, Donnelly MJ. Malaria and anaemia among children in two communities of Kumasi, Ghana: a cross-sectional survey. Malar J. 2006;5:105.
Kinung’hi SM, Magnussen P, Kaatano GM, Kishamawe C, Vennervald BJ. Malaria and helminth co-infections in school and preschool children: a cross-sectional study in Magu district, north–western Tanzania. PLoS One. 2014;9:e86510.
Cotter C, Sturrock HJW, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11.
O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10(8):545–55.
Ayeh-Kumi P, Obeng-Nkrumah N, Baidoo N, Teye J, Asmah R. High levels of urinary schistosomiasis among children in Bunuso, a rural community in Ghana : an urgent call to intensify surveillance and control programs. J Parasit Dis. 2013. doi:10.1007/s12639-013-0411-5.
Verhoef H, West CE, Veenemans J, Beguin Y, Kok FJ. Stunting may determine the severity of malaria-associated anemia in African children. Pediatrics. 2002;110:e48.
Trabelsi S, Aouinet A, Khaled S. Procedure and indications of stool examination in parasitology. Tunis Med. 2012;90:431–4.
Authors’ contributions
PFAK, SKA, PBTQ conceived the study; participated in its design, coordination, and collation of laboratory data. KAO participated in the study design, co-ordination and collation of laboratory data, and helped to draft the manuscript. NON, GAM, HNAA, KOD helped in collation of data, performed the statistical analysis and helped to draft the manuscript. AF, participated in the study design and coordination and helped to draft the manuscript. RHA, MC, LA participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the following for their support: Sogakope district assembly office, staff of the Comboni hospital and microbiology laboratory, staff of iDEC and Plan Ghana. This study was supported with Grant from the postgraduate research fund at the University of Ghana college of Health Sciences.
Availability of data and materials
The authors do not have the permission/consent of parents/guardians to share data in publicly available repositories. We may however consider sharing our findings per individual requests.
Competing interests
The authors declare that they have no competing interests.
Consent to publish
Not applicable.
Ethics, consent and permissions
Approval for research was sought from the ethical review and Protocol Committee of the University of Ghana Medical School, Korle-bu. At the study area, approval was sort from both the district assembly office, district education office, and the Heads that oversee the selected schools. Prior to the commencement of work, the research team conducted meetings with Heads of Schools, leaders, teachers and community members of the selected study area to explain the objectives of our study including benefits, potential risks and discomforts. Subsequently, informed written consent for children who participated in the study was sought from parents or legal guardians. In addition, children were requested to give assent to their involvement; with the right to refuse participation or withdraw at any time during the study. Samples and accompanying data were de-identified and allotted arbitrary numbers to ensure anonymity. Children found infected with parasites as well as those found with ailments not targeted by the project were referred to hospital for treatment.
Funding
This study was supported with Grant from the postgraduate research fund at the University of Ghana college of Health Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional file
13104_2016_2025_MOESM1_ESM.pdf
Additional file 1. Questionnaire for data collection. Data on participants characteristics were collected via interviews using standardized questionnaires. The questionnaire abstracted information regarding the following demographics (gender and age), child’s knowledge of causes of malaria, helminth infections and schistosomiasis, deworming, availability of toileting facilities in household, sources of household water, child’s activity in any river and parent/guardian educational level. We also determined the household socio-economic status using proxy measures based on the World Bank asset scores for Ghana. For each enrolee, anthropometric measurements including height and weight were determined.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ayeh-Kumi, P.F., Addo-Osafo, K., Attah, S.K. et al. Malaria, helminths and malnutrition: a cross-sectional survey of school children in the South-Tongu district of Ghana. BMC Res Notes 9, 242 (2016). https://doi.org/10.1186/s13104-016-2025-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-016-2025-3