Abstract
Background
Resistance development in human malaria parasites against commonly used antimalarial drugs has necessitated the scientific exploration of traditionally used antimalarial plants. Plant derivatives have been used for curing malaria historically. The present study involves in vitro evaluation of two medicinally important plants Aristolochia griffithii and Thalictrum foliolosum DC used in antimalarial chemotherapy by the tribes of northeast India.
Method
Chloroform, ethyl acetate and n–butanol extracts of Aristolochia griffithii and Thalictrum foliolosum DC were evaluated in vitro against chloroquine sensitive (SS) and chloroquine resistance strains (RS) of P. falciparum. The tests were conducted following WHO standard method and the inhibition of parasite (IC50) was calculated.
Results
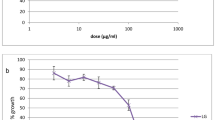
In A. griffithii, the IC50 value for ethyl acetate extracts against SS was 6.2 ± 0.02 μg/ml and found to be lower than chloroform extracts, which exhibited an IC50 value of 14.1 ± 0.1 μg/ml (t = 191.1; p < 0.0001). The IC50 values of both chloroform and ethyl acetate extracts for RS were higher as compared to the SS (p < 0.0001). In T. foliolosum DC the IC50 concentration of chloroform extracts for SS and RS were 0.5 ± 0.0 and 1.1 ± 0.0 μg/ml respectively (t = 54.2; p < 0.0001).
Conclusion
The present findings, although preliminary, but scientifically demonstrate that identification and isolation of active compounds of these two plant materials and testing against different Plasmodium species could lead to the development of potential antimalarial drugs for future.
Similar content being viewed by others
Background
Approximately 214 million cases and 0.44 million deaths were reported due to malaria worldwide in 2015 [1]. Plasmodium falciparum, the life threatening malaria parasite, has developed resistance to many antimalarials, which has entailed the urgent need of developing new and effective antimalarial drugs that are affordable to the developing countries [2–7]. Medicinal plants have been a source of many antimalarial compounds such as quinine, artemisinin that have been used in developing potential antimalarial drugs. Artemisinin derivatives have been recommended in combination with other antimalarial drugs, such as amodiaquine, mefloquine, sulphadoxine–pyrimethamine (SP) for the treatment of malaria in most of the endemic countries. In order to identify potential antimalarial compounds, several studies have been conducted in malaria endemic countries to evaluate the suppressive effects of various plant derivatives on malaria parasite [8–10].
Malaria is commonly reported in many states of India and neighbouring countries and the problem of reduced susceptibility to commonly used antimalarial drugs specifically in P. falciparum has been a growing concern for malaria control programme in India [11, 12]. In addition to the threat to life, malaria has been found to have detrimental effect on the prosperity of the society by impeding the overall development, including effect on population growth, productivity and medical cost [13]. In India, the resistance to chloroquine was first detected in Assam, since then it prevailed into other parts of the country attributing countless malaria related casualties. The tremendous pressure of chloroquine resistance has led to switch-over to artesunate-based combination therapy (ACT) as first line of treatment of uncomplicated malaria cases. Therefore, it is important that traditionally used antimalarial plants are investigated to discover new antimalarial drugs, in order to tackle the resurging of antimalarial resistance in Plasmodium parasite. In the recent years, various studies have explored the antimalarial activity of phyto-drugs based on the traditional reputation of plants in malaria treatment [2, 8–10, 14, 15].
Northeast region of India has great plant biodiversity potential and many plants are used in traditional medicine system for the treatment of various ailments [16–19]. Among the tribes of Assam, especially Bodo, Karbi, Mishing and Dimasa, the plant Aristolochia griffithii is used against insect and snake bite, skin problem, stomach problems and fever. However, Adi and Monpa tribes of Arunachal Pradesh use Thalictrum foliolosum DC for the treatment of nematode worms, stomach problem, fever and pain. Both the plants materials are used traditionally in treatment of malaria by the tribals of the region but experimental data to support the activity against malaria parasite is lacking. Therefore after collecting basic information about antimalarial activity of both the plant materials from the local tribals, we have evaluated the anti-plasmodial activity of A. griffithii, Hook and T. foliolosum DC extracts in vitro against P. falciparum in the present study.
Methods
Plants and chemicals
Aristolochia griffithii Hook, commonly called Nagbal, was collected from Kalamati foot Hills of Sonitpur district of Assam, whereas Thalictrum foliolosum DC, commonly called Yangchira, was collected from the hills of Bomdila, West Kameng district of Arunachal Pradesh (Table 1). The aqueous extracts of root portion of both the plants has been used in the traditional treat of malaria for many years. Therefore, roots were used as testing material in the present study. The information about the use of plants in malaria treatment was gathered from locals through group discussions. The plants used in the study were identified by Dr. Ashish Kar of Tata Energy Research Institute, Guwahati, India and the voucher specimens of the plant material have been preserved in Defence Research Laboratory, Tezpur, India. Chloroform, ethyl acetate and n-butanol extracts were prepared using HPLC grade chemicals obtained from Merck, India Ltd., whereas water extracts were prepared using Millipore (Merck Millipore, USA) filtered water.
Preparation of extracts and maintenance of P. falciparum culture
Plant materials were dried under the shade and grinded using an electric grinder. One hundred gram of individual material was macerated into 1 L of each solvent and thoroughly mixed using a sterilized glass rod at room temperature. The solution was kept for 24 h and mixed after every 2 h. Extracts were filtered through Whatman No. 1 filter paper, freeze dried and kept at 4 °C in well-closed containers for use in the anti-plasmodial assay. The extracts were dissolved in dimethylsulfoxide (DMSO) at concentration of 1 mg/ml and then diluted with incomplete medium (without serum) to achieve required concentrations of 0.1, 0.5, 1, 5, 10, 20 and 30 μg/ml for evaluation against known P. falciparum chloroquine sensitive strain, 3D7 (SS) and chloroquine resistance strains, LS1 (RS). P. falciparum culture was maintained in vitro on human erythrocytes (blood group O+) in RPMI-1640 medium (Sigma) supplemented with 10 % AB+ human serum, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES buffer; Sigma Aldrich), 25 mM NaHCO3 and 60 µg/ml gentamicin sulfate (7.2 pH) at 37 °C and 5 % CO2 in a CO2 incubator (HERA cell 240i, Thermo Scientific).
P. falciparum sensitivity study
Plasmodium falciparum was synchronized for the ring stages [20] and diluted with fresh uninfected human RBC’s to adjust the level of parasitaemia to 1000–8000/µl of blood to study the sensitivity against plant extracts. The tests were done in 96 well microtitre plates for 24–36 h in duplicate following standard methods [21, 22]. An aliquot of parasite culture (100 µl) was added into the each well of the plate and then 100 µl of incomplete culture medium containing extracts at various concentrations was added into the well plates. Titre plates were kept in CO2 incubator at 37 °C for 24–36 h. After 24–36 h, the growth was monitored by preparing thick smear from each test as well as from the control well. The films were stained 10 % Giemsa solution (pH 7.3) and observed under light microscope (100×). The number of schizonts was counted per 200 asexual stages of parasite and values were compared between test wells and control well. The percent inhibition of parasite was calculated as follows:
where S = (No. of schizonts in the test well/No. of schizonts in control well) × 100 [23].
The concentration at which 50 % inhibition obtained was recorded as IC50 value and determined using log dose probit method.
Data analysis
Comparison of IC50 values among sensitive and resistant strain and two extracts was done using paired students ‘t’ test, while among different extracts were made using one way Krusal Wallis (K) test following Dunn test of multiple comparison. All the data obtained were tested for normality using Kolmogorov and Smirnov (KS) method.
Results
The results of in vitro antimalarial evaluation of A. griffithii have been shown in (Table 2). In SS, the IC50 concentration for ethyl acetate extracts was 6.2 ± 0.02 μg/ml and found to be statistically lower than the chloroform extracts, which exhibited IC50 value of 14.1 ± 0.1 μg/ml (t = 191.1; p < 0.0001). Similarly, for RS, the IC50 value for ethyl acetate extracts was lower as compared to chloroform extracts, however the difference was not very much significant (t = 3.6; p = 0.005). Furthermore, the IC50 values of both chloroform and ethyl acetate extracts for RS were higher as compared to the SS (t = 49.6; p < 0.0001 for chloroform; t = 223.8; p < 0.0001 for ethyl acetate). Water soluble extracts and n-butanol extracts of A. griffithii did not show considerable activity. The IC50 values of water extracts for SS and RS strains were 274.0 ± 8.2 and 390.3 ± 1.7 respectively, while the IC50 concentration of n-butanol extracts for SS and RS were 90.7 ± 1.1 and 139.7 ± 0.3 μg/ml respectively.
The extracts of T. foliolosum DC were also found to be effective against P. falciparum in the present study (Table 3). The IC50 concentration of T. foliolosum DC chloroform extracts for SS and RS were 0.5 ± 0.0 and 1.1 ± 0.0 μg/ml respectively, which differed statistically (t = 54.2; p < 0.0001). For SS, the IC50 value was found to be lowest in chloroform extracts and higher in ethyl acetate extracts (K = 21.7; p < 0.0001). The chloroform extracts and n-butanol extracts showed antimalarial inhibition at very low concentration, however significant different was observed between the IC50 values of both the extracts for SS (t = 189.3; p < 0.0001) and RS (t = 405.1; p < 0.0001). The water soluble extracts exhibited an IC50 concentration of 11.0 ± 0.1 and 10.8 ± 0.1 μg/ml for SS and RS respectively, indicating that the activity of water extracts was higher against RS as compared to the SS (t = 5.8; p = 0.001).
Discussion
Although many antimalarial drugs have been proved to be very effective against malaria parasite, but emerging resistance to these drugs have highlighted the need for new therapeutic agents, which could replace existing antimalarial drugs, if required. In this investigation, in vitro anti-plasmodial activity of two Indian medicinal plants, A. griffithii and T. foliolosum DC root extracts was studied against a well known human malaria parasite. The plant material extracts were found to be effective against SS as well as RS of P. falciparum parasite. In A. griffithii, the chloroform and ethyl acetate extracts showed IC50 values of 14.1 ± 0.1 and 6.2 ± 0.0 for SS and 16.2 ± 0.0 and 16.0 ± 0.10 for RS respectively. However the IC50 values for n-butanol and water extracts were significantly high as compared to the chloroform and ethyl acetate extracts, indicating that both chloroform and ethyl acetate extracts have high parasiticidal activity and were more effective against the malaria parasite. A. griffithii is a shrub climbing plant with heart shape leaves, approximately 10–28 cm long, 8–26 cm wide and distributed at an altitude of 2000–2900 m. This plant is perennial, deciduous and fragrant and has abundant distribution in Arunachal Pradesh and Sikkim states of northeastern India. Many species of Aristolochia has been used in traditional medicine for the treatment of seizures, snakebite, intestinal pain, gallbladder pain, arthritis, gout, rheumatism, eczema, weight loss and wounds, however the antimalarial activity of this plant has not been evidenced by any scientific experimentation [24].
Further, in T. foliolosum DC, the chloroform and n-butanol extracts were more effective than ethyl acetate and water soluble extracts against both SS and RS of P. falciparum. The chloroform extracts of T. foliolosum DC were found to have highest activity against P. falciparum in present study, as the IC50 values for chloroform extracts against SS and RS were 0.5 ± 0.0 and 1.1 ± 0.0 respectively. T. foliolosum DC is a perennial plant with a height of about 2.5 m. The plant is distributed in the temperate Himalayas from 1500 to 2400 m, in the Khasi hills and in Kashmir, Punjab, Delhi, Uttar Pradesh, Bihar and Orissa. The root contains alkaloids, which has a stimulant action on the movements of the gastrointestinal tract, a depression of both the auricles and ventricles, distinct dilatation of the heart and induces hypotension [17]. T. foliolosum DC is a medicinally important plant and has been used as antipyretic, diuretic, febrifuge, ophthalmic, purgative and stomachic. It is considered to be a good remedy for atonic dyspepsia and is also useful in treating peptic ulcers, indigestion, toothache, hemorrhoids, for convalescence after acute diseases and liver disorders. The juice of the leaves is applied to boils and pimples [17]. Many studies have evaluated the antimalarial activity of plant derivatives and indicated their efficacy against the Plasmodium species using in vivo and in vitro methods [2, 8–10, 14, 15, 18, 19]. A recent study conducted in Thailand has suggested the promising activity of Plumbago indica Linn against both chloroquine resistant and chloroquine-sensitive strains of P. falciparum [25]. The study also indicated that the activity of plumbagin was relatively higher against chloroquine-resistant P. falciparum as compared to the chloroquine-sensitive P. falciparum, which suggests that the plants based compound may be more useful in clearing the resistant malaria parasite.
The majority of extracts used in the current study displayed convincing in vitro anti-plasmodial activity against known chloroquine susceptibility and resistant strains. The aqueous extracts and decoction of both the plant materials are traditionally used in malaria treatment in the study areas, however the organic extracts displayed excellent anti-plasmodial activity currently compared to aqueous counterparts. The present study using two indigenous plants has indicated that both have intrinsic anti-plasmodial activity. These plants have been used in the treatment of human malaria for many years by the tribes of northeastern India, however the current results have provided the scientific reason behind the folkloric use of A. griffithii and T. foliolosum DC in the treatment of malaria.
Conclusion
Continuous spreading of multi-drug resistant malaria parasite has entailed that there is a need of exploring and evaluating new antimalarial molecules to combat malaria. Current study, aimed for searching new and effective anti-plasmodial drugs, indicated the potential in vitro antimalarial activity of A. griffithii and T. foliolosum DC collected from the northeastern region of India. Although these plants have been used in traditional medicine system of the region, but the antimalarial activity has been demonstrated for the first time. The selected extracts of the plants were very effective against SS and RS of P. falciparum in laboratory condition, which may be exploited for the treatment of chloroquine sensitive as well as resistance malaria patients. The present findings, however preliminary, but scientifically evidences the antimalarial potential of both the plants evaluated currently. Identification, isolation of active compound and testing against different Plasmodium species could lead to the development of more effective antimalarials for the future.
Abbreviations
- SS:
-
chloroquine sensitive strain
- RS:
-
chloroquine resistance strain
- IC:
-
inhibitory concentration
- WHO:
-
World Health Organisation
- μg:
-
microgram
- ml:
-
mililitre
- SP:
-
sulphadoxine–pyrimethamine
- ACT:
-
artesunate-based combination therapy
- HPLC:
-
high performance liquid chromatography
- DMSO:
-
dimethylsulfoxide
- RPMI:
-
Roswell Park Memorial Institute medium
- RBC:
-
red blood corpuscles
- KS:
-
Kolmogorov Smirnov
References
World Health Organization. World Malaria Report. Geneva: WHO; 2015.
Bhat PG, Surolia N. In vitro antimalarial activity of extracts of three plants used in the traditional medicine in India. Am J Trop Med Hyg. 2001;65(4):304–8.
Miller LH. The challenge of malaria. Science. 1992;257:36–7.
Vial H. Recent development and rationale towards new strategies for malarial chemotherapy. Parasite. 1996;3:3–23.
Wernsdorfer WH, Trigg PI. Recent progress of malaria research: chemotherapy. In: Wernsdorfer WH, McGregor I, editors. Malaria principle and practice of malariology (2). London: Churchill Livingstone; 1988. p. 1569–674.
Andrade-Neto VF, Brandao MG, Stehmann JR, Oliveira LA, Krettli AU. Antimalarial activity of Cinchona-like plants used to treat fever and malaria in Brazil. J Ethnopharmacol. 2003;87:253–6.
Saxena NPS, Jain DC, Bhakuni RS. Antimalarial agents from plant sources. Curr Sci. 2003;85:1314–29.
Bagavan A, Rahuman AA, Kaushik NK, Sahal D. In-vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol Res. 2011;108(1):15–22.
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. In-vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol. 2004;92:177–91.
Ramazani A, Zakeri S, Sardari S, Khodakarim N, Djadid ND. In-vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malaria J. 2010;9:124.
Goswami D, Dhiman S, Rabha B, Kumar D, Baruah I, Veer V, Bhola RK, Sharma DK. High prevalence of pfcrt K76T and mdr1 N86Y mutations in Sonitpur district of Assam. India. J Parasit Dis. 2013. doi:10.1007/s12639-013-0298-1.
Nath MJ, Bora AK, Yadav K, Talukdar PK, Dhiman S, Baruah I, Singh L. Prioritizing areas for malaria control using geographical information system in Sonitpur district, Assam. India. Public Health. 2013;127:572–8.
Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–5.
Azas N, Laurencin N, Delmas F, Di Giorgio C, Gasquet M, Laget M, Timon-David P. Synergistic in vitro antimalarial activity of plant extracts used as traditional herbal remedies in Mali. Parasitol Res. 2002;88:165–71.
Caraballo A, Caraballo B, Rodriguez-Acosta A. Preliminary assessment of medicinal plants used as antimalarial in the Southeastern Venezuelan Amazon. Rev Soc Bras Med Trop. 2004;37(2):186–8.
Shankar R, Deb S, Sharma BK. Anti-malarial plants of NE India: an overview. J Ayurveda Integr Med. 2012;3(1):10–6.
Shankar R, Devalla RB. Conservation of folk healing practices and commercial medicinal plants with special reference to Nagaland. Int J Biodiv Conserv. 2012;4(3):155–63.
Namsa ND, Mandal M, Tangjang S, Mandal SC. Ethnobotany of the Monpa ethnic group at Arunachal Pradesh. India. J Ethnobiol Ethnomed. 2011;7:31.
Namsa ND, Mandal M, Tangjang S. Anti-malarial herbal remedies of northeast India, Assam: an ethnobotanical survey. J Ethnopharmacol. 2011;133(2):565–72.
Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65(3):418–20.
WHO. In vitro micro test (Mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine aartemisinin. Geneva: WHO.CTD/MAL/97,20; 2001.
Rickman K, Campbell HGH, Sax L, Mrema JE. Drug sensitivity of Plasmodium falciparum. An in vitro microtech. Lancet. 1978;1:22–3.
Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nature Rev Drug Disc. 2004;3:509–20.
Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MS. Local uses of Aristolochia species and content of nephrotoxic ristolochic acid 1 and 2—a global assessment based on bibliographic sources. J Ethnopharmacol. 2009;125(1):108–44.
Sumsakul W, Plengsuriyakarn T, Chaijaroenkul W, Viyanant V, Karbwang J, Na-Bangchang K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Comp Alternat Med. 2014;14:15.
Authors’ contributions
NG, BR and PKT conceived the idea. BR, DG, NG and PKT collected the plant material and conducted the experimental study. DG, SD and BR interpreted and analysed the data. SD, BR prepared the manuscript. NG, DG and PKT critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Authors are thankful to Dr. Kavita Yadav of Defence Research Laboratory, Tezpur, India for inputs and support in revising the manuscript for important intellectual content. Technical help of Dr. Ashish Kar of Tata Energy Research Institute, Guwahati, India in identifying the plant material is highly acknowledged. The help rendered by the local village heads and villagers in during the study is also acknowledged.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Das, N.G., Rabha, B., Talukdar, P.K. et al. Preliminary in vitro antiplasmodial activity of Aristolochia griffithii and Thalictrum foliolosum DC extracts against malaria parasite Plasmodium falciparum . BMC Res Notes 9, 51 (2016). https://doi.org/10.1186/s13104-016-1862-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-016-1862-4