Abstract
Background
Hypertension, type 2 diabetes, and cardiovascular disease affect the activities of daily living at varying degree. While the effects of aerobic exercise on functional capacity are well-documented, the extent of change for different types of exercise in these chronic conditions remains unexplored. Additionally, there is conflicting evidence regarding the role of exercise in reducing body weight.
Methods
We conducted systematic review with meta-analysis and trial sequential analysis and searched various databases from inception to July 2020. We included randomised clinical trials adding any form of trialist defined exercise to usual care versus usual care in people with either hypertension, type 2 diabetes, and/or cardiovascular disease irrespective of setting, publication status, year, and language. The outcomes assessed were i) functional capacity assessed through different scales separately i.e., Maximal Oxygen Uptake (VO2max), 6-min walk test (6MWT), 10-m walk test (10MWT), and ii) body weight.
Results
We included 950 studies out of which 444 trials randomising 20,098 participants reported on various functional outcomes (355 trials) and body weight (169 trials). The median follow-up was 3 months (Interquartile ranges (IQR): 2.25 to 6). Exercise added to the usual care, improved VO2max (Mean Difference (MD):2.72 ml/kg/min; 95% Confidence Interval (CI) 2.38 to 3.06; p < 0.01; I2 = 96%), 6MWT (MD: 42.5 m; 95%CI 34.95 to 50.06; p < 0.01; I2 = 96%), and 10MWT (MD: 0.06 m/s; 95%CI 0.03 to 0.10; p < 0.01; I2 = 93%). Dynamic aerobic and resistance exercise showed a consistent improvement across various functional outcomes, whereas body-mind therapies (MD: 3.23 ml/kg/min; 95%CI 1.97 to 4.49, p < 0.01) seemed especially beneficial for VO2max and inspiratory muscle training (MD: 59.32 m; 95%CI 33.84 to 84.80; p < 0.01) for 6MWT. Exercise yielded significant reduction in body weight for people with hypertension (MD: -1.45 kg; 95%CI -2.47 to -0.43; p < 0.01), and type 2 diabetes (MD: -1.53 kg; 95%CI -2.19 to -0.87; p < 0.01) but not for cardiovascular disease with most pronounced for combined exercise (MD: -1.73 kg; 95%CI -3.08 to -0.39; p < 0.05). The very low certainty of evidence warrants cautious interpretations of the results.
Conclusion
Exercise seemed to improve functional capacity for people with hypertension, type 2 diabetes, and/or cardiovascular disease but the effectiveness seems to vary with different forms of exercise. The potentially superior improvement in VO2max and 6MWT by body-mind therapies and inspiratory muscle training calls for further exploration. Additionally, prescribing exercise for the sole purpose of losing weight may be a potential strategy for people with hypertension and type 2 diabetes. The extent of improvement in functional capacity and body weight reduction differed with different exercise regimens hence personalised exercise prescriptions tailored to individual needs may be of importance.
PROSPERO registration
PROSPERO registration number: CRD42019142313.
Similar content being viewed by others
Background
Functional capacity in broad terms refers to an individual’s ability to perform physical tasks such as walking, climbing, and other daily activities without experiencing undue fatigue or physical stress [1]. Hypertension, type 2 diabetes, and cardiovascular disease are the leading non-communicable disease globally that affect the activities of daily living at varying degree [2]. For instance, patients with cardiovascular disease, especially for conditions like coronary artery disease, heart failure, or cardiomyopathy is characterized by reduced cardiac output leading to shortness of breath, fatigue and muscle weakness depending on severity of the condition [1, 3]. Likewise, the most common consequence of stroke leads to hemiparesis or spasticity which limits individual’s mobility and may have severe cognitive impairment affecting the autonomy in activities of daily living [4]. Hypertension may lead to hypertension-related structural and functional changes in target-organs like heart, kidneys and brain [5]. impacting an individual’s stamina and mobility. In case of type 2 diabetes, complications like neuropathy can hamper neuromuscular function leading to difficulties in walking and other fine motor skills [6].
Evidence have shown that impaired functional capacity is an effective predictor of cardiovascular disease risk and even mortality [7, 8]. Functional capacity is objectively measured through maximal/peak oxygen uptake (VO2max). VO2max is the uptake or consumption of maximal oxygen during exercise and is considered gold standard to evaluate individuals’ cardiovascular fitness level. Walk tests such as six-minute walk test (6MWT), or ten-meter walk test (10MWT) [8,9,10]. are comparatively simple, inexpensive, safe, and reproducible tools for assessing aerobic fitness [8, 11]. Apart from this, muscular strength and balance are other important elements of functional capacity [12].
Regular exercise is considered an important element in enhancing functional capacity for individuals with hypertension, type 2 diabetes, and cardiovascular disease. These three conditions, while distinct, share a common underlying pathophysiology in how exercise influences overall wellbeing [13]. Previous reviews have reported the beneficial effect of exercise in increasing functional capacity for hypertension [14]. type 2 diabetes [15]. or cardiovascular disease [1, 8, 16]. However, such findings are often limited to common forms of exercise like aerobic or resistance exercise, and the effect of diverged forms of exercise remains inconsistent. Thus, an umbrella summary of the effect of different types of exercise on functional capacity in these cardiometabolic conditions seems necessary. We have not identified any systematic reviews that have included all forms of exercise and comprehensively assessing the effect on different functional capacity outcomes.
Additionally, individuals with hypertension, type 2 diabetes, and cardiovascular disease are recommended to engage in exercise not only for maintaining a healthy body weight but also for weight reduction [17,18,19]. particularly for overweight and obese individual [20, 21]. There is evidence both supporting and contradicting [20, 22,23,24]. the effectiveness of exercise as a standalone strategy for reducing body weight. Thus, an overview of different forms of exercise on body weight for these leading cardiometabolic conditions could significantly contribute to the existing pool of evidence.
Therefore, we aimed to perform a systematic review with meta-analysis and trial sequential analysis to assess the effect of different forms of exercise on functional capacity and body weight for people with hypertension, type 2 diabetes or cardiovascular disease when added to their usual care. Additionally, we also wanted to investigate if the exercise induced changes in functional capacity can explain the reduction in all-cause mortality reported in our previously published paper [25].
Methods
We described our methodology in detail in our protocol registered and published prior to the systematic literature search [26]. We reported this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [27]. We included all randomised clinical trials assessing the effect of adding any of form exercise (as defined by trialists) to usual care (as defined by trialists-any routine care received by the patients) versus usual care (same usual care as in the intervention group). We included any form of co-interventions, if the co-intervention is intended to be delivered similarly to the intervention and control groups. We included people with either hypertension, type 2 diabetes, and/or cardiovascular disease irrespective setting, trial duration, publication status, publication year, and language.
We searched the database Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), Science Citation Index Expanded on Web of Science, BIOSIS, google scholar and clinicaltrials.gov from inception till July 2020. Additionally, we also manually searched reference lists of previously published reviews for relevant publications.
The detailed search strategy can be found in (text S1).
Data extraction strategy
We extracted data using standardised data extraction sheet. Five authors (AR, TBA, SD, MM, RP) extracted information on trials’ characteristics (gender, country, number of participants in intervention and control, length of intervention, follow-up period, baseline information- age, body mass index, medication) and characteristics of exercise intervention such as type of exercise, volume of exercise (hours/week), intensity of exercise.
Information on exercise intensity if not explicitly mentioned in the trials were categorised to low, moderate or vigorous based on Oxygen uptake Reserve (VO2R%), Heart Rate Reserve (HRR%), Age- predicted maximal heart rate (HRmax%), Ratings of perceived exertion (RPE) parameters as per guidelines presented in General Principles of Exercise Prescription [28]. which has been adapted from American College of Sports Medicine Guidelines for Exercise Testing, 8th edition [29]. and Prescription and Physical Activity Guidelines Advisory Committee Report, USA [30]. We resolved disagreements through discussion or consulting with a third author (JCJ or EEN). If data were missing or unclear, we attempted to contact authors through email.
Risk of bias
We assessed risk of bias using the Cochrane Risk of Bias- version 1 (RoB1) [31]. and assessed the following bias domains: random sequence generation, allocation concealment, blinding of people and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, for profit bias, and other risks of bias. We classified trials as being at overall “high risk of bias”, if any of the bias domains are classified as “unclear” or “high risk of bias”.
Outcomes and subgroup analyses
Our outcomes were i) functional capacity assessed through VO2max (ml/kg/min), 6MWT (m), 10MWT/Gait velocity (m/s), Berg balance scale, Timed Up and Go Test (TUGT,seconds), Exercise Capacity (measured in Watt and MET) and ii) body weight (kg) reported at maximum follow-up.
Primary outcomes (all-cause mortality, serious adverse events, quality of life) and other secondary outcomes from this review has been published elsewhere [25].
We prespecified several subgroup analyses (see Results): 1) different types of exercise, 2) different disease groups (hypertension, type 2 diabetes, or cardiovascular disease as defined by trialists or cardiovascular disease as defined by WHO that includes cerebrovascular disease, rheumatic heart disease, deep vein thrombosis, pulmonary thrombosis, coronary artery disease such as myocardial infarction, and heart failure), 3) High Income countries (HICs) vs. Low-middle income countries (LMICs), 4) trials at high risk of bias compared to trials at low risk of bias.
In addition, we have added further post-hoc subgroup analyses: 1) trials including biological male compared to biological female compared to trials including both biological sexes, 2) short term follow up (≤ median follow-up) compared to long term follow up (> median follow-up). We also additionally conducted subgroup analysis for VO2max, 6 MWT, 10 MWT, and body weight based on 3) age in years (≤ median age compared to > median age) 4) baseline Body Mass Index (BMI) (normal < 25 kg/m2; overweight ≥ 25 to ≤ 29.9 kg/m2; obese ≥ 30 kg/m2) 5) size of trials (trials with ≤ 100 people compared to trials with > 100 people) 6) type of control (usual care compared to no intervention compared to co-intervention).
Data Analysis
We used STATA 17 (StataCorp) for all statistical analyses [32]. We considered a p value of 0.05 as the threshold for statistical significance for functional capacity and body weight due to the hypothesis generating nature of analyzing predefined exploratory outcomes [26]. We conducted both fixed-effect and random-effects meta-analysis and primarily reported the most conservative result and considered the less conservative result as sensitivity analysis [26, 33]. We analyzed different functional capacity measures separately to avoid the methodological problems with using standardized mean difference [34]. The predetermined minimal important difference for functional capacity and body weight was calculated as the mean difference of the observed Standard Deviation (SD) divided by two in the control group [33, 35]. We investigated possible heterogeneity by visual inspection of forest plots, by calculating inconsistency (I2), and by performing subgroup analysis (test of interaction).
In order to further assess the potential sources of heterogeneity we performed random effect stepwise meta-regression [36]. with forward selection. We regressed intervention/exercise specific co-variates (length of exercise program, volume of exercise) and patient specific co-variates (type of participants, age of participants and body mass index) separately (univariate regression) against the functional capacity measures and body weight to select variables for inclusion in meta-regression models. We used a significance level of 10% to select variables for the multivariable models; however only those with a p < 0.05 were considered significant in the final model. If one of the categories of categorical variable was statistically significant, all the categories of the variable were kept in the model.
Additionally, a random effect model regression analysis was performed to evaluate the association between change in VO2max and all-cause mortality, reported previously. The logarithm of relative risk of each trial was regressed against the difference in mean VO2max for participants assigned to exercise intervention and control group at the end of maximum follow-up and the statistically significant was assessed using the Wald test [36].
We assessed small study bias through funnel plots and regression asymmetry test (Egger’s test) [37]. We performed trial sequential analysis to control for the risks of type I errors and type II errors [38]. We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) to assess the certainty of evidence [39, 40].
Result
Characteristics of study
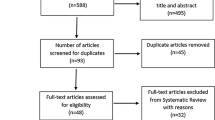
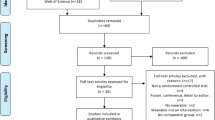
We identified 32,739 potentially relevant references through our literature search conducted on July 6, 2020. We included 950 studies out of which 444 unique studies randomising 20,098 people reporting on functional outcomes (355 trials) and body weight (169 trials) were meta-analysed (Fig. 1) in this review.
Most trials included both male and female participants. The number of people in each trial ranged from 10 [41]. to 380 [42]. The median intervention follow-up period was 3 months (IQR: 2.5 to 6 months). Most trials (60%) included people with cardiovascular diseases. The most frequently reported exercise intervention was dynamic aerobic exercise in (60%) trials. The majority of included trials (76%) were conducted in HICs (Table 1). The median duration of the exercise interventions was 135 min/week (IQR: 90 to 180 min/week) and intensity varied from low to vigorous. The mean age of participants in intervention group was 58.6 (± 8.3) years and they were overweight with a BMI of 28.7 (± 5.4) kg/m2. The baseline characteristics of included studies is presented in Table 2. Further information on included trials is described in Table S1.
Effect of exercise on VO2max
A total of 251 studies randomising 11,075 people reported on VO2max with median follow-up of 3 months (IQR: 2.5 to 7.5). Meta-analysis showed that exercise significantly improve VO2max (MD: 2.72 ml/kg/min; 95%CI 2.38 to 3.06; p < 0.01) and the effect is higher than predetermined level of minimal importance (2 ml/kg/min). Visual inspection of forest plot (Fig. 2) and I2 statistics indicated substantial signs of heterogeneity which could not be resolved (I2 = 96.6%). Trial sequential analysis showed that there was enough information to confirm that exercise improved VO2max (Fig. 3). Funnel plot and egger’s test (p = 0.49) indicated no small study bias (Figure S1). We assessed this outcome result as high risk of bias (Figures S2 and S3) and the certainty of evidence as very low (Table 3).
Test of interaction showed evidence of difference when comparing trials randomising different types of exercise (Q = 31.91; p < 0.05) (Fig. 4). When analysed separately, the meta-analysis showed that exercise improved VO2max for participants following body mind therapies(MD: 3.23 ml/kg/min; 95%CI 1.97 to 4.49, p < 0.01), dynamic aerobic exercise (MD: 3.09 ml/kg/min; 95%CI 2.67 to 3.50; p < 0.01), dynamic resistance exercise (MD: 1.58 ml/kg/min; 95%CI 0.74 to 2.41; p < 0.01) and combined exercise(MD: 2.09 ml/kg/min; 95%CI 1.34 to 2.84; p < 0.01), but not for inspiratory muscle training (MD: 0.79 ml/kg/min; 95%CI -0.02 to 1.59; p = 0.05) and isometric resistance exercise (MD: 1.85 ml/kg/min; 95%CI -0.38 to 4.08; p = 0.10).
None of the remaining planned subgroup analysis showed evidence of a difference (Fig. 4 and Table S2).
Effect of exercise on 6MWT
A total of 117 trials randomising 6,301 people reported on 6MWT with median follow up of 3 months (IQR: 2.5 to 6 months). Meta-analysis showed that exercise significantly improve 6MWT (MD: 42.5 m; 95%CI 34.95 to 50.06; p < 0.01). The pre-determined minimal important difference 45 m lies within the CI of the effect estimate. Visual inspection of forest plot (Fig. 5) and I2 statistics indicated substantial heterogeneity which could not be resolved (I2 = 93.5%). Trial sequential analysis showed that there was enough information to confirm that exercise improved 6MWT (Fig. 6). Funnel plot and egger’s test (p = 0.36) indicated no small study bias (Figure S4). We assessed this outcome result as high risk of bias (Figures S5-S6) and the certainty of evidence as very low (Table 3).
Test of interaction showed evidence of difference when comparing trials randomising different types of exercise (Q = 13.31; p < 0.05) (Fig. 7) When analysed separately, the meta-analysis showed that inspiratory muscle training (MD: 59.32 m; 95%CI 33.84 to 84.80; p < 0.01), dynamic aerobic exercise (MD: 45.57 m; 95% CI 35.62 to 55.52; p < 0.01), combined exercise (MD: 45.45 m; 95%CI 28.48 to 62.42; p < 0.01), dynamic resistance exercise (MD: 35.21 m; 95%CI 8.60 to 61.82; p < 0.05) improved 6MWT, but body mind therapies (MD: 11.11 m; 95%CI -8.09 to 30.32;p = 0.25) and stroke functional exercise (MD: 20.86 m; 95%CI -23.94 to 65.66; p = 0.36) did not.
Test of interaction showed evidence of difference when comparing trials randomising baseline BMI category (117 trials; Q = 6.44, p < 0.05). When analyzed separately, the meta-analysis showed greater improvement in 6MWT for people with obesity (MD = 57.4 m; 95%CI 29.74 to 85.06; p < 0.01) and overweight (MD: 50.09 m; 95% CI 32.34 to 67.86; p < 0.01) compared to people with normal BMI (MD: 20.96 m; 95%CI 1.52 to 40.4; p < 0.05).
None of the remaining planned subgroup analysis showed evidence of a difference (Fig. 7 and Table S3).
Effect of exercise on 10MWT
A total of 39 trials randomising 2646 people reported on 10 MWT with median follow up of 3 months (IQR: 1 to 6.5 months). Meta-analysis showed that exercise significantly improved 10MWT (MD: 0.06 m/s; 95%CI 0.03 to 0.10; p < 0.01), but the effect was lower than the predetermined minimal clinical important difference (0.14 m/s). Visual inspection of forest plot (Fig. 8) and I2 statistics indicated substantial signs of heterogeneity which could not be resolved (I2 = 89.6%). Trial sequential analysis showed that there was not enough information to confirm that exercise improved 10MWT (Fig. 9). Funnel plot and egger’s test (p = 0.05) indicated no small study bias (Figure S7). We assessed this outcome result as high risk of bias (Figures S8 and S9) and the certainty of evidence as very low (Table 3).
Test of interaction showed evidence of difference when comparing trials randomising different types of exercise Q = 14.89; p < 0.05 (Fig. 10). When analysed separately, the meta-analysis showed that exercise improved 10MWT for participants following stroke functional exercise (MD: 0.18 m/s; 95% CI 0.09 to 0.27; p < 0.01), dynamic resistance exercise (MD: 0.07 m/s; 95%CI 0.006 to 0.14; p < 0.05), dynamic aerobic exercise (MD: 0.06 m/s; 95%CI 0.01 to 0.12; p < 0 0.05) but not for combined exercise (MD: 0.03 m/s; 95%CI -0.01 to 0.15; p = 0.69), body mind therapies (MD: -0.1 m/s; 95%CI -0.23 to 0.03;p = 0.14) nor isometric resistance exercise(MD: -0.1 m/s; 95%CI -0.34to 0.14; p = 0.42).
Test of interaction showed evidence of difference when comparing trials randomising different types of participants Q = 5.27; p < 0.05 (Fig. 10). When analysed separately, the meta-analysis showed that exercise improved 10MWT for people with cardiovascular disease (MD: 0.07 m/s; 95% 0.03 to 0.11; p < 0.01), but not for people with type 2 diabetes (MD: -0.05 m/s; 95% CI -0.15 to 0.05; p = 0.29). There were no trials including people with hypertension.
None of the remaining planned subgroup analysis showed evidence of a difference (Fig. 10 and Table S4).
Effect of exercise on other functional outcomes
Meta-analyses of other scales namely Berg Balance Scale (MD: 2.90; 95%CI 2.01 to 3.79; p < 0.01; I2 = 86.3%; 36 trials), Exercise Capacity(Watt) (MD: 23.76 W; 95%CI 16.87 to 30.64; p < 0.01; I2 = 90%; 8 trials), and Exercise Capacity(Metabolic Equivalent of Task(MET)) (MD: 1.24 MET; 95%CI 0.67 to 1.82; p < 0.05; I2 = 57.2%; 6 trials) reported statistically significant improvements in functional capacity after exercise intervention, but not for TUGT scale (MD: -1.88 s; 95%CI -3.86 to 0.09; p = 0.06; I2 = 97.9%; 15 trials).
All meta-analyses and the corresponding figures are included in Figures S10-S13.
Effect of exercise on body weight
One hundred sixty-nine trials randomising 7,535 people reported on body weight with median follow up of 3 months (IQR: 3 to 6 months). Meta-analysis showed that exercise did not significantly reduce the body weight (MD: -1.42 kg; 95%CI -1.91 to -0.92; p < 0.01) and the estimate was far below the pre-determined minimal clinical important difference (-5 kg). Visual inspection of forest plot (Fig. 11) and I2 statistics indicated substantial signs of heterogeneity which could not be resolved (I2 = 86.5%). Trial sequential analysis showed that there was enough information to confirm that exercise reduced body weight (Figs. 12). Funnel plot and egger’s test (p = 0.30) indicated no small study bias (Figure S14). We assessed this outcome result as high risk of bias (Figures S15 and S16) and the certainty of evidence as very low (Table 3).
Test of interaction showed evidence of difference when comparing trials randomising different type of people (Q = 24.56; p < 0.05) (Fig. 13). When analysed separately, the meta-analysis showed exercise significantly reduced body weight for people with hypertension (MD: -1.45 kg; 95%CI -2.47 to -0.43; p < 0.01), people with type 2 diabetes (MD: -1.53 kg; 95%CI -2.19 to -0.87; p < 0.01) and people with hypertension and type 2 diabetes (MD: -3.48 kg; 95%CI -4.15 to -2.81; p < 0.01), but not for people with cardiovascular diseases (MD: -0.87 kg; 95%CI -2.20 to 0.35; p = 0.15).
Test of interaction showed evidence of difference when comparing trials randomising different type of exercise (Q = 14.70; p < 0.05) (Fig. 13). When analysed separately, the meta-analyses showed exercise significantly reduced body weight following combined exercise (MD: -1.73 kg; 95%CI -3.08 to -0.39; p < 0.05), body mind therapies (MD: -1.63 kg; 95%CI -3.14 to -0.12; p < 0.05), and dynamic aerobic exercise (MD: -1.50 kg; 95%CI -2.16 to -0.84; p < 0.01) but not for dynamic resistance exercise (MD: -0.04 kg; 95%CI -0.65 to 0.58; p = 0.91) and isometric resistance exercise (MD: -2.60 kg; 95% CI -5.30 to 0.10; p = 0.06).
Test of interaction showed evidence of difference when comparing trials randomising people with different baseline BMI category (58 trials; Q = 12.96, p < 0.05) (Table S5). When analysed separately, the meta-analysis showed exercise significantly reduced body weight for people with normal BMI (MD: -3.09 kg; 95%CI -5.53 to -0.64; p < 0.05) and people with overweight BMI (MD: -2.86 kg; 95%CI -4.24 to -1.48; p < 0.01) but not for obese people (MD: 0.35 kg; 95%CI -0.96 to1.65; p = 0.60).
None of the remaining planned subgroup analysis showed evidence of a difference (Fig. 13 and Table S5).
Meta-regression
For the outcomes VO2max, 6MWT, 10MWT, and body weight the meta-regression on exercise led change in effect estimate, we did not observe statistically significant regression coefficients using intervention specific co-variates (length of exercise program, volume of exercise) and patient specific co-variates (type of participants, age, body mass index) (Tables S6-S9).
Additionally, A total of 27 out of 251 trials reporting VO2max also reported on all-cause mortality. Meta regression showed that the exercise-induced change in VO2max was not significantly associated with decrease in risk of all-cause mortality (RR: 0.94; 0.82 to 1.09; p = 0.423).
Discussion
In this review, we analysed 355 trials assessing the effects of exercise on functional capacity and 169 trials assessing the effects of exercise on body weight. Our meta-analyses showed that exercise added to the usual care improved functional capacity as measured by VO2max, 6MWT and 10MWT for people with hypertension, type 2 diabetes, and/or cardiovascular disease. The effect estimates for VO2max and 6MWT was higher than the pre-determined minimal important difference but not for 10MWT and body weight as the effect sizes were small and may be clinically minimal. The effectiveness of improvement in functional outcomes varied with different modalities of exercise but it was notable that dynamic aerobic exercise, dynamic resistance exercise was found to consistently improve various functional capacity outcomes. Body mind therapies and inspiratory muscle training reported greater improvement for VO2max and 6MWT respectively compared to other forms of exercise. The observed estimates for functional capacity outcomes were independent of follow up duration, economic region, age of participants, size of trials and baseline BMI. Additionally, exercise added to usual care seemed to reduce body weight for people with hypertension and type 2 diabetes but not for people with cardiovascular disease and the reduction was higher for combined exercise and people with normal or overweight BMI but not for obese individual. However, it is important to acknowledge that the very low certainty of evidence underscores careful interpretation of the summarised evidence.
Effect of exercise in functional outcomes
Exercise-induced improvement in VO2max and 6MWT was higher than the predetermined level of minimal important difference indicating clinical significance of the reported estimate. The VO2max reported here is lower than previous meta-analysis that reported increment in cardiorespiratory fitness of 3.5 ml/kg/min (≈1 MET) lowered the risk of all-cause mortality and cardiovascular disease by 13% and 15% among healthy people [43]. However, even the modest improvement in 1–2 ml/kg/min VO2max has been associated with lowering clinical outcomes and better cardio-respiratory fitness among people with cardiovascular disease [44, 45]. and hypertension [7].
Likewise, our reported estimate on exercise-led improvement of 6MWT is similar to another meta-analysis assessing exercise-based rehabilitation for heart failure [46]. In case of 10MWT, though statistically significant was lower than minimal important difference predetermined in this review so it may have minimal clinical relevance. However, this could also be due to the inherent limitation of minimal clinical importance difference used in our review which is based on distributional method- Cohen’s D definition i.e., SD/2 in the control group [35]. Nevertheless, the estimates reported here is still much lower than minimal clinical importance difference obtained through distributional and discriminative methods for 0.16 m/s for the 10-m walk test in stroke patients [47].
Exercise-specific considerations
The subgroup analysis demonstrated evidence of a difference in different type of exercise on cardiorespiratory fitness measured by VO2max, 10MWT, and 6MWT. Higher improvement in VO2max was reported for body mind therapies and dynamic aerobic exercise. It is surprising that exercises like yoga and tai chi which includes both psychological and physical mechanisms may be as effective in improving cardiorespiratory fitness as dynamic aerobic exercise and even more effective than other prominent types of exercise. Though it should be noted that there were only five trials involving body mind therapies and information on the intensities of such kind of exercise were usually not mentioned in the trials hence comparison with other types of exercise could be futile. Studies have shown that dynamic aerobic exercise in general augment 10–30% of VO2max by increasing maximal stroke volume and arteriovenous oxygen difference [48].
Likewise, for 6MWT higher improvement was reported for inspiratory muscle training followed by dynamic aerobic exercise. However, there were only four studies assessing inspiratory muscle training so emergence of trials involving such exercise intervention will further add to our understanding of the effects of such trainings on functional capacity outcomes. Our result reported that exercise yielded greater improvement in 6MWT for obese and overweight individuals as compared to people with normal BMI. Thus, tailoring exercise interventions addressing the unique needs of obese and overweight individuals with coexisting cardiometabolic conditions can be particularly beneficial for improving functional capacity.
For 10MWT, larger improvement was reported for stroke functional exercise and patients with cardiovascular disease with majority being stroke patients. This specialized exercise regimen is specifically tailored to address the distinct functional limitations often severely experienced by stroke patient [49]. Common forms of exercise like aerobic exercise, resistance exercise and combination of both consistently reported improvement in functional capacity measures. Hence, the results reiterate that different forms of exercise can be recommended to individuals with hypertension, type 2 diabetes, and cardiovascular disease, but it should be prescribed with consideration of patient goals, preferences, and capabilities. Our results are concurrent with another meta-analysis that also showed that different forms of exercise have beneficial effect on improving functional capacity and reducing disability in patient with non-communicable diseases [50]. assessed through various functional capacity measures.
As the improvement of functional capacity differs according to type of exercise it is likely that the characteristics of exercise- frequency, intensity and duration may also explain the magnitude of change in functional capacity. However, in this review, the information on frequency, volume and intensity on exercise was sparse and our meta-regression among subset of trials where information was available did not show significant effect of length of intervention or volume of exercise on effect estimate. Existing evidence suggest that higher intensity exercise [51,52,53]. as compared to moderate intensity or traditional endurance training [54]. may elicit greater changes in cardiorespiratory fitness. A gradual progression to higher intensity may be more beneficial in reducing discomfort, maximizing safety and increasing adherence [53].
Functional Outcomes and all-cause mortality
We did not find significant association between exercise induced VO2 max increment and all-cause mortality published by us [25]. One of the reasons could be that only 27 trials reported both VO2max and all-cause mortality thus making the meta-analysis underpowered to detect any association between exercise induced VO2max changes and all-cause mortality. Moreover, a long term change in cardiorespiratory fitness may provide us with a more significant assessment of its association with all-cause mortality [55].
Effect of exercise on body weight
The meta-analysis showed that exercise when added to usual care seemed to reduce body weight minimally for people with hypertension and type 2 diabetes with normal or overweight BMI but not for cardiovascular disease and obese people. Empirical evidence suggests that even the modest reduction of 5–10% of initial body weight or weight loss of < -5 kg has been associated with clinically significant improvement in CVD risk factors for individuals with type 2 diabetes [21]. and adults with overweight and obese respectively [56]. while the extent of cardiovascular benefit of weight loss varies [57, 58]. This result further highlights the assertion that reducing body weight is often complex and strongly influenced by diet and genetics [23]. and exercise alone may not be sufficient [59]. One of the reasons for absence of reduced body weight especially for obese individuals with cardiovascular disease after exercise intervention could be attributed to the fact that exercise may reduce visceral body fat but at the same time increase muscle mass leading to no or insignificant loss in overall body weight [60]. Thus, prescribing exercise as a sole purpose of losing weight may not be an optimal strategy for obese patients with cardiovascular disease and may be a discouraging element in adherence to exercise regimen [61].
Like previous reviews, our result showed that the body weight reduction was more pronounced for combined exercise, body mind therapies and aerobic exercise and not for resistance and isometric exercise previous review [56]. While the comparable effect of body mind therapies presents a potential adjunct therapy to lose weight for people with hypertension, type 2 diabetes, and cardiovascular disease but the effect of such exercise as weight management strategy remains insufficiently investigated [62].
Implications for low- and middle-income countries
Our result did not suggest evidence of difference between trials from low- and middle-income countries compared to trials from high income countries for effect of exercise on functional capacity and body weight. The consistent beneficial impact of exercise on functional capacity across different economic contexts is promising; however, it is essential to acknowledge that this finding may be influenced by the limited statistical power of the analysis. It was important to note that the evidence generated from these regions were disproportionately fewer (25%) compared to high income countries. Specifically, for VO2max, a mere 20% (49 out of 251) of the included trials originated from low- and middle-income countries. This review like other studies [1, 63, 64]. reiterates the need of more representative data from low-and middle-income economic region to have a better understanding of the role of exercise in different socio-economic context.
Strengths of this review
The present review has various strengths. We followed the pre-published protocol, which was registered and published before the literature search ended [26]. We assessed the risk of bias using the Cochrane Risk of Bias version 1 tool and trial sequential analysis to control the risks of random errors. We included trials irrespective of any language, setting, or publication status. To minimise inaaccuracis in data extraction a team of five authors were involved in data extraction using standardized data extraction sheet and risk of bias assessment. We also did not identify signs of small study bias in our review. To our knowledge, the review is the first of its kind to assess the effect of all forms of exercise in people with hypertension, type 2 diabetes, or cardiovascular disease- the leading non-communicable disease on various functional capacity measures and body weight.
Limitations of this review
The limitation of this review needs to be considered. All the trials included in this review were assessed as having high risk of bias. For instance, in many of the trials, there was a lack of sufficient description regarding the random allocation of people and the concealment procedure. Additionally, participants in most trials were aware of their allocation to the exercise or control group, or the descriptions were unclear, which could have influenced the overall impact of the intervention. Furthermore, information on lost to follow-up and reporting bias due to selective outcome reporting was generally unavailable. Most of the trials were small, predominantly with less than 100 participants. Our post-hoc subgroup analysis did not show evidence of difference for functional capacity measures and body weight based on size of trials. It was also surprising to observe that the majority of the trials did not report on baseline characteristics, especially age, medication use, and details on exercise frequency, intensity, and adherence which further limits the discussion of the results. Though our meta-regression did not show significant effect of exercise-specific and disease-specific characteristics on functional capacity and body weight, but it has to be considered that inferences was limited due to lack of trials reporting those co-variates.
Furthermore, we have pooled different types of exercise and different people which may lead to clinical heterogeneity and hence needs to be considered while interpreting the results. Our results reported high statistical heterogeneity, but this phenomenon is inevitable for meta-analyses of continuous outcomes with a large number of trials [65]. We reported the results primarily based on the random-effects model while results from the fixed effect model have been presented as sensitivity analysis. The results for VO2max, 6MWT and body weight for both models were comparably similar however, the results varied for 10MWT indicating significant heterogeneity (text S3). Some of the heterogeneity was explained by several planned and post-hoc subgroup analyses, however, the heterogeneity could not be fully resolved even after multivariate meta-regression. We also acknowledge that the variation of usual care between trials may have impacted the estimates of functional capacity measures and body weight reported in this review. However, our post-hoc subgroup analysis did not suggest evidence of difference for different variation in control reported in this review (usual care/ no intervention /with co-interventions).
We did not find significant differences in exercise induced improvement in functional capacity between the different groups of participants (hypertension, type 2 diabetes, cardiovascular disease). However, it is possible that duration of illness, variation in medication use, and severity of these conditions could potentially play an important role in effect of exercise on functional capacity which could not be comprehensively explored in this review and calls for further exploration in future research.
Conclusion
In conclusion, our meta-analysis and trial sequential analysis further substantiated that adding exercise to usual care seemed to improve functional capacity and may potentially be recommended for people with hypertension, type 2 diabetes, or cardiovascular disease. Notably, dynamic aerobic and resistance exercise consistently enhanced various functional capacity outcomes while the superiority of body-mind therapies for VO2max and inspiratory muscle training for 6MWT calls for further investigation. Furthermore, prescribing exercise for the sole purpose of losing weight could be a potential strategy for people with hypertension and type 2 diabetes but not for cardiovascular disease. The extent of improvement in functional capacity and reduction of body weight varied with the specific exercise regimen employed thus highlighting the importance of personalised exercise prescriptions tailored to individual needs.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- 10MWT:
-
10-Meter walk test
- 6MWT:
-
6-Minute walk test
- BMI:
-
Body Mass Index
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- IQR:
-
Inter-Quartile Range
- Kg:
-
Kilo gram
- M:
-
Meter
- MET:
-
Metabolic Equivalent of Task
- MD:
-
Mean Difference
- ml/kg/min:
-
Milliliter/kilogram/minute
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SD:
-
Standard deviation
- Sec:
-
Seconds
- VO2max:
-
Maximal Oxygen Uptake
References
Arena R, Cahalin LP, Borghi-Silva A, Phillips SA. Improving functional capacity in heart failure: the need for a multifaceted approach. Curr Opin Cardiol. 2014;29(5):467–74.
Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34(5):575–84.
Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart Disease and Stroke Statistics—2014 Update. Circulation. 2014;129(3):e28–292.
Tadic M, Ivanovic B. Why is functional capacity decreased in hypertensive patients? From mechanisms to clinical studies. J Cardiovasc Med. 2014;15(6):447–55.
Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6):1507–12.
Kokkinos P, Manolis A, Pittaras A, Doumas M, Giannelou A, Panagiotakos DB, Faselis C, Narayan P, Singh S, Myers J. Exercise Capacity and Mortality in Hypertensive Men With and Without Additional Risk Factors. Hypertension. 2009;53(3):494–9.
Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, Collins E, Fletcher G. Assessment of Functional Capacity in Clinical and Research Settings. Circulation. 2007;116(3):329–43.
Guyatt GH, Thompson PJ, Berman LB, Sullivan MJ, Townsend M, Jones NL, Pugsley SO. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38(6):517–24.
Teasell R, Salbach NM, Foley N, Mountain A, Cameron JI, Jong Ad, Acerra NE, Bastasi D, Carter SL, Fung J et al: Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int J Stroke 2020, 15(7):763–788.
Casillas JM, Hannequin A, Besson D, Benaïm S, Krawcow C, Laurent Y, Gremeaux V. Walking tests during the exercise training: Specific use for the cardiac rehabilitation. Ann Phys Rehabil Med. 2013;56(7):561–75.
Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46(2):239–48.
Nystoriak MA, Bhatnagar A. Cardiovascular Effects and Benefits of Exercise. Front Cardiovasc Med. 2018;5:135.
Ramos RdA, Ferreira AdS. Functional capacity in adults with hypertension as assessed by the six-minute walk distance test: systematic review. Fisioter Pesq. 2014;21(3):257–63.
Pfeifer LO, De Nardi AT, da Silva LXN, Botton CE. do Nascimento DM, Teodoro JL, Schaan BD, Umpierre D: Association Between Physical Exercise Interventions Participation and Functional Capacity in Individuals with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med Open. 2022;8(1):34.
Fuentes-Abolafio IJ, Stubbs B, Pérez-Belmonte LM, Bernal-López MR, Gómez-Huelgas R, Cuesta-Vargas AI. Physical functional performance and prognosis in patients with heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2020;20(1):512.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021–104.
Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934.
WHO guidelines on physical activity and sedentary behaviour. In. Geneva: World Health Organization; 2020.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–79.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, et al. Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals With Type 2 Diabetes. Diabetes Care. 2011;34(7):1481–6.
Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114(10):1557–68.
Lee HS, Lee J: Effects of Exercise Interventions on Weight, Body Mass Index, Lean Body Mass and Accumulated Visceral Fat in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Environ Res Public Health 2021, 18(5).
Recchia F, Leung CK, Yu AP, Leung W, Yu DJ, Fong DY, Montero D, Lee C-H, Wong SHS, Siu PM: Dose–response effects of exercise and caloric restriction on visceral adiposity in overweight and obese adults: a systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine 2023:bjsports-2022–106304.
Rijal A, Nielsen EE, Adhikari TB, Dhakal S, Maagaard M, Piri R, Neupane D, Gæde PH, Olsen MH, Jakobsen JC. Effects of adding exercise to usual care in patients with either hypertension, type 2 diabetes or cardiovascular disease: a systematic review with meta-analysis and trial sequential analysis. Br J Sports Med. 2023;57:930–9.
Rijal A, Nielsen EE, Hemmingsen B, Neupane D, Gæde PH, Olsen MH, Jakobsen JC. Adding exercise to usual care in patients with hypertension, type 2 diabetes mellitus and/or cardiovascular disease: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst Rev. 2019;8(1):330.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Skinner J S: General Principles of Exercise Prescription. Exercise testing and exercise prescription for special cases 2005:22–37.
Medicine ACoS: ACSM's guidelines for exercise testing and prescription: Lippincott williams & wilkins; 2013.
Physical Activity Guideline Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report In. Washington DC: U.S. Department of Health and Human Services; 2018.
Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated 2011) [www.handbook.cochrane.org]
StataCorp. 2019: Stata Statistical Software: Release 17. In.: College Station, TX: StataCorp LLC.
Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol. 2014;14:120.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA: Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2019.
Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic Press; 1988.
Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39–39.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Verity LS, Ismail AH. Effects of exercise on cardiovascular disease risk in women with NIDDM. Diabetes Res Clin Pract. 1989;6(1):27–35.
Askim T, Langhammer B, Ihle-Hansen H, Gunnes M, Lydersen S, Indredavik B, Group LC. Efficacy and Safety of Individualized Coaching After Stroke: the LAST Study (Life After Stroke): A Pragmatic Randomized Controlled Trial. Stroke. 2018;49(2):426–32.
Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-analysis. JAMA. 2009;301(19):2024–35.
Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou MC, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5(5):579–85.
Bensimhon DR, Leifer ES, Ellis SJ, Fleg JL, Keteyian SJ, Piña IL, Kitzman DW, McKelvie RS, Kraus WE, Forman DE, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing). Am J Cardiol. 2008;102(6):712–7.
Ciani O, Piepoli M, Smart N, Uddin J, Walker S, Warren FC, Zwisler AD, Davos CH, Taylor RS. Validation of Exercise Capacity as a Surrogate Endpoint in Exercise-Based Rehabilitation for Heart Failure: A Meta-Analysis of Randomized Controlled Trials. JACC Heart Fail. 2018;6(7):596–604.
Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, Duncan PW. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90(2):196–208.
Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, O’Connor FC, Gerstenblith G, Becker LC, Katzel LI, Lakatta LE. Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation. 1996;94(3):359–67.
Eng JJ. Fitness and Mobility Exercise (FAME) Program for stroke. Top Geriatr Rehabil. 2010;26(4):310–23.
Pasanen T, Tolvanen S, Heinonen A, Kujala UM. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomised controlled trials. Br J Sports Med. 2017;51(20):1459–65.
Ismail H, McFarlane JR, Dieberg G, Smart NA. Exercise training program characteristics and magnitude of change in functional capacity of heart failure patients. Int J Cardiol. 2014;171(1):62–5.
Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94.
Taylor JL, Bonikowske AR, Olson TP: Optimizing Outcomes in Cardiac Rehabilitation: The Importance of Exercise Intensity. Front Cardiovasc Med 2021, 8.
Milanović Z, Sporiš G, Weston M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med. 2015;45(10):1469–81.
Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-up Study. Mayo Clin Proc. 2016;91(9):1183–8.
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–7.
Zhao Y, Yu BY, Liu Y, Tong T, Liu Y. Weight reduction and cardiovascular benefits: Protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(50): e13246.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss. Circulation. 2006;113(6):898–918.
Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of Weight Reduction on Blood Pressure. Hypertension. 2003;42(5):878–84.
Recchia F, Leung CK, Yu AP, Leung W, Yu DJ, Fong DY, Montero D, Lee C-H, Wong SHS, Siu PM: Dose–response effects of exercise and caloric restriction on visceral adiposity in overweight and obese adults: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2023:bjsports-2022–106304.
Cox CE. Role of Physical Activity for Weight Loss and Weight Maintenance. Diabetes Spectr. 2017;30(3):157–60.
Koithan M. Mind-Body Solutions for Obesity. J Nurse Pract. 2009;5(7):536–7.
Mamataz T, Uddin J, Ibn Alam S, Taylor RS, Pakosh M, Grace SL. Effects of cardiac rehabilitation in low-and middle-income countries: A systematic review and meta-analysis of randomised controlled trials. Prog Cardiovasc Dis. 2022;70:119–74.
Pesah E, Turk-Adawi K, Supervia M, Lopez-Jimenez F, Britto R, Ding R, Babu A, Sadeghi M, Sarrafzadegan N, Cuenza L, et al. Cardiac rehabilitation delivery in low/middle-income countries. Heart. 2019;105(23):1806–12.
Alba AC, Alexander PE, Chang J, MacIsaac J, DeFry S, Guyatt GH. High statistical heterogeneity is more frequent in meta-analysis of continuous than binary outcomes. J Clin Epidemiol. 2016;70:129–35.
Acknowledgements
Not applicable.
Funding
Open access funding provided by University of Southern Denmark The salary of the first author was supported by a research grant from the Danish Diabetes Academy PhD012-18, which is funded by the Novo Nordisk Foundation, grant number NNF17SA0031406. They had no role in study design, data collection, data analysis, interpretation or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
AR, MHO and JCJ conceived this systematic review. AR, TBA and SD conducted the literature search. AR, TBA, SD, MM, and RP extracted data independently and assessed the risk of bias using the Cochrane Risk of Bias-version 1 (RoB1) in pairs. AR conducted the data analysis and data interpretation and wrote the first draft with input from MHO. JCJ, EEN, DN provided valuable comments and amended the article. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Text S1. Detail Search strategy. Text S2. Other functional capacity Berg Balance Scal. Table S1. Characteristics of included studies. Text S3. Sensitivity Analysis (Fixed Model; Inverse Variance). Table S2. Subgroup analysis for VO2 max for age and baseline BMI. Table S4. Subgroup analysis for 10MWT for age, baseline BMI and size of trials. Table S5. Subgroup analysis for body weight for age and baseline BMI. Table S6. Meta-regression on effect of exercise on VO2max. Table S7. Meta-regression on effect of exercise on 6MWT. Table S8. Meta-regression on effect of exercise on 10MWT. Table S9. Meta-regression on effect of exercise on body weight.
Additional file 2:
figure S1. Funnel plot for trials reporting VO2max, figure S2. Risk of bias graph for VO2max: review authors’ judgements about each risk of bias item presented as percentages across all included studies, figure S3. Risk of bias summary for VO2max. figure S4. Funnel plot on trials reporting 6MWT, figure S5. Risk of bias graph for 6MWT. figure S6. Risk of bias summary for 6MWT. figure S7. Funnel plot on trials reporting 10MWT. figure S8. Risk of bias graph for 10MWT, figure S9. Risk of bias summary for 10MWT. figure S10. Forest plot on trials reporting berg balance scale. figure S11. Forest plot on trials reporting TUGT. figure S12. Forest plot on trials reporting exercise capacity (watt). figure S13. Forest plot on trials reporting exercise capacity(MET). figure S14. Funnel plot on trials reporting body weight. figure S15. Risk of bias graph for body weight. figure S16. Risk of bias summary for body weight
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rijal, A., Adhikari, T.B., Dhakal, S. et al. Effect of exercise on functional capacity and body weight for people with hypertension, type 2 diabetes, or cardiovascular disease: a systematic review with meta-analysis and trial sequential analysis. BMC Sports Sci Med Rehabil 16, 38 (2024). https://doi.org/10.1186/s13102-024-00829-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-024-00829-1