Abstract
Background
Physical activity is indicated as a treatment for Long COVID, but prevention is unknown. This study aimed to investigate the relationship between physical activity (PA) before and after acute SARS-Cov-2 infection and the presence of Long COVID symptoms in adults.
Methods
We used data from the Sulcovid-19, a longitudinal study carried out with individuals who were infected by SARS-Cov-2 between December/2020 and March/2021. Participants were asked about 19 symptoms commonly associated with long COVID. Three PA variables were built, as follows: (1) remained inactive; (2) became inactive; (3) remained active.
Results
2.919 people were interviewed. The prevalence of individuals who had at least one symptom of Long COVID is 48.3% (95%CI 46.5–51.1). Our results showed that 71.8% (95%CI 70.1–73.4) of the individuals remained inactive, 14.9% (95%CI 13.6–16.2) became inactive and 13.3% (95% CI 12.1–14.6) remained active. The likelihood of experiencing long COVID symptoms was reduced in the musculoskeletal (PR 0.70; 95%CI 0.49–0.99), neurological (PR 0.61; 95%CI 0.43–0.88), and respiratory (PR 0.58; 95%CI 0.35–0.96) systems in those who remained active. In addition, the likelihood of experiencing Long COVID symptoms was 7% less in those who remained active.
Conclusions
Continuous PA practice showed important protection effect for Long COVID symptoms in adults.
Similar content being viewed by others
Introduction
The societal, economic, and public health impacts of the COVID-19 pandemic have been extensive [1]. Since the pandemic was declared until January 2023, there have been more than 657 million reported cases of COVID-19 worldwide, resulting in over 6.6 million deaths. In Brazil the number of cases has exceeded 36.4 million, with over 694 thousand deaths [2].
Long COVID refers to the persistence of symptoms for a minimum of three months, lasting at least two months without an alternative diagnosis [3, 4].This condition can affect individuals who experienced mild or severe forms of the disease [5]. Among non-hospitalized individuals, the prevalence of Long COVID can reach as high as 34.0% [6].
Physical activity (PA) is widely recognized as an effective non-pharmacological approach to prevent and treat several chronic physical and mental diseases [7]. Previous research suggested an association between physical inactivity and COVID-19, demonstrating that physically active individuals have a 20.0–26.0% reduced likelihood of testing positive for the virus [8]. Recommendations highlight the importance of PA in managing Long COVID, as it has shown promise as an effective non-pharmacological therapy for mitigating persistent COVID-19 symptoms [9]. However, studies evaluating PA as a form of prevention of Long COVID are scanty. Therefore, this study aimed to investigate the relationship between PA before and after acute SARS-CoV-2 infection and the presence of Long COVID symptoms in adults.
Methodology
We analyzed the baseline data of the Sulcovid-19, a longitudinal study that monitors the health indicators of individuals infected with COVID-19 in the city of Rio Grande, Rio Grande do Sul, Brazil. Participants should be 18 or older, have received a COVID-19 diagnosis through RT-PCR testing between December 2020 and March 2021, experienced COVID-19 symptoms, and have received medical care in Rio Grande. The Health Research Ethics Committee (CEPAS) of the Federal University of Rio Grande (FURG) (CAAE:39081120.0.0000.5324) approved the study protocol.
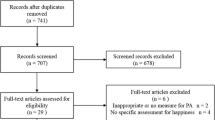
To identify adults who had been infected with SARS-CoV-2 contact was established with the Epidemiological Health Surveillance department of the Rio Grande. Subsequently, a list was compiled, consisting of 4,014 individuals who had tested positive for SARS-CoV-2 through RT-PCR, along with their corresponding information such as name, address, telephone number, and presence of symptoms. Following the creation of this list, inclusion and exclusion criteria were applied, resulting in 3,822 individuals being deemed eligible for the study. More information can be found in Flowchart 1 in the supplementary file.
Data collection was conducted through telephone interviews carried out by trained interviewers who underwent a rigorous selection process and received comprehensive training and qualification. The questionnaire used can be verified in the supplementary file. When necessary, home visits were offered as an alternative for face-to-face data collection. Further information regarding the study design and recruitment process can be found elsewhere [10].
We investigated a total of 19 symptoms commonly associated with Long COVID. These symptoms included headache, dyspnea (shortness of breath), dry cough, cough with phlegm, pain or discomfort when breathing, ageusia (loss of taste), anosmia (loss of smell), change in sensation (such as numbness, tingling, needling, pressure, cold/heat), fatigue, sore throat, coryza (runny nose), nasal congestion, diarrhea, nausea, arthralgia (joint pain), myalgia (muscle pain), memory loss, attention loss and alteration of the skin.
To assess Long COVID symptoms, participants were asked if they had experienced any of these symptoms after their SARS-CoV-2 infection and whether they were still experiencing them at the time of the survey. We considered a Long COVID symptom when the participant answered “yes” to both questions. Each of the 19 symptoms was analyzed as an individual outcome, and a composite variable for Long COVID was created if at least one of the symptoms was reported.
Furthermore, we categorized the symptoms into groups based on the affected body systems. These groupings included digestive symptoms (nausea and/or vomiting), musculoskeletal symptoms (muscle pain, joint pain, and/or fatigue), neurological symptoms (headache, memory loss, and/or loss of attention), respiratory symptoms (shortness of breath, dry cough, cough with phlegm, pain or discomfort in breathing, coryza, and/or nasal congestion), and sensory symptoms (ageusia, anosmia, and/or altered sensitivity). This categorization allowed for a more comprehensive analysis of the specific systems impacted by Long COVID.
We further grouped the symptoms into four broader categories, as follows: (1) musculoskeletal, neurological, and/or respiratory; (2) musculoskeletal and/or neurological; (3) musculoskeletal and/or respiratory; and (4) neurological and/or respiratory. These categories were treated as dichotomous variables, indicating whether the participant experienced at least one symptom or not.
PA was assessed based on participants self-reported frequency (days per week) and duration (time per day). Firstly, participants were asked about their PA practice in the 12 months before their SARS-CoV-2 infection. Secondly, they were asked about their PA after their infection. Those who engaged in 150 min/week or more of PA were considered active, following the WHO guidelines [11]. The independent variable was operationalized into three categories considering both timepoints, as follows: (1) remained inactive, (2) became inactive, and (3) remained active. The category “became active” was excluded due to the small number of participants (n = 14) [12].
To control for confounding the following variables were used: sex (male/female), age (18–59 years/60 years or more), income (R$ 0-1000/1001–2000/2001–4000/4001 or more in Brazilian Real), body mass index (BMI) [13] (eutrophic/low weight; overweight/obese), self-reported medical diagnosis of comorbidity (depression, hypertension, diabetes mellitus, heart problems, renal failure, respiratory problems - asthma, chronic obstructive pulmonary disease, osteoporosis, arthritis, arthrosis, or rheumatism), and hospitalization (no/yes).
All analyses were performed in Stata® 15.0. Descriptive data were presented as proportions along with their corresponding 95% confidence intervals (95%CI). We used Poisson regression to investigate the relationship between Long COVID symptoms and PA. Adjusted analyses were performed by Poisson regression with robust variance adjustment. Associations were considered statically significant when the 95% CIs did not overlap between categories.
Results
Overall, 3,822 individuals who had tested positive for COVID-19 were eligible for the survey; after losses (631) and refusals (272), 2,919 individuals were interviewed. The interviews took place 6 to10 months after the participants infection had been diagnosed by RT-PCR testing. The prevalence of participants who experienced at least one symptom of Long COVID was 48.3% (95%CI 46.5–51.1) and most were women (58.9 95%CI 56.6–61.3) and people with a monthly income of 0-1000 (54.5 95%CI 50.7–58.3). No significant differences were found between age, ethnicity, and education level (Table 1). Regarding PA before and after infection, 71.8% (95%CI 70.1–73.4) of the participants remained inactive, 14.9% (95%CI 13.6–16.2) became inactive, and 13.3% (95% CI 12.1–14.6) remained active.
Participants who remained active showed reduced prevalence of dyspnea (3.1% 95%CI 1.8–5.4), arthralgia (4.9% 95%CI 3.2–7.6), myalgia (5.2% 95%CI 3.4–7.9), headache (6.8% 95%CI 4.7–9.8), fatigue (9.1% 95%CI 6.6–12.5) and memory loss (11.7% 95%CI 8.8–15.3). Additionally, reduced prevalence on this group was observed when combining symptoms for body systems, such as respiratory (9.0% 95%CI 6.8–12.7), musculoskeletal (13.8% 95%CI 10.7–17.7) and neurological (16.8% 95%CI 13.3–20.9) and any combinations of these systems (Table 2).
Participants who became inactive had a higher probability of experiencing headache (PR 1.44, 95%CI 1.02; 2.03). Conversely, remaining active reduced the probability of experiencing headache by 74.0% (95%CI 0.23; 0.90). Furthermore, participants who remained active were less likely to experience fatigue (PR 0.63, 95%CI 0.41; 0.97) and at least one long COVID symptom (PR 0.80, 95%CI 0.63; 0.99) (Table 3).
Remaining active also reduced the probability of experiencing Long COVID symptoms in the musculoskeletal (PR 0.70; 95%CI 0.49; 0.99), neurological (PR 0.61; 95%CI 0.43; 0.88), and respiratory system (PR 0.58; 95%CI 0.35; 0.96). Also, when considering the grouping of these systems together, the probability of experiencing Long COVID symptoms in those who remained active was reduced by 32–39% (Table 4).
Discussion
Almost half of participants experienced at least one Long COVID symptom, and only 13.3% remained active. We revealed that remaining active reduced the probability of experience Long COVID symptoms, and specific symptoms such as fatigue. Also, continuous practice of PA reduced the probability of experiencing symptoms on respiratory, musculoskeletal and neurological systems.
One of the reasons behind the association between continuous PA and respiratory symptoms is the affinity of the SARS-Cov-2 virus with pericytes, leading to their elimination [14]. Consequently, individuals infected with COVID-19 tend to have lower cardiorespiratory fitness compared to healthy individuals. This is due to reduced peripheral oxygen extraction rate, increased venous saturation [15], and a resultant decrease in exercise tolerance [14]. Engaging in PA is crucial since enhances mitochondrial activity and improves oxygen uptake, thereby preserving energy production during cellular respiration [16]. Fatigue, which is reported as one of the primary symptoms following SARS-CoV-2, with a prevalence of 47.0% [17, 18], can be prevented by engaging in PA, as supported by our data.
Myalgia, the third most prevalent symptom observed in individuals following acute infection, has a prevalence of 25.0% [17]. This can be attributed to the affinity of the SARS-CoV-2 for angiotensin-converting enzyme 2 (ACE2), which is present in muscle tissue, leasing to direct damage to muscle tissue [19, 20]. Regular PA provides benefits to the osteomuscular system by strengthening the immune system, which aids in the detection and elimination of infected cells, particularly in tissues with ACE2 expression, such as muscle tissues [21, 22].
Neurological symptoms, such as memory and attention loss, can be attributed to two main factors. Firstly, possible vascular, hemorrhagic, and ischemic injuries, as well as microglial activation in the white matter, T-cell invasion, and regional neuronophagy in the locus coeruleus, which is responsible for brain functions such as attention and memory [23,24,25]. Secondly, there may be a psychosomatic effect due to the trauma of contracting the virus [26]. Individuals who have recovered from COVID-19 often report feeling unlike themselves, experiencing short-term memory loss, confusion, difficulty concentrating, and a general sense of being different compared to before the infection [26, 27].
Engaging in continuous PA has beneficial impact on neurogenesis and synaptic plasticity associated, which can help alleviate minor neurological complications. Notably, PA promotes the production of astrocytes, which are central nervous system cells responsible for maintaining brain homeostasis [28, 29]. Our data supports the potential advantages of maintaining regular physical activity in the context of Long COVID. These findings are crucial as approximately 50% of individuals affected by COVID-19 may experience the syndrome [5].
The etiology of headache as a residual symptom is complex and multifactorial, involving direct infection effects, cerebrovascular disease (including hypercoagulation), physiological impairments (e.g., hypoxia), medication side effects, and social aspects related to having a life-threatening illness [30]. Continuous PA appears to play a role maintaining brain health by regulating neurotrophic factors and anti-inflammatory cytokines [31]. Thus, the significance of our findings underscores the potential benefits of continuous PA in the prevention of Long COVID symptoms [32].
Despite the challenges posed by the closure of exercise spaces due to social distancing requirements [33], coupled with the difficulties faced by individuals with Long COVID in resuming PA due to their symptoms, our findings indicate that regular PA should be considered as a non-pharmacological strategy to reduce the likelihood of developing Long COVID. In other words, PA promoting policies should be included in first response action in pandemic scenarios, such as the COVID-19. It is important to highlight the magnitude of protection that PA can provide for the various symptoms, considering that the estimated overall prevalence of Long COVID in non-hospitalized patients can reach values of 34.0% (95% CI, 25.0–46.0) [4].
The limitations of our study should be listed. Firstly, we did not assess asymptomatic individuals during the acute phase of SARS-CoV-2 infection. Consequently, sample bias cannot be discarded. Secondly, PA was assessed retrospectively, and memory bias might be a concern. Thirdly, the questionnaire focused on the 19 most prevalent symptoms reported in the literature. Even though there are more than 200 Long COVID symptoms reported, we were unable to cover all of them in the questionnaire due to interview restraints [5]. Fourthly, we only analyzed individuals who survived COVID-19, and survival bias cannot be discarded. However, our sample consisted of individuals with COVID-19 who were diagnosed using the gold standard test (RT-PCR), and we achieved a high response rate (> 75%). Furthermore, different from other studies, we evaluated different PA levels in individuals who were not hospitalized.
Conclusion
The continuous PA practice showed a protective effect against Long COVID symptoms, particularly those affecting the musculoskeletal, neurological and respiratory systems. These findings enhance our understanding of the association between PA and Long COVID symptoms, providing a theoretical basis for healthcare managers to promote PA during pandemic scenarios. To facilitate continuous PA strategies, it is recommended to encourage home-based exercises and utilize digital platforms that provide guidance from exercise science professionals. Implementing these initiatives aims to assist individuals in sustaining an active lifestyle despite the challenges posed by the pandemic scenario.
Data Availability
Datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- PA:
-
Physical activity
- CEPAS:
-
Health Research Ethics Committee
- FURG:
-
Federal University of Rio Grande
- RT-PCR:
-
Reverse transcription polymerase chain reaction
References
Onyeaka H, Anumudu CK, Al-sharify ZT, Egele-godswill E. COVID-19 pandemic: a review of the global lockdown and its far-reaching effects. Sci Prog. 2021;104(2):1–18.
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [Internet]. WHO Coronavirus (COVID-19) Dashboard. 2023. p. 1. Available from: https://covid19.who.int/.
Lippi G, et al. COVID-19 and its long-term sequelae: what do we know in 2023? Pol Archives Intern Med vol. 2023;133(4):16402. https://doi.org/10.20452/pamw.16402.
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of Post COVID-19 Condition or Long COVID: a Meta-analysis and systematic review. J Infect Dis. 2022.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1).
Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect [Internet]. 2021;27(10):1507–13. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1198743X21002810.
Luan X, Tian X, Zhang H, Huang R, Li N, Chen P et al. Exercise as a prescription for patients with various diseases. J Sport Heal Sci [Internet]. 2019;8(5):422–41. Available from: https://www.sciencedirect.com/science/article/pii/S2095254619300493.
Zhang X, Li X, Sun Z, He Y, Xu W, Campbell H et al. Physical activity and COVID-19: an observational and mendelian randomisation study. J Glob Health. 2020;10(2).
Faghy MA, Arena R, Stoner L, Haraf RH, Josephson R, Hills AP, et al. The need for exercise sciences and an integrated response to COVID-19: a position statement from the international HL-PIVOT network. Prog Cardiovasc Dis. 2021;67:2–10.
Saes M, de O, Rocha JQS, Rutz AAM, Silva CN, da, Camilo L dos, de Oliveira S et al. Aspectos metodológicos e resultados da linha de base do monitoramento da saúde de adultos e idosos infectados pela covid-19 (Sulcovid-19) [Internet]. SciELO Preprints; 2023. https://doi.org/10.1590/scielopreprints.5334.
Organização Mundial da Saúde. Diretrizes Da OMS para atividade física e comportamento sedentário. Gr Psychother Students Teach (RLE Gr Ther; 2020.
Feter N, Caputo EL, Smith EC, Doring IR, Cassuriaga J, Leite JS et al. Association between physical activity and subjective memory decline triggered by the COVID-19 pandemic: findings from the PAMPA cohort. Prev Med (Baltim). 2021;145.
Obesity WC. on. OBESITY: PREVENTING AND MANAGING THE GLOBAL EPIDEMIC. WHO Tech Rep Ser. 2000.
Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063.
Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, et al. Persistent Exertional Intolerance after COVID-19: insights from Invasive Cardiopulmonary Exercise Testing. Chest. 2022;161(1):54–63.
Apabhai S, Gorman GS, Sutton L, Elson JL, Plötz T, Turnbull DM, et al. Habitual physical activity in mitochondrial Disease. PLoS ONE. 2011;6(7):e22294.
Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, Complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–42.
Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(1):89–95.
Hu Z, Yang Z, Li Q, Zhang A, Huang Y. Infodemiological study on COVID-19 epidemic and COVID-19 infodemic. 2020;(March).
McFarland AJ, Yousuf MS, Shiers S, Price TJ. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6(1):e885.
Zbinden-Foncea H, Francaux M, Deldicque L, Hawley JA. Does high Cardiorespiratory Fitness Confer some Protection Against proinflammatory responses after Infection by SARS-CoV-2? Obes (Silver Spring). 2020;28(8):1378–81.
Damiot A, Pinto AJ, Turner J, Gualano B. Immunological implications of Physical Inactivity among older adults during the COVID-19 pandemic. Gerontology. 2020;66(5):431–8.
Agrawal S, Farfel JM, Arfanakis K, Al-Harthi L, Shull T, Teppen TL et al. Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases. Acta Neuropathol Commun [Internet]. 2022;10(1):186. https://doi.org/10.1186/s40478-022-01493-7.
Plini ERG, O’Hanlon E, Boyle R, Sibilia F, Rikhye G, Kenney J et al. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells [Internet]. 2021;10(7). Available from: https://www.mdpi.com/2073-4409/10/7/1829.
Granholm A-C. Long-Term effects of SARS-CoV-2 in the brain: clinical consequences and Molecular mechanisms. J Clin Med. 2023;12(9).
Junior SSD, Guarnier2 GFF, Rodrigues IB et al. Cardoso3, Felicio4 F de C, Pereira5 JS,. Recuperação De Déficit De Memória Pós-Covid-19: Uma Revisão Recovery From Post Covid-19 Memory Deficit: a Review. Rev Ciências Biológicas e da Saúde. 2021;1–10.
Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and Meta-analysis of one-year Follow-Up studies on Post-COVID symptoms. Pathogens. 2022;11(2).
Huang S, Fishell G. In SARS-CoV-2, astrocytes are in it for the long haul. Proc Natl Acad Sci U S A. 2022;119(30):e2209130119.
Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry [Internet]. 2020;7(7):611–27. https://doi.org/10.1016/S2215-0366(20)30203-0.
Baldini T, Asioli GM, Romoli M, Carvalho Dias M, Schulte EC, Hauer L, et al. Cerebral venous Thrombosis and severe acute respiratory syndrome coronavirus-2 Infection: a systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3478–90.
Feter N, Alt R, Dias MG, Rombaldi AJ. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci Sports [Internet]. 2019;34(5):293–304. Available from: https://www.sciencedirect.com/science/article/pii/S0765159719300309.
Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F et al. Sánchez-Alcaraz Martínez BJ,. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int J Environ Res Public Health. 2021;18(10).
Becker RC. Covid-19 treatment update: follow the scientific evidence. Vol. 50, Journal of thrombosis and thrombolysis. Netherlands; 2020. p. 43–53.
Acknowledgements
The authors would like to thank FAPERGS (Research Support Foundation of the State of RS) and the Coordination for the Improvement of Higher Education Personnel (CAPES).
Funding
The study was carried out with financial support from FAPERGS - FResearch Support Foundation of Rio Grande do Sul, Brazil grant number 21/2551-0000107-0 Research Program for the SUS: shared management in health - PPSUS).
Author information
Authors and Affiliations
Contributions
J.Q.S.R, E.L.C, and M.O.S planned and conducted the analyses, Y.P.V,J.Q.S.R and M.S.A prepared the manuscript and performed the data collection, M.O.S and S.M.S.D developed the questionnaire and performed the reviews. All the authors contributed to the writing of the article, reviewed and approved the content of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research involves human participants and was carried out in accordance with the relevant guidelines and regulations of the Declaration of Helsinki and this study protocol was approved by the Ethics Committee in Health Research of the Federal University of Rio Grande (Certificate of Submission for Ethical Evaluation nº 39081120.0 0.0000.5324). This research complied with the specific resolution of the National Health Council (466/2012) and informed consent was obtained from all subjects in accordance with the resolution of the Free and Informed Consent Term of the National Health Council.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rocha, J.Q.S., Caputo, E.L., Vieira, Y.P. et al. Physical activity status prevents symptoms of long covid: Sulcovid-19 survey. BMC Sports Sci Med Rehabil 15, 170 (2023). https://doi.org/10.1186/s13102-023-00782-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-023-00782-5