Abstract
Bacteria form a highly complex ecosystem in the gastrointestinal (GI) tract. In recent years, mounting evidence has shown that bacteria can release nanoscale phospholipid bilayer particles that encapsulate nucleic acids, proteins, lipids, and other molecules. Extracellular vesicles (EVs) are secreted by microorganisms and can transport a variety of important factors, such as virulence factors, antibiotics, HGT, and defensive factors produced by host eukaryotic cells. In addition, these EVs are vital in facilitating communication between microbiota and the host. Therefore, bacterial EVs play a crucial role in maintaining the GI tract’s health and proper functioning. In this review, we outlined the structure and composition of bacterial EVs. Additionally, we highlighted the critical role that bacterial EVs play in immune regulation and in maintaining the balance of the gut microbiota. To further elucidate progress in the field of intestinal research and to provide a reference for future EV studies, we also discussed the clinical and pharmacological potential of bacterial EVs, as well as the necessary efforts required to understand the mechanisms of interaction between bacterial EVs and gut pathogenesis.
Similar content being viewed by others
Introduction
The gastrointestinal (GI) tract serves as the primary site for food digestion and absorption while also acting as a gateway for toxin invasion. It performs the dual functions of digestion and absorption as well as intestinal defense, which cannot be accomplished by the digestive system alone [1]. Hidden within the GI tract is a unique "organ" called the intestinal flora, which plays a critical role in digesting chyme and protecting the intestines [2,3,4]. The intestinal flora consists of thousands of species and over 10 trillion bacteria, which lack a cell connection and stable extracellular matrix with host cells. As a result, communication between surface proteins and secretory proteins becomes challenging. Extracellular vesicles (EVs) produced by intestinal bacteria serve as the primary mode of material transport and communication [5], and they remain active in the intestinal microenvironment while passing through the vascular barrier to enter the bloodstream [6]. Using specific surface structures, EVs recognize and bind to either bacteria or host cells, transmitting substances and signals. Intestinal bacteria and host cells continuously secrete and accept extracellular vesicles in the intestinal microenvironment, thereby affecting the viability of bacteria and the physiological function of host cells. These vesicles transport substances and information between the two types of cells [5]. In recent years, mounting evidence has confirmed that bacteria can secrete EVs as phages, antibiotics, and eukaryotic host defense actors [7, 8]. Additionally, EVs play crucial roles in intercellular communication, virulence factor transport, horizontal gene transfer (HGT), nutrient and electron transport, and biofilm formation [9,10,11]. Detecting bacterial EVs enables us to understand the composition of intestinal flora and aid in diagnosis. Transforming bacterial EVs can help us develop bacterial vaccines or immune adjuvants, as well as target drug delivery. However, future work should focus on regulating intestinal flora and protecting host cells through bacterial EV preparations [12, 13].
Composition and distribution of the microflora in the gut
More than 99% of the microbiota in the intestine is composed of intestinal flora, and the number of bacteria in the intestine exceeds the number of human cells. There are at least 1000 to 150 species present in the intestinal tract, and more than 500 species can be cultured. These are concentrated in 5–7 bacterial phyla, of which Bacteroidetes and Firmicutes make up about 95% and are part of the predominant microflora (Fig. 1) [14]. Typically, these bacteria are obligate anaerobes and specialize in colonizing the intestine. Most of them are probiotics and have a symbiotic relationship with the host [15]. However, most of the sub-dominant microflora belong to foreign or transient flora with high mobility. They are mainly aerobic bacteria or facultative anaerobic bacteria and may have potential pathogenicity that can cause harm to the host [15]. The intestinal flora is complex and diverse, and its composition can be influenced by various factors, such as age, diet, exercise, heredity, drugs, living environment, regional climate, and health status [3, 4, 15,16,17,18,19,20]. The leading indicators for evaluating intestinal flora include the concentration of colonic bacteria, the diversity of intestinal bacteria, and the ratio of probiotics [21]. In healthy individuals, probiotics are usually the dominant bacterial species in the intestine. Studies have shown that the proportion of probiotics in the intestine is about 70% in healthy individuals, 25% in average individuals, and 15% in those with constipation. However, the proportion of probiotics is only about 10% in the intestine of cancer patients [21, 22]. Figure 1 provides a visual representation of the microbial density and species present in the gut.
The bacterial flora inhabits in regions of human gastrointestinal tract constitute a complex ecosystem. More than 10 [14] microorganisms, 500 bacteria species have been identified in GI. The upper gastrointestinal tract (stomach, duodenum, jejunum, and upper ileum) is usually contained Lactobacillus, with bacterial concentrations less than 10 [4] microorganisms/ml. By contrast, bacteria in the large intestine are dramatic increase as 10 million bacteria. Anaerobic bacteria (A. bacteria) such as Bacteroides, Enterobacter, Anaerobic Streptococcus (A. Streptococcus), Clostridium and Lactobacillus are 1000 times more abundant than facultative anaerobes such as E. coli. It is a general trend that bacteria increase in complexity and concentration as they enter the gastrointestinal tract
The intestinal microbiota is closely intertwined with the physical and chemical environment of the gut and the host cells, collectively constituting the intestinal micro-ecosystem. These elements have a reciprocal and restrictive influence on each other, always striving to maintain a dynamic equilibrium, known as intestinal microecological homeostasis [23]. An imbalance in the intestinal flora can disrupt the host cells and the physical and chemical environment of the intestine, leading to intestinal micro-ecosystem disorders. This imbalance can cause not only acute and chronic inflammatory reactions, GI dysfunction, digestive tract tumors, and other digestive system diseases but also extraintestinal diseases, such as obesity, type 2 diabetes, liver disease, atherosclerosis, infectious diseases, allergic diseases, and mental and neurological dysfunction [24].
Biogenesis of bacterial EVs
Vesicle transport is the primary means by which cells transport macromolecules. EVs are a diverse array of vesicles released by cells [25,26,27,28]. Bacteria are unicellular prokaryotes. Bacterial EVs are secreted and transported by bacteria, facilitating the transfer of information and energy conversion. This mode of transport differs from small molecule transmembrane transport and the bacterial protein I–IX secretion systems [27, 29,30,31].
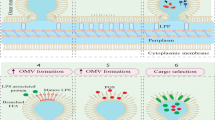
Bacteria can be categorized into Gram-negative and Gram-positive based on Gram staining, and they differ in their secretion of EVs [27, 32, 33]. Gram-negative bacteria possess an outer membrane structure, and it is commonly believed that EVs are vesicles formed by the extrusion of the bacterial outer membrane, known as outer membrane vesicles (OMVs) [34, 35]. Most Gram-negative bacteria secrete OMVs, and it is believed that the size of bacterial OMVs is similar to that of eukaryotic microvesicles, resulting from the pinching off of the outer membrane [36]. The specific mechanism of exfoliation is not clear, but proposed models mainly include the dissociation of stable cross-links of cell walls, the enlargement of the distance between the inner and outer membranes of bacteria, the local bulging and breakage of the outer membranes, and the orderly exfoliation of OMVs under the regulation of bacterial genes [32, 33, 37,38,39]. Moreover, a small proportion of cells are lysed to form outer-inner membrane vesicles (OIMVs) and explosive outer membrane vesicles (EOMVs) [28, 40]. Cell lysis can be triggered by DNA damage or by the partial degradation of the peptidoglycan layer of the cell wall by autolysin to form pores, where the inner and outer membranes protrude outward to wrap the cytoplasmic components, forming vesicles that are eventually squeezed out of the bacterial surface to form OIMVs [40]. When cell death and lysis occur, membrane fragments produced by explosive lysis can re-aggregate and randomly encapsulate cytoplasmic components to form vesicles, known as EOMVs [41]. Figure 2 provides further details on the occurrence of OMVs, OIMVs, and EOMVs.
Biogenesis model and composition of bacterial extracellular vesicles (bEVs). The composition of bEVs includes biphospholipid layers, proteins, glycoproteins, metabolites, and nucleic acids. A bEVs derived from Gram-negative bacteria can be released through the outer membrane; (i) by reducing outer membrane-peptidoglycan protein linkages; (ii) lipid/LPS differential assembly in specific regions of the outer membrane; (iii) swelling pressure by accumulation of protein or peptidoglycan fragments in the periplasmic space; (iiii) blast by cell lysis. B bEVs derived from Gram-positive bacteria can be released from the swelling pressure caused by EV accumulation through the cell wall composed of peptidoglycan; these turgor pressure promotes membrane curvature, then bEVs are released by peptidoglycan-degrading enzymes
On the other hand, the cell wall of Gram-positive bacteria lacks an outer membrane structure and is encased with a thick peptidoglycan layer. Currently, it is widely accepted that the weakening of the peptidoglycan layer by cell wall degrading enzymes and the increase of bacterial internal pressure allow for the release of the bacterial inner membrane, and the bacterial plasma membrane wraps the cytoplasmic components and bulges outward to form vesicles known as cytoplasmic membrane vesicles (CMVs). Only a small fraction of Gram-positive bacteria secrete CMVs [27, 32, 42,43,44,45,46,47]. OMVs and CMVs differ in generation, morphology, and function, as summarized in Table 1 [48, 49]. OMVs and CMVs are collectively referred to as membrane vesicles (MVs) [29, 30]. While MVs and EVs secreted by eukaryotic cells share similarities in structure, they differ in composition and function, as outlined in Table 2 [43, 50].
The production and secretion of bacterial EVs are influenced by the expression and regulation of bacterial genes, as well as the growth environment of bacteria [51, 52]. Bacterial genetic performance, such as bacterial adhesion, reproduction, and resistance to digestive enzymes and antibiotics, is closely related to the EVs secreted by bacteria [52, 53]. The production and secretion of EVs by bacteria are also affected by host age, dietary habits, antibiotic use, GI function, immune response, stress response, intestinal physical and chemical environment, and intestinal microbial composition [54,55,56,57,58,59,60,61]. Studies have found that the more vigorous the cell metabolism and the more stimulation the cell receives, the more EVs are secreted [30, 62, 63].
EVs can be categorized into different subtypes based on their pathogenesis and morphological structure. Each subtype represents different physiological or pathological states of cells and has different purposes and functions. Similar to eukaryotic EVs, bacterial EVs can be classified into exosomes, microvesicles, apoptotic bodies, and oncosomes. There may be additional subtypes of bacterial EVs that have yet to be discovered, and further research into the mechanisms of their occurrence can reveal different physiological states of bacteria and uncover a wider range of functions of EVs [8, 27, 40, 62].
Components of bacterial EVs
Bacterial EVs are nanoscale spherical transporters that are composed of a phospholipid bilayer membrane and have a diameter ranging from 20 to 250 nm. The membrane envelops nucleic acids, proteins, lipids, and other substances, while specific lipopolysaccharides and outer membrane proteins are embedded in the outer layer of the membrane. The asymmetry and fluidity of the membrane structure are responsible for the specific structure and function of the membrane surface [9, 64, 65], which is influenced by genetic and growth environments. EVs from different bacteria have different contents, with a focus on protein sorting, protein proportion, and DNA or RNA with different functions [66].
Proteins
Proteomic analysis has identified more than 3,500 proteins associated with OMVs [67, 68]. OMVs have been found to contain a large number of outer membrane proteins, including OmpA, OmpC, and OmpF, as well as periplasmic proteins, such as AcrA and alkaline phosphatase [67, 68]. Additionally, OMVs contain various adhesins and exotoxins [69]. The majority of OMV proteins are virulence factors that aid in the survival of bacteria by promoting bacterial growth, inhibiting competitive bacteria, evading host immunity, and resisting environmental toxins. OMVs also contain carrier proteins and channel proteins that are responsible for transport, accounting for a large proportion of OMV proteins [69]. Proteins carried by OMVs are strictly sorted based on their amino acid sequences, which contain special signal sequences known as signal peptides [70]. These signal peptides act as sorting signals that guide proteins to their target compartments. Each sorting signal is recognized by a corresponding sorting receptor. Proteins synthesized by bacterial ribosomes are transported to the inner and outer membranes, periplasm, or outside of the cell by various secretion systems. If a protein is loaded into a small vesicle, the corresponding receptor on the vesicle membrane must recognize its sorting signal signal [10, 68, 71]. The protein content of OMVs is subject to change due to alterations in gene expression and the growth environment of bacteria [70].
Nucleic acids
Bacterial EVs have been found to contain multiple types of nucleic acids [72]. EVs can carry DNA both in the lumen and on the membrane surface. The DNA in the lumen retains its antigenicity even after treatment with DNase, distinguishing it from the membrane-bound DNA. OMVs also contain miRNAs, mRNAs, and other non-coding RNAs. Several different forms of luminal DNA have been identified in OMVs secreted by Escherichia coli (E. coli), Neisseria gonorrhoeae (N. gonorrhoeae), Pseudomonas aeruginosa (P. aeruginosa), and Haemophilus influenzae (H. influenzae) [73].
OMVs have been found to contain mRNA, which can be transferred and translated after entering the host cell. Retrotransposons and other non-coding RNAs have also been reported in OMVs [74, 75]. The discovery of various nucleic acid types in OMVs highlights their importance as carriers and transmitters of genetic information, although the mechanism by which nucleic acids enter OMVs remains unclear. Similar to the intracellular transport of proteins, it is speculated that the intracellular transport of nucleic acids may involve corresponding recognition sequences through which nucleic acids are selected to enter OMVs [76, 77].
Lipids
The basic structure of EV membranes is composed of lipids, primarily phospholipids, and lipopolysaccharides. In E. coli OMVs, glycerophospholipid, phosphatidylglycerol, and phosphatidylethanolamine are important lipid components that contribute to the curvature of the OMVs [78]. Lipopolysaccharide (LPS) is an endotoxin and serves as an important antigen and ligand on the membrane surface, playing a crucial role in adhesion and activating the immune response. LPS is composed of three parts: lipid A, core polysaccharide, and O antigen. Lipid A is the most toxic component, while the O antigen is exposed on the membrane surface and serves as an important antigenic determinant [79, 80].
Role of bacterial EVs in the gut
EVs secreted by intestinal bacteria can diffuse in the intestinal microenvironment or enter the bloodstream. They are capable of recognizing specific molecules present in the environment through ligands on their membrane surface and can also be recognized and bound by specific receptors on the membrane surface of target cells. Once inside the cells, they can transmit substances and activate specific signaling pathways to transmit information [81, 82]. EVs are powerful tools that can deliver, bind, and transform substances (Fig. 2) [53, 83]. These vesicles carry various substances that have been screened by bacteria, representing the bacteria’s viability. They play a similar role to bacteria and have a significant impact on host cells, intestinal microorganisms, and the intestinal environment [12, 84].
EVs have different effects on the growth, reproduction, and colonization of bacteria of the same species. They also have both advantages and disadvantages for other bacteria and host cells. For example, they can promote the colonization of probiotics and regulate the immune response, which is beneficial to the host. However, they can also destroy the host mucosal barrier and cause inflammatory storms that are harmful to the host. The current study has identified the role of bacterial EVs in the gut, which is summarized in Table 3. This includes related studies on the role of EVs from known intestinal bacteria in the intestine [13].
Host cells
Intestinal immune cells
EVs recognize and stimulate immune cells through pathogen-associated molecular patterns (PAMPs), mainly including specific antigens, such as LPS, peptidoglycan on the surface of the membrane, and DNA in the cell. They combine with pattern recognition receptors (PRRs) for target recognition [85, 86]. PRRs associated with bacterial EVs include Toll-like receptors (TLRs) on the cell surface, such as TLR4 activated by LPS, and NOD-like receptors (NLRs) in cells, such as NOD1 and NOD2, activated by peptidoglycan components. Cysteine-containing aspartic proteolytic enzymes (Caspases), such as Caspase-11, which act as intracellular receptors for LPS, mediate the activation of an intracellular inflammatory pathway in OMVs (Fig. 3) [87].
When bacterial EVs recognize and bind to immune cells, whether they are beneficial to host immune regulation depends on the source of bacteria and the substances they carry [88,89,90]. Firstly, LPS and peptidoglycan on the surface can stimulate intestinal immune cells, trigger an inflammatory response, and maintain normal intestinal immune function. However, they can also trigger immunosuppression, an excessive immune response, or induce immune tolerance, which may lead to bacterial invasion or infection of the host and enable the bacteria to evade the host's immune response [91, 92]. Secondly, sDNA or DNA antigenicity or specific virulence factors in EVs can invade immune cells by endocytosis or endocytosis, affect the expression of inflammatory factors in immune cells, induce apoptosis of immune cells, and create conditions for bacterial invasion or infection [87, 93].
EVs derived from probiotics have mostly been shown to have beneficial effects on host immune regulation. For example, OMVs secreted by Bacteroides fragilis (B. fragilis) carry polysaccharide A (PSA) and are delivered to intestinal dendritic cells, which can induce CD4 + regulatory T cells (Tregs) to produce IL-10, down-regulating inflammatory responses and effectively ameliorating DSS-induced colitis of the colon [94,95,96]. Similarly, OMVs secreted by Akkermansia muciniphila (A. muciniphila) have been shown to significantly down-regulate DSS-induced colitis in mice and play an important role in regulating inflammatory immune response and maintaining the intestinal immune barrier [97]. Lactobacillus paracasei (L. paracasei) is a probiotic with anti-inflammatory properties, and in vitro studies have shown that EVs from L. paracasei (LpEVs) can down-regulate the expression of proinflammatory cytokines, such as IL-1α, IL-1β, IL-2, and TNF-α, and up-regulate the expression of anti-inflammatory cytokines, such as IL-10 and TGF-β. Additionally, LpEVs can inhibit the activation of inflammatory proteins, such as COX-2, iNOS, NF-κB, and nitric oxide (NO), in signal transduction pathways and significantly inhibit the inflammatory response of human colon adenocarcinoma HT-29 cells induced by LPS. Animal experiments have also demonstrated that oral administration of LpEVs can significantly prevent the reduction in body weight, colon length, and disease activity index (DAI), thus attenuating clinical signs in DSS-induced mice [98].
According to Kim et al. [99], EVs derived from pathogenic bacteria often result in host immune abnormalities [99]. For example, E. coli OMVs transmit virulence factors to host intestinal macrophages, which up-regulate the expression of proinflammatory cytokines, such as IL-6 and TNF-α. This leads to systemic inflammatory response syndrome (SIRS) and sepsis. Additionally, the heat-labile enterotoxin (LT) on the surface of enterotoxigenic E. coli OMVs interacts directly with host cells through PRRs, activating proinflammatory signaling pathways and chemokines expressed by host cells and ultimately causing inflammatory responses [100,101,102]. Furthermore, Vibrio cholerae (V. cholerae) O395 OMVs are taken up by intestinal epithelial cells through caveolin-mediated endocytosis of outer membrane porins (OmpU and OmpT). This induces the expression of proinflammatory cytokines (such as IL-8 and GM-CSF) and chemokines (such as CCL2 and CCL20), leading to the polarization of T cells to Th2/Th17 and causing an inflammatory response [103, 104]. In the case of Helicobacter pylori (H. pylori), OMVs bind to human monocytes and deliver virulence factors, such as vacuolating cytotoxin (VacA), strongly up-regulating the expression of inflammatory cytokines, such as IL-6 and IL-10. This inhibits the proliferation of CD4 + T cells and induces T cell apoptosis [105]. Flagellated bacteria, such as Salmonella typhimurium (S. typhimurium) and Pseudomonas aeruginosa, release OMVs that cause strong NLRC4-mediated caspase-1 activation and IL-1β secretion in macrophages in an endocytosis-dependent manner, promoting an inflammatory response [106]. Besides, Acinetobacter baumannii (A. baumannii) OMVs containing the virulence factor OmpA target mitochondria in mice and disrupt the mitochondrial morphology of mouse macrophages [107, 108].
Intestinal epithelial cells
Bacterial EVs are capable of being recognized by intestinal epithelial cells and can enter them through various mechanisms, including macropinocytosis and clathrin-mediated endocytosis, in order to transmit substances or signals [10]. Depending on the components of OMVs, they may provide nutrients and digestive enzymes that are necessary for metabolism and help repair the intestinal epithelial barrier. However, they may also have harmful effects, such as damaging the intestinal epithelial cells, destroying the intestinal epithelial barrier, and inducing intestinal epithelial cell death, including apoptosis, necrosis, autophagy, and other harmful effects [99, 109,110,111,112,113,114].
Bacteroides OMVs have been discovered to carry human therapeutic keratinocyte growth factor 2 (KGF-2), which promotes the repair of intestinal epithelial cells in DSS-induced colitis in mice after oral administration [115]. Other studies have demonstrated that the oral administration of EVs secreted by E. coli Nissle 1917 (EcN), L. paracasei, and B. fragilis can promote the repair of intestinal epithelial cells and the intestinal mucosal barrier in DSS model mice, significantly improving the inflammatory response [98, 116, 117]. OMVs secreted by EcN and a human E. coli strain containing the tcpC gene (ECOR 63) can up-regulate ZO1 and claudin14 while down-regulating claudin2 in intestinal epithelial cells, which helps enhance the tight junction between intestinal epithelial cells and reduce intestinal permeability (Fig. 3) [116].
Studies have demonstrated that OMVs secreted by various enterobacteria contain OMV-related virulence factors that can trigger the death of human intestinal epithelial cells, as well as the release of inflammatory factors [118]. The outbreak strain E. coli O104: H4 has been found to release OMVs carrying virulence factors, including Shiga toxin (Stx2a), which enters into intestinal epithelial cells, targets the mitochondria, and induces the release of cytochrome C. This, in turn, activates the caspase-9 and caspase-3 pathways, leading to the formation of apoptotic bodies and the apoptosis of intestinal epithelial cells [113]. [113]. Enterohemorrhagic E. Coli (EHEC) releases OMVs containing the virulence factor EHEC hemolysin (EHEC-Hly), which is taken up into lysosomes through endocytosis by intestinal epithelial cells. EHEC-Hly then escapes from lysosomes and targets mitochondria, causing a decrease in mitochondrial transmembrane potential and the release of cytochrome C. This induces the formation of apoptotic bodies and triggers apoptosis of intestinal epithelial cells by activating caspase-9 conduction channels [119, 120].
In Crohn’s disease (CD), the endoplasmic reticulum-localized stress response protein (ER-localized stress response protein, Gp96) is overexpressed on the surface of ileal epithelial cells. PAMP molecules carried by OMVs derived from Adherent-invasive E. coli (AIEC) recognize and bind to Gp96 receptors on the surface of intestinal epithelial cells, promoting AIEC invasion and mediating the destruction of intestinal mucosal epithelial cells in CD) [110, 121]. Fusobacterium nucleatum (F. nucleatum) secretes EVs containing a variety of virulence factors that promote M1 polarization of macrophages, leading to oxidative stress injury of intestinal epithelial cells. These EVs also activate receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3), ultimately leading to the activation of caspase-3-related signaling pathways. This promotes programmed cell necrosis of intestinal epithelial cells and destruction of the intestinal epithelial barrier [122].
GI tumor cells
Research on the effect of bacterial EVs on GI tumor cells is still in its early stages. However, current studies have found that some bacterial EVs can invade host cells, causing chronic inflammatory responses, damaging genetic material, and increasing the risk of host cell canceration [123]. Bacterial EVs have also been found to be capable of crossing physiological barriers and selectively accumulating near tumor cells, potentially altering the tumor microenvironment (TME) [124, 125].
Bacterial EVs use enhanced permeation and retention (EPR) effects and EPMP antigen molecules to induce tumor immune responses. Whether EVs can recognize introduced cells in tumor tissues and induce targeted tumor cell death requires further research. Nevertheless, modifying bacterial EVs to load chemotherapeutic drugs or anti-tumor components may be a new direction for anti-tumor therapy in the future [126, 127]. Animal experiments have shown that a mixture of Bifidobacterium lactis (B. lactis) and Lactobacillus rhamnosus (L. rhamnosus) can improve the level of intestinal butyrate, reduce the proliferation of cancerous cells, and decrease the activity of enzymes related to rectal cancer occurrence, thereby reducing the colon cancer morbidity of rats [128].
In mouse models, several intestinal microflorae have been found to promote colorectal cancer (CRC). For example, Enterotoxigenic B. fragilis (ETBF) and E. coli both carry PKS islands to promote toxin production, while F. nucleatum promotes CRC growth by stimulating inflammation and activating the β-catenin pathway [128, 129].
Exo-intestinal somatic cells
Some bacterial EVs can be absorbed into the blood and circulate to extraintestinal target cells, such as vascular endothelial cells, blood cells, and central neurons, causing diseases in cardiovascular, metabolic, and central nervous system (CNS) diseases [130]. Additionally, bacterial EVs can alter intestinal microecology and cause multi-system diseases. For instance, OMVs secreted by Bacteroides thetaiotaomicron (Bt) can be endocytosed and captured by intestinal epithelial cells through paracellular pathways and migrate to the submucosal and circulatory systems [131, 132].
Intestinal microorganisms
The composition of the intestinal microbiota is not solely determined by competition among microorganisms but also by their ability to sense and adapt to the intestinal microenvironment. The viability and defense mechanisms of microorganisms determine their ability to colonize and thrive in the gut. EVs derived from probiotic bacteria can support the growth and colonization of beneficial microorganisms while inhibiting the growth and colonization of foreign microorganisms, which are typically pathogenic or opportunistic. These EVs are crucial for maintaining the stability of the intestinal microbiota. When the microbiota becomes dysbiotic, pathogenic EVs can inhibit the growth and colonization of probiotics, alter the structure of the microbiota, and disrupt the homeostasis of the intestinal microecology.
Bacteria secrete EVs to deliver essential nutrients, functional genes, and enzymes with varying functions to the same flora. This enables the bacteria to enhance their viability and survive in the changing microenvironment of the intestine [133, 134]. Bifidobacterial EVs contain mucin-binding proteins that promote the colonization of bifidobacteria in the intestinal mucosa [135]. Similarly, Bacteroides ovatus (B. ovatus) OMVs carry inulin-degrading enzymes, which can degrade inulin and produce nutrients to support the growth of other Bacteroides species that cannot utilize inulin [136]. Proteomic analysis has revealed that OMVs of B. fragilis and Bacteroides thetaiotaomicron (B. thetaiotaomicron) selectively package a large number of carbohydrate hydrolysis and proteolytic enzymes that can digest and absorb various polysaccharides. These OMVs provide nutrients for bacteria that cannot decompose polysaccharides and help maintain the stability of the intestinal microecology [136, 137].
Furthermore, OMVs secreted by normal H. pylori have been found to promote biofilm formation in non-membranous H. pylori strains, demonstrating their ability to enhance biofilm formation [136].
EVs secreted by bacteria have the ability to transfer virulence factors to competitive flora and host cells. This can result in structural damage or dysfunction of competitive flora and host cells and even lead to cell death [138]. However, host cells and competing flora have corresponding defense mechanisms to resist the destructive effects of virulence factors, and they are constantly fighting and evolving, with the winner surviving and the loser migrating [139].
For instance, OMVs isolated from the foodborne pathogen E. coli O157:H7 have been shown to transfer virulence factors and other genetic material to recipient bacteria, such as E. coli JM109 or Salmonella enterica serovar irritable bowel [140]. This transfer enhances the cytotoxicity and defense ability of recipient bacteria [141]. Burkholderia cepacia (B. cepacia) have been shown to have strong antibacterial activity against A. baumannii, S. aureus, multidrug-resistant A. baumannii, methicillin-resistant S. aureus, and fungal pathogens. Further chemical analysis of OMVs derived from Burkholderia tylanica reveals that they carry peptidoglycan hydrolase and proteolytic enzymes, as well as antibacterial molecules, such as 4-hydroxy-2-alkylquinoline and rhamnolipid compounds. These molecules can affect ionophores, iron chelation, immunomodulation, and intercellular communication [142].
In addition, EVs secreted by bacteria can also carry defense factors, including virulence factors produced by various bacteria, toxic molecules in the environment, phage invasion, and host immune response. For instance, P. aeruginosa OMVs carry extracellular DNA, which not only mediates evasion of the host immune response but also promotes resistance to aminoglycoside antimicrobial agents [143]. Moreover, bacterial EVs are the primary means of HGT for spreading antibiotic resistance genes (ARGs), leading to reduced therapeutic efficiency of antibiotics and posing a serious threat to human health [144,145,146]. EVs can also transfer β-lactam resistance genes into and out of bacterial species, enhancing resistance to β-lactam antibiotics in many bacteria [147, 148]. Additionally, the number of active bacteriophages is significantly reduced by about 90% after co-incubation of T4 bacteriophage and EVs, indicating that EVs can combine irreversibly with T4 bacteriophages and thus significantly reduce their numbers in the environment. This, in turn, reduces the chance of bacterial infection by bacteriophages, as measured by the number of plaque-forming units [149].
Intestinal environment
The contents of the intestine primarily include chyme, mucus, and bacteria. Mucus is the exocrine fluid secreted by host cells, while the chyme is the digested food by the GI tract and serves as a shared resource for both host and bacteria. Each bacterium competes for high-quality resources and degrades harmful substances. Bacteria secrete EVs that carry digestive enzymes and transformed nutrients, which integrate into the intestinal chemical environment, digest chyme, provide nutrients, and transform harmful substances to bacteria, such as immune antibodies, antibiotics, toxic molecules, and phages, to improve the chemical environment for bacteria. Additionally, OMVs contain numerous enzymes that can degrade biological macromolecules. Therefore, when macromolecular substances are present in the living environment of bacteria, the release of OMVs can degrade them, enabling the bacteria to absorb and utilize these nutrients effectively [5, 27]. Secondly, OMVs have the ability to adsorb and bind antibiotics, thereby reducing their concentration, carrying antibiotic hydrolase, degrading antibiotics, and horizontally transferring ARGs to enhance the antibiotic resistance of bacteria [5, 7]. For instance, EVs released from S. Aureus under the stress of ampicillin contain a large number of proteases that can degrade β-lactam antibiotics and neutralize them in the environment [58]. Furthermore, EVs secreted by Bacteroides spp. containing β-lactamase can hydrolyze β-lactam antibiotics, reduce the concentration of antibiotics in the intestinal microenvironment, and improve the survival rate of intestinal symbiotic bacteria [150]. In a study, it has been reported that polymyxin treatment can induce sewage bacterial communities to produce a large number of EVs in the real environment, and these EVs can potentially reduce the concentration of antibiotics in water [151]. It has also been observed that polymyxin B and colistin, which are polypeptide antibiotics, can induce E. coli to release EVs that can adsorb antimicrobial peptides, thus eliminating the killing effect of these peptides on bacteria, possibly due to the binding of LPS carried by EVs to antimicrobial peptides [149, 152, 153].
Although excreted feces still contain a significant number of intestinal bacterial EVs, these EVs can remain stable and active in vitro. They carry a diverse range of enzymes and active molecules that degrade intestinal chyme, which can alter the characteristics of intestinal contents or feces, stimulate the mechanical movement of the intestine, and influence defecation patterns. In general, probiotics and their EVs can stimulate GI motility, improve stool characteristics, and promote regular bowel movements. In cases of dysbiosis, pathogenic bacterial EVs can weaken GI motility, result in dry stools, and cause constipation or diarrhea.
Potential application of bacterial EVs in GI
Bacterial EVs play a wide range of roles in the GI system. Although still in the basic research stage, bacterial EVs exhibit greater diversity and functionality than somatic EVs. They possess strong immunogenicity and can be conveniently detected in feces, urine, blood, and other bodily fluids. Obtaining bacterial EVs is relatively easy, thanks to mature bacterial culture and strain isolation technologies. Moreover, ideal EVs can be obtained through regulation. Bacterial EVs hold immense potential in disease diagnosis, vaccine or immune adjuvant development, intestinal microecology maintenance, and drug delivery. Currently, research technology presents the biggest obstacle to realizing this potential [154, 155]. However, as EV research technology matures, bacterial EVs will likely demonstrate even greater application potential (Fig. 4).
Diagnostic biomarkers
By detecting changes in bacterial EVs in bodily fluids, such as blood, feces, urine, and others, we can gain insight into the composition of the gut microbiota and identify imbalances in a timely manner. These bacterial EVs carry a large number of specific biological macromolecules that serve as the basis for the directional recognition and immunogenicity of bacterial EVs and are also key molecules in the development of intestinal and extraintestinal diseases. Discovering new biomarkers for the diagnosis of GI and extraintestinal diseases, as well as monitoring disease progression and treatment efficacy, is of great importance [156, 157]. For example, the levels of antibacterial EV antibodies in the blood of patients with bronchial asthma, chronic obstructive pulmonary disease (COPD), and lung cancer are found to be significantly higher than those of healthy controls, indicating that measuring EVs in these patients may serve as a diagnostic biomarker [158]. It is believed that in the near future, specific components of bacterial EVs in human bodily fluids can be identified to aid in the diagnosis of intestinal diseases.
Therapeutics
Bacterial EVs have a significant impact on host cells and intestinal microorganisms, rendering them a potential therapeutic tool for regulating gut microbiota imbalances and improving immune function. By modulating the balance of the gut microbiota and maintaining intestinal immune barriers, bacterial EVs can indirectly improve the intestinal microenvironment, protect the intestinal epithelial and mucus barriers, and enhance intestinal digestion, absorption, and defecation functions.
Regulation of flora disorder
Dysbiosis is considered to be the primary factor or a concurrent change in the development of various diseases, and the regulation of gut microbiota is becoming a key aspect of disease treatment. Traditionally, antibiotics, oral probiotics, and other methods have been used to control pathogenic bacteria. However, in some patients, these methods are ineffective and may even result in antibiotic-associated diarrhea. Fecal microbiota transplantation (FMT) has been successful in treating refractory diarrhea [159]. Compared to the direct administration of microbial agents, using bacterial EVs as a therapeutic approach has several advantages, such as the ability to cross the intestinal barrier, low toxicity, high plasticity, and specificity [155]. The future direction is to use EVs to control bacteria and maintain the homeostasis of gut microbiota through EVs, which can be more accurate and effective. The introduction of EVs from probiotics can not only inhibit the reproduction and colonization of pathogenic bacteria but also provide nutrients for normal gut microbiota and resist harmful substances, such as reactive oxygen species, antibiotics, and antimicrobial peptides [53].
Regulation of immune function
Bacterial EVs are known to carry specific immunogens and PAMP molecules that are related to the parent bacteria. They have strong immunogenicity and immune cell recognition ability, which makes them suitable for developing bacterial outer membrane vesicle vaccines or immune adjuvants [85, 93, 160]. For example, some countries, such as Cuba, Norway, New Zealand, and the Netherlands, have developed monovalent vaccines against local prevalent strains of group B meningococcal (MenB) using detergent-extracted outer membrane vesicles (dOMVs) as one of the primary antigens [161,162,163,164]. The process of dOMV detergent extraction reduces LPS/lipoprotein content and hence OMV endotoxicity. It can also induce the production of bactericidal antibodies against cell-surface outer-membrane proteins (OMPs) [165]. Based on this, a polyvalent MenB vaccine (MenB-4C) was developed by Novartis (China) Biomedical Research Co. in 2013. This vaccine contains dOMV and three recombinant proteins, and it can cover 66–91% of MenB strains worldwide [165,166,167]. EVs are known to carry a significant amount of LPS, which not only acts as a potent immunogen but also generates a strong heat source. LPS is a key component of virulence factors, which can cause damage to host cells, trigger immune cell inflammation, and even lead to death [168, 169]. However, lysozyme can bind strongly to LPS, and the complex formed by lysozyme can help to inhibit inflammatory responses. As a result, OMVs can be detoxified by lysozyme [170, 171].
Currently, OMVs are treated with detergents, such as sodium deoxycholate, to remove a large amount of LPS. However, this process can also cause the loss of some immunogenic lipoproteins [172]. To address this issue, researchers have introduced OMVs of Neisseria meningitidis (Nm) LpxL 1 mutant gene, which converts lipid A from six fatty acyl chains to five fatty acyl chains. This modification has been shown to reduce pyrogen toxicity to the host while retaining moderate immunogenicity of LPS, thus ensuring the effectiveness and safety of vaccine products. This approach is still in the clinical research Stage [173, 174]. In other studies, OMV vaccines have been developed against Mycobacterium tuberculosis (M. tuberculosis) and Staphylococcus aureus, which produce protective cellular and humoral immune responses in mice [175, 176]. Furthermore, vaccination with S. aureus OMVs has been shown to protect against active S. aureus infection [177]. Currently, OMV vaccines against other bacteria, including H. pylori, V. cholerae, and Klebsiella pneumoniae (K. pneumoniae), and genetically engineered Bacteroides OMV vaccines are in the late stages of research and development [115, 178, 179].
Another promising approach in vaccine development involves mimicking the mechanism of OMVs and incorporating specific immunogens of pathogenic bacteria into the ideal OMV vector [180,181,182]. For example, non-pathogenic E. coli OMVs are engineered to express Streptococcus pneumoniae (S. pneumoniae) surface glycans. These OMVs can generate immune responses comparable to commercially available Streptococcus pneumoniae vaccines [183].
To address bacterial resistance, the development of bacterial vaccines should focus on innovative solutions. In one study, bovine serum albumin (BSA) is encapsulated with OMVs to create a BSA-OMV nano-vaccine. This approach significantly improves the survival rate of mice infected with a lethal dose of carbapenem-resistant Klebsiella pneumoniae (CRKP) [184].
Drug delivery
Bacterial EVs possess stability and targetability, allowing them to recognize specific molecules and cells with an EPR effect. As non-toxic drug carriers with good human compatibility, bacterial EVs can improve the efficacy of drugs [155, 185, 186]. To prepare bacterial EVs for drug delivery, screening of suitable bacterial EVs should be done first and then transformed and modified to recognize and load drugs or bioactive substances. Specific PAMP molecules should be present on the surface of vector EVs for directionally recognizing target cells and introducing drugs and bioactive substances. Synthetic nanomaterial carriers lack the ability to replicate the surface features of vesicles, lack intercellular interaction, and lack targeting recognition ability [187]. OMVs derived from B. fragilis have the potential to be used as drug carriers for the treatment of intestinal diseases [188]. EVs secreted by Bacillus subtilis can transport across the GI epithelium, which is useful for food, nutrition, health care products, and clinical treatment [189]. [190]. Combining OMVs with miRNA can be used to treat cancer, such as intestinal cancer, where OMVs extracted from intestinal bacteria can encapsulate anti-tumor miRNA and be delivered orally to cancer tissues in the GI tract [190]. The combination of OMVs and miRNA can effectively inhibit metastatic tumor cells. E. coli OMVs encapsulating mediating pore silica and combined with 5-fluorouracil can enhance the drug concentration at a colon part and release the drug centrally in the TME, resulting in reduced systemic adverse reactions and improved treatment of CRC [191]. Levofloxacin-loaded A. baumannii OMVs can effectively invade E. coli, P. aeruginosa, and A. baumannii, kill E. coli, and produce good therapeutic effects in a mouse intestinal E. coli infection model [192]. Exogenous siRNA carried by cellular EVs can inhibit oncogene expression by targeting mRNA. Synthetic nanocarriers have made some progress in silencing oncogene expression with exogenous siRNA, and bacterial EVs are under research for this purpose [123, 193]. OMVs can also be adapted for genetic engineering and chemical engineering methods similar to eukaryotic exosomes for targeting delivery [194,195,196,197,198,199]. Targeting intestinal tissue delivery of OMVs is particularly meaningful for treating IBD [200].
Conclusions
The intestinal flora, which has coexisted with humans for hundreds of millions of years, is closely intertwined with human health and disease. The relationship between humans and microorganisms will continue to shape human health and disease in the future. Therefore, we must expand our research and knowledge of microorganisms and comprehend their evolution and variations, as this is the path towards ensuring human survival indefinitely.
EVs derived from somatic cells have shown promising results in treating GI diseases. In particular, EVs derived from intestinal epithelial cells, macrophages, and mesenchymal stem cells are currently undergoing clinical trials. However, bacterial EVs have even more diverse types and functions, stronger immunogenicity, and greater plasticity than human-derived EVs. OMVs are natural immune adjuvants that play a critical role in vaccine production, infection prevention and control, tumor treatment, and drug delivery. Bacterial EVs offer several advantages in maintaining intestinal microecology, regulating immunity, and serving as drug carriers, making them a promising candidate for a broad range of applications (Fig. 4).
However, there are several challenges that need to be addressed: (1) The biological mechanisms and structural components of bacterial EVs are not fully understood, necessitating further research. (2) Identifying OMV components is complex, and the functions of various components need to be determined. (3) Techniques for extracting, identifying, shaping, and preserving EVs are not yet ideal and can limit the development of related clinical applications. (4) Further research is required to mitigate the adverse effects of reducing the activity and toxicity of OMV surface antigens.
To overcome these obstacles, the development of small-molecule inhibitors or novel strains with bacterial toxin activity knocked out on the surface of OMV may be an effective strategy for reducing the immunogenicity of OMVs. It is reasonable to believe that with the advancement of technology, these challenges will be overcome, and OMV-based nanotechnology will develop into a powerful toolkit for intestinal targeted delivery, GI disease diagnosis, treatment, and other related fields.
Availability of data and materials
All relevant data were included in the paper.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- DMAIDs:
-
Disease-modifying anti-IBD drugs
- PDENs:
-
Plant-derived exosome-like nanoparticles
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- DDSs:
-
Drug delivery systems
- EXPO:
-
Exocyst-positive organelle
- MVBs:
-
Multivesicular bodies, ESCRT, endosomal sorting complex required for transport
- PA:
-
Phosphatidic acid
- PC:
-
Phosphatidylcholines
- DGDG:
-
Digalactosyldiacylglycerol
- MGDG:
-
Monogalactosyldiacylglycerol
- TEM:
-
Transmission electron microscopy
- SEM:
-
Scanning electron microscopy
- AFM:
-
Atomic force microscopy
- DLS:
-
Dynamic light scattering
- PE:
-
Phosphatidylethanolamine
- mTOR:
-
Mammalian target of rapamycin
- MAPK:
-
Mitogen-activated protein kinase
- mRNA:
-
MicroRNA
- sRNA:
-
Small RNA
- PEG:
-
Polyethylene glycol
- GDENs:
-
Grape-derived exosome-like nanoparticles
- DSS:
-
Dextran sulphate sodium
- GFDENs:
-
Grapefruit-derived exosome-like nanoparticles
- HO-1:
-
Heme oxygenase-1
- GDENs:
-
Ginger-derived exosome-like nanoparticles
- IECs:
-
Intestinal epithelial cells
- LGG:
-
Lactobacillus rhamnosus
- TLDENs:
-
Tea leaves-derived exosome-like nanoparticles
- BDENs:
-
Broccoli-derived exosome-like nanoparticles
- AMPK:
-
Adenosine monophosphate activated protein kinase
- MBDENs:
-
Mulberry bark-derived exosome-like nanoparticles
- AhR:
-
Aryl hydrocarbon receptor
- COPS8:
-
Constitute photomorphogenic homolog subunit 8
- CDENs:
-
Carrots-derived exosome-like nanoparticles
- TET8:
-
Tetraspanin-8
- PEN1:
-
PENETRATION 1
- EC1:
-
Extracellular domains 1
- TL:
-
Targeting ligand
- DSPE-PEG:
-
1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly (ethylene glycol)
- E. coli :
-
Escherichia coli
- N. gonorrhoeae :
-
Neisseria gonorrhoeae
- P. Aeruginosa :
-
Pseudomonas aeruginosa
- H. influenzae :
-
Haemophilus influenzae
- B. fragiles :
-
Bacteroides fragilis
- A. muciniphila :
-
Akkermansia muciniphila
- L. paracasei :
-
Lactobacillus paracasei
- V. cholera :
-
Vibrio cholerae
- H. pylori :
-
Helicobacter pylori
- S. typhimurium :
-
Salmonella typhimurium
- A. baumannii :
-
Acinetobacter baumannii
- S. toxin :
-
Shiga toxin
- F. nucleatum :
-
Fusobacterium nucleatum
- B. lactis :
-
Bifidobacterium lactis
- L. rhamnoses :
-
Lactobacillus rhamnosus
- B. ovatus :
-
Bacteroides ovatus
- B. thetaiotaomicron :
-
Bacteroides thetaiotaomicron
- S. enterica :
-
Salmonella enterica
- B. cepacia :
-
Burkholderia cepacia
- S. aureus :
-
Staphylococcus aureus
- MenB :
-
B Meningococcal
- Nm :
-
Neisseria meningitidis
- M. tuberculosis :
-
Mycobacterium tuberculosis
- K. pneumonia :
-
Klebsiella pneumoniae
- S. pneumonia :
-
Streptococcus pneumoniae
References
Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol life Sci CMLS. 2019;76:473–93. https://doi.org/10.1007/s00018-018-2943-4.
Gilbert JA, et al. Current understanding of the human microbiome. Nat Med. 2018;24:392–400. https://doi.org/10.1038/nm.4517.
Shetty SA, Hugenholtz F, Lahti L, Smidt H, de Vos WM. Intestinal microbiome landscaping: insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol Rev. 2017;41:182–99. https://doi.org/10.1093/femsre/fuw045.
Nogacka AM, et al. Xenobiotics formed during food processing: their relation with the intestinal microbiota and colorectal cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20082051.
Jahromi LP, Fuhrmann G. Bacterial extracellular vesicles: understanding biology promotes applications as nanopharmaceuticals. Adv Drug Deliv Rev. 2021;173:125–40. https://doi.org/10.1016/j.addr.2021.03.012.
Unal CM, Schaar V, Riesbeck K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin Immunopathol. 2011;33:395–408. https://doi.org/10.1007/s00281-010-0231-y.
Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev Microbiol. 2021;75:609–30. https://doi.org/10.1146/annurev-micro-052821-031444.
Pérez-Cruz C, Delgado L, López-Iglesias C, Mercade E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS ONE. 2015;10:e0116896. https://doi.org/10.1371/journal.pone.0116896.
Uddin MJ, et al. The role of bacterial membrane vesicles in the dissemination of antibiotic resistance and as promising carriers for therapeutic agent delivery. Microorganisms. 2020. https://doi.org/10.3390/microorganisms8050670.
Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–19. https://doi.org/10.1038/nrmicro3525.
Li DF, et al. Extracellular vesicles: the next generation theranostic nanomedicine for inflammatory bowel disease. Int J Nanomedicine. 2022;17:3893–911. https://doi.org/10.2147/ijn.S370784.
Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161. https://doi.org/10.1002/jev2.12161.
Bose S, Aggarwal S, Singh DV, Acharya N. Extracellular vesicles: an emerging platform in gram-positive bacteria. Microbial Cell. 2020;7:312–22. https://doi.org/10.15698/mic2020.12.737.
Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. https://doi.org/10.1126/science.1058709.
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. https://doi.org/10.1038/nature11234.
Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. https://doi.org/10.1038/nature11053.
Rowland I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. https://doi.org/10.1007/s00394-017-1445-8.
Wan MLY, Ling KH, El-Nezami H, Wang MF. Influence of functional food components on gut health. Crit Rev Food Sci Nutr. 2019;59:1927–36. https://doi.org/10.1080/10408398.2018.1433629.
Verbeke KA, et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28:42–66. https://doi.org/10.1017/s0954422415000037.
Hills RD Jr, et al. Gut microbiome: profound implications for diet and disease. Nutrients. 2019. https://doi.org/10.3390/nu11071613.
Del Chierico F, et al. Phylogenetic and metabolic tracking of gut microbiota during perinatal development. PLoS ONE. 2015;10:e0137347. https://doi.org/10.1371/journal.pone.0137347.
Schanche M, et al. High-resolution analyses of overlap in the microbiota between mothers and their children. Curr Microbiol. 2015;71:283–90. https://doi.org/10.1007/s00284-015-0843-5.
Filyk HA, Osborne LC. The multibiome: the intestinal ecosystem’s influence on immune homeostasis, health, and disease. EBioMedicine. 2016;13:46–54. https://doi.org/10.1016/j.ebiom.2016.10.007.
DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22:1137–50. https://doi.org/10.1097/mib.0000000000000750.
Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24–43. https://doi.org/10.15252/embr.201439363.
Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol. 2006;61:839–46. https://doi.org/10.1111/j.1365-2958.2006.05272.x.
Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13–24. https://doi.org/10.1038/s41579-018-0112-2.
Roier S, Zingl FG, Cakar F, Schild S. Bacterial outer membrane vesicle biogenesis: a new mechanism and its implications. Microbial Cell. 2016;3:257–9. https://doi.org/10.15698/mic2016.06.508.
Wang M, Nie Y, Wu XL. Extracellular heme recycling and sharing across species by novel mycomembrane vesicles of a gram-positive bacterium. ISME J. 2021;15:605–17. https://doi.org/10.1038/s41396-020-00800-1.
Obana N, et al. Immunoactive clostridial membrane vesicle production is regulated by a sporulation factor. Infect Immun. 2017. https://doi.org/10.1128/iai.00096-17.
Guerrero-Mandujano A, Hernández-Cortez C, Ibarra JA, Castro-Escarpulli G. The outer membrane vesicles: secretion system type zero. Traffic. 2017;18:425–32. https://doi.org/10.1111/tra.12488.
Meldolesi J. Extracellular vesicles, news about their role in immune cells: physiology, pathology and diseases. Clin Exp Immunol. 2019;196:318–27. https://doi.org/10.1111/cei.13274.
Furuyama N, Sircili MP. Outer membrane vesicles (OMVs) produced by gram-negative bacteria: structure, functions, biogenesis, and vaccine application. Biomed Res Int. 2021;2021:1490732. https://doi.org/10.1155/2021/1490732.
Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84. https://doi.org/10.1146/annurev.micro.091208.073413.
Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–55. https://doi.org/10.1101/gad.1299905.
Michel LV, Gaborski T. Outer membrane vesicles as molecular biomarkers for gram-negative sepsis: taking advantage of nature’s perfect packages. J Biol Chem. 2022;298:102483. https://doi.org/10.1016/j.jbc.2022.102483.
Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–33. https://doi.org/10.1128/jb.181.16.4725-4733.1999.
Avila-Calderón ED, et al. Outer membrane vesicles of gram-negative bacteria: an outlook on biogenesis. Front Microbiol. 2021;12:557902. https://doi.org/10.3389/fmicb.2021.557902.
Yu YJ, Wang XH, Fan GC. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin. 2018;39:514–33. https://doi.org/10.1038/aps.2017.82.
Pérez-Cruz C, et al. New type of outer membrane vesicle produced by the gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Appl Environ Microbiol. 2013;79:1874–81. https://doi.org/10.1128/aem.03657-12.
Turnbull L, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220. https://doi.org/10.1038/ncomms11220.
Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620–30. https://doi.org/10.1038/nrmicro3480.
Vitse J, Devreese B. The contribution of membrane vesicles to bacterial pathogenicity in cystic fibrosis infections and healthcare associated pneumonia. Front Microbiol. 2020;11:630. https://doi.org/10.3389/fmicb.2020.00630.
Murase K, et al. Biological effect of Streptococcus pyogenes-released extracellular vesicles on human monocytic cells, induction of cytotoxicity, and inflammatory response. Front Cell Infect Microbiol. 2021;11:711144. https://doi.org/10.3389/fcimb.2021.711144.
Kopparapu PK, et al. Lipoproteins are responsible for the pro-inflammatory property of staphylococcus aureus extracellular vesicles. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22137099.
Liu Y, Defourny KAY, Smid EJ, Abee T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front Microbiol. 2018;9:1502. https://doi.org/10.3389/fmicb.2018.01502.
Toyofuku M, et al. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun. 2017;8:481. https://doi.org/10.1038/s41467-017-00492-w.
Ñahui Palomino RA, Vanpouille C, Costantini PE, Margolis L. Microbiota-host communications: bacterial extracellular vesicles as a common language. PLoS Pathog. 2021;17:e1009508. https://doi.org/10.1371/journal.ppat.1009508.
Cai W, et al. Bacterial outer membrane vesicles, a potential vaccine candidate in interactions with host cells based. Diagn Pathol. 2018;13:95. https://doi.org/10.1186/s13000-018-0768-y.
Gill S, Catchpole R, Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev. 2019;43:273–303. https://doi.org/10.1093/femsre/fuy042.
Bonnington KE, Kuehn MJ. Outer membrane vesicle production facilitates LPS remodeling and outer membrane maintenance in salmonella during environmental transitions. MBio. 2016. https://doi.org/10.1128/mBio.01532-16.
Schwechheimer C, Sullivan CJ, Kuehn MJ. Envelope control of outer membrane vesicle production in gram-negative bacteria. Biochemistry. 2013;52:3031–40. https://doi.org/10.1021/bi400164t.
Caruana JC, Walper SA. Bacterial membrane vesicles as mediators of microbe- microbe and microbe- host community interactions. Front Microbiol. 2020;11:432. https://doi.org/10.3389/fmicb.2020.00432.
Toyofuku M, et al. Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environ Microbiol. 2014;16:2927–38. https://doi.org/10.1111/1462-2920.12260.
Prados-Rosales R, et al. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J Bacteriol. 2014;196:1250–6. https://doi.org/10.1128/jb.01090-13.
Keenan JI, Davis KA, Beaugie CR, McGovern JJ, Moran AP. Alterations in Helicobacter pylori outer membrane and outer membrane vesicle-associated lipopolysaccharides under iron-limiting growth conditions. Innate Immun. 2008;14:279–90. https://doi.org/10.1177/1753425908096857.
Baumgarten T, et al. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol. 2012;78:6217–24. https://doi.org/10.1128/aem.01525-12.
Kim SW, et al. Significant increase in the secretion of extracellular vesicles and antibiotics resistance from methicillin-resistant Staphylococcus aureus induced by ampicillin stress. Sci Rep. 2020;10:21066. https://doi.org/10.1038/s41598-020-78121-8.
Bos J, Cisneros LH, Mazel D. Real-time tracking of bacterial membrane vesicles reveals enhanced membrane traffic upon antibiotic exposure. Sci Adv. 2021. https://doi.org/10.1126/sciadv.abd1033.
Orench-Rivera N, Kuehn MJ. Environmentally controlled bacterial vesicle-mediated export. Cell Microbiol. 2016;18:1525–36. https://doi.org/10.1111/cmi.12676.
Devos S, et al. Membrane vesicle secretion and prophage induction in multidrug-resistant Stenotrophomonas maltophilia in response to ciprofloxacin stress. Environ Microbiol. 2017;19:3930–7. https://doi.org/10.1111/1462-2920.13793.
Woith E, Fuhrmann G, Melzig MF. Extracellular vesicles-connecting kingdoms. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20225695.
Andreoni F, et al. Antibiotics stimulate formation of vesicles in staphylococcus aureus in both phage-dependent and -independent fashions and via different Routes. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/aac.01439-18.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. https://doi.org/10.1038/nrm.2017.125.
Quaglia M, et al. Extracellular vesicles as mediators of cellular crosstalk between immune system and kidney graft. Front Immunol. 2020;11:74. https://doi.org/10.3389/fimmu.2020.00074.
Chen Y, Liu L, Fu H, Wei C, Jin Q. Comparative proteomic analysis of outer membrane vesicles from Shigella flexneri under different culture conditions. Biochem Biophys Res Commun. 2014;453:696–702. https://doi.org/10.1016/j.bbrc.2014.09.142.
Kim YS, et al. Extracellular vesicles, especially derived from gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2013;43:443–54. https://doi.org/10.1111/cea.12085.
Cahill BK, Seeley KW, Gutel D, Ellis TN. Klebsiella pneumoniae O antigen loss alters the outer membrane protein composition and the selective packaging of proteins into secreted outer membrane vesicles. Microbiol Res. 2015;180:1–10. https://doi.org/10.1016/j.micres.2015.06.012.
Altindis E, Fu Y, Mekalanos JJ. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc Natl Acad Sci USA. 2014;111:E1548-1556. https://doi.org/10.1073/pnas.1403683111.
Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053. https://doi.org/10.3389/fmicb.2017.01053.
Lee J, Kim OY, Gho YS. Proteomic profiling of gram-negative bacterial outer membrane vesicles: current perspectives. Proteomics Clin Appl. 2016;10:897–909. https://doi.org/10.1002/prca.201600032.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. https://doi.org/10.1038/ncb1596.
Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. https://doi.org/10.1038/ncb1800.
Wei Z, et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8:1145. https://doi.org/10.1038/s41467-017-01196-x.
Hoen Nolte-’t EN, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–85. https://doi.org/10.1093/nar/gks658.
Cicero AL, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Biol. 2015;35:69–77. https://doi.org/10.1016/j.ceb.2015.04.013.
Gyorgy B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–64. https://doi.org/10.1146/annurev-pharmtox-010814-124630.
Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275:12489–96. https://doi.org/10.1074/jbc.275.17.12489.
Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from gram-negative bacteria. Microbiology. 2014;160:2109–21. https://doi.org/10.1099/mic.0.079400-0.
Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. https://doi.org/10.1146/annurev.biochem.71.110601.135414.
Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms21010107.
Bitto NJ, et al. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep. 2017;7:7072. https://doi.org/10.1038/s41598-017-07288-4.
Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. https://doi.org/10.3402/jev.v4.27066.
Larabi A, Barnich N, Nguyen HTT. Emerging role of exosomes in diagnosis and treatment of infectious and inflammatory bowel diseases. Cells. 2020. https://doi.org/10.3390/cells9051111.
Kuipers ME, Hokke CH, Smits HH, Nolte-’t Hoen ENM. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: an overview. Front Microbiol. 2018;9:2182. https://doi.org/10.3389/fmicb.2018.02182.
Cecil JD, et al. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol. 2017;8:1017. https://doi.org/10.3389/fimmu.2017.01017.
Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–87. https://doi.org/10.1038/nri3837.
Schlatterer K, et al. The mechanism behind bacterial lipoprotein release: phenol-soluble modulins mediate toll-like receptor 2 activation via extracellular vesicle release from Staphylococcus aureus. MBio. 2018. https://doi.org/10.1128/mBio.01851-18.
Johnston EL, Heras B, Kufer TA, Kaparakis-Liaskos M. Detection of bacterial membrane vesicles by NOD-like receptors. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22031005.
Elizagaray ML, et al. Canonical and non-canonical inflammasome activation by outer membrane vesicles derived from Bordetella pertussis. Front Immunol. 2020;11:1879. https://doi.org/10.3389/fimmu.2020.01879.
Layre E. Trafficking of mycobacterium tuberculosis envelope components and release within extracellular vesicles: host-pathogen interactions beyond the wall. Front Immunol. 2020;11:1230. https://doi.org/10.3389/fimmu.2020.01230.
Schetters STT, et al. Outer membrane vesicles engineered to express membrane-bound antigen program dendritic cells for cross-presentation to CD8(+) T cells. Acta Biomater. 2019;91:248–57. https://doi.org/10.1016/j.actbio.2019.04.033.
Lu Q, et al. Immunology of inflammatory bowel disease: molecular mechanisms and therapeutics. J Inflamm Res. 2022;15:1825–44. https://doi.org/10.2147/jir.S353038.
Chu H, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–20. https://doi.org/10.1126/science.aad9948.
Mazmanian SK. Capsular polysaccharides of symbiotic bacteria modulate immune responses during experimental colitis. J Pediatr Gastroenterol Nutr. 2008;46(Suppl 1):E11-12. https://doi.org/10.1097/01.mpg.0000313824.70971.a7.
Shen Y, et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–20. https://doi.org/10.1016/j.chom.2012.08.004.
Kang CS, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE. 2013;8:e76520. https://doi.org/10.1371/journal.pone.0076520.
Choi JH, et al. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med. 2020;52:423–37. https://doi.org/10.1038/s12276-019-0359-3.
Kim JH, Lee J, Park KS, Hong SW, Gho YS. Drug repositioning to alleviate systemic inflammatory response syndrome caused by gram-negative bacterial outer membrane vesicles. Adv Healthcare Mater. 2018;7:e1701476. https://doi.org/10.1002/adhm.201701476.
Mirhoseini A, Amani J, Nazarian S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it. Microb Pathog. 2018;117:162–9. https://doi.org/10.1016/j.micpath.2018.02.032.
Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23:4538–49. https://doi.org/10.1038/sj.emboj.7600471.
Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem. 2002;277:32538–45. https://doi.org/10.1074/jbc.M203740200.
Zingl FG, et al. Outer membrane vesicles of vibrio cholerae protect and deliver active cholera toxin to host cells via porin-dependent uptake. MBio. 2021;12:e0053421. https://doi.org/10.1128/mBio.00534-21.
Chatterjee D, Chaudhuri K. Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. J Biol Chem. 2013;288:4299–309. https://doi.org/10.1074/jbc.M112.408302.
Winter J, Letley D, Rhead J, Atherton J, Robinson K. Helicobacter pylori membrane vesicles stimulate innate pro- and anti-inflammatory responses and induce apoptosis in Jurkat T cells. Infect Immun. 2014;82:1372–81. https://doi.org/10.1128/iai.01443-13.
Yang J, et al. Bacterial outer membrane vesicle-mediated cytosolic delivery of flagellin triggers host NLRC4 canonical inflammasome signaling. Front Immunol. 2020;11:581165. https://doi.org/10.3389/fimmu.2020.581165.
Lindmark B, et al. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009;9:220. https://doi.org/10.1186/1471-2180-9-220.
Tiku V, et al. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Sci Rep. 2021;11:618. https://doi.org/10.1038/s41598-020-79966-9.
Filip R. An update on the role of extracellular vesicles in the pathogenesis of necrotizing enterocolitis and inflammatory bowel diseases. Cells. 2021. https://doi.org/10.3390/cells10113202.
Deo P, et al. Mitochondrial dysfunction caused by outer membrane vesicles from gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat Microbiol. 2020;5:1418–27. https://doi.org/10.1038/s41564-020-0773-2.
Fulsundar S, et al. Molecular characterization of outer membrane vesicles released from Acinetobacter radioresistens and their potential roles in pathogenesis. Microb Pathog. 2015;83–84:12–22. https://doi.org/10.1016/j.micpath.2015.04.005.
Losier TT, et al. AMPK promotes xenophagy through priming of autophagic kinases upon detection of bacterial outer membrane vesicles. Cell Rep. 2019;26:2150-2165.e2155. https://doi.org/10.1016/j.celrep.2019.01.062.
Kunsmann L, et al. Virulence from vesicles: novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci Rep. 2015;5:13252. https://doi.org/10.1038/srep13252.
Mondal A, et al. Cytotoxic and inflammatory responses induced by outer membrane vesicle-associated biologically active proteases from vibrio cholerae. Infect Immun. 2016;84:1478–90. https://doi.org/10.1128/iai.01365-15.
Carvalho AL, et al. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J Extracell Vesicles. 2019;8:1632100. https://doi.org/10.1080/20013078.2019.1632100.
Alvarez CS, Badia J, Bosch M, Giménez R, Baldomà L. Outer membrane vesicles and soluble factors released by probiotic Escherichia coli nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 2016;7:1981. https://doi.org/10.3389/fmicb.2016.01981.
Fábrega MJ, et al. Intestinal Anti-inflammatory effects of outer membrane vesicles from Escherichia coli nissle 1917 in DSS-experimental colitis in mice. Front Microbiol. 2017;8:1274. https://doi.org/10.3389/fmicb.2017.01274.
Chen S, et al. Dysregulated hemolysin liberates bacterial outer membrane vesicles for cytosolic lipopolysaccharide sensing. PLoS Pathog. 2018;14:e1007240. https://doi.org/10.1371/journal.ppat.1007240.
Bielaszewska M, et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013;9:e1003797. https://doi.org/10.1371/journal.ppat.1003797.
Bielaszewska M, et al. Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic Escherichia coli O157: intracellular delivery, trafficking and mechanisms of cell injury. PLoS Pathog. 2017;13:e1006159. https://doi.org/10.1371/journal.ppat.1006159.
Rolhion N, et al. Abnormally expressed ER stress response chaperone Gp96 in CD favours adherent-invasive Escherichia coli invasion. Gut. 2010;59:1355–62. https://doi.org/10.1136/gut.2010.207456.
Liu L, Liang L, Yang C, Zhou Y, Chen Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes. 2021;13:1–20. https://doi.org/10.1080/19490976.2021.1902718.
Chen Y, et al. Extracellular vesicles in inter-kingdom communication in gastrointestinal cancer. Am J Cancer Res. 2021;11:1087–103.
Raman M, et al. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut microbes. 2013;4:181–92. https://doi.org/10.4161/gmic.23919.
Qing S, et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32:e2002085. https://doi.org/10.1002/adma.202002085.
Amatya SB, Salmi S, Kainulainen V, Karihtala P, Reunanen J. Bacterial extracellular vesicles in gastrointestinal tract cancer: an unexplored territory. Cancers. 2021. https://doi.org/10.3390/cancers13215450.
Zhang Y, Fang Z, Li R, Huang X, Liu Q. Design of outer membrane vesicles as cancer vaccines: a new toolkit for cancer therapy. Cancers. 2019. https://doi.org/10.3390/cancers11091314.
Femia AP, et al. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–60. https://doi.org/10.1093/carcin/23.11.1953.
Rubinstein MR, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. https://doi.org/10.1016/j.chom.2013.07.012.
Cecil JD, Sirisaengtaksin N, O’Brien-Simpson NM, Krachler AM. Outer membrane vesicle-host cell interactions. Microbiol Spectr. 2019. https://doi.org/10.1128/microbiolspec.PSIB-0001-2018.
Jones EJ, et al. The Uptake, trafficking, and biodistribution of Bacteroides thetaiotaomicron generated outer membrane vesicles. Front Microbiol. 2020;11:57. https://doi.org/10.3389/fmicb.2020.00057.
Durant L, et al. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome. 2020;8:88. https://doi.org/10.1186/s40168-020-00868-z.
Roszkowiak J, et al. Interspecies outer membrane vesicles (OMVs) modulate the sensitivity of pathogenic bacteria and pathogenic yeasts to cationic peptides and serum complement. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20225577.
Domingues S, Nielsen KM. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr Opin Microbiol. 2017;38:16–21. https://doi.org/10.1016/j.mib.2017.03.012.
Nishiyama K, et al. Extracellular vesicles produced by Bifidobacterium longum export mucin-binding proteins. Appl Environ Microbiol. 2020. https://doi.org/10.1128/aem.01464-20.
Rakoff-Nahoum S, Coyne MJ, Comstock LE. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol CB. 2014;24:40–9. https://doi.org/10.1016/j.cub.2013.10.077.
Elhenawy W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. MBio. 2014;5:e00909-00914. https://doi.org/10.1128/mBio.00909-14.
Rueter C, Bielaszewska M. Secretion and delivery of intestinal pathogenic Escherichia coli virulence factors via outer membrane vesicles. Front Cell Infect Microbiol. 2020;10:91. https://doi.org/10.3389/fcimb.2020.00091.
Koeppen K, et al. A Novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 2016;12:e1005672. https://doi.org/10.1371/journal.ppat.1005672.
Yaron S, Kolling GL, Simon L, Matthews KR. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl Environ Microbiol. 2000;66:4414–20. https://doi.org/10.1128/aem.66.10.4414-4420.2000.
Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology. 1999;145(Pt 8):2051–60. https://doi.org/10.1099/13500872-145-8-2051.
Wang Y, et al. Burkholderia thailandensis outer membrane vesicles exert antimicrobial activity against drug-resistant and competitor microbial species. J Microbiol. 2020;58:550–62. https://doi.org/10.1007/s12275-020-0028-1.
Sarkar S. Release mechanisms and molecular interactions of Pseudomonas aeruginosa extracellular DNA. Appl Microbiol Biotechnol. 2020;104:6549–64. https://doi.org/10.1007/s00253-020-10687-9.
Pruden A, Pei R, Storteboom H, Carlson KH. Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environ Sci Technol. 2006;40:7445–50. https://doi.org/10.1021/es060413l.
Allen HK, et al. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8:251–9. https://doi.org/10.1038/nrmicro2312.
Nazarian P, Tran F, Boedicker JQ. Modeling multispecies gene flow dynamics reveals the unique roles of different horizontal gene transfer mechanisms. Front Microbiol. 2018;9:2978. https://doi.org/10.3389/fmicb.2018.02978.
Rumbo C, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:3084–90. https://doi.org/10.1128/aac.00929-10.
Chatterjee S, Mondal A, Mitra S, Basu S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J Antimicrob Chemother. 2017;72:2201–7. https://doi.org/10.1093/jac/dkx131.
Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258. https://doi.org/10.1186/1471-2180-11-258.
Stentz R, et al. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J Antimicrob Chemother. 2015;70:701–9. https://doi.org/10.1093/jac/dku466.
Maestre-Carballa L, et al. Insights into the antibiotic resistance dissemination in a wastewater effluent microbiome: bacteria, viruses and vesicles matter. Environ Microbiol. 2019;21:4582–96. https://doi.org/10.1111/1462-2920.14758.
Steimle A, Autenrieth IB, Frick JS. Structure and function: Lipid A modifications in commensals and pathogens. Int J Med Microbiol IJMM. 2016;306:290–301. https://doi.org/10.1016/j.ijmm.2016.03.001.
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50. https://doi.org/10.1038/nrmicro1098.
Chen SZ, Chen Y. The role of intestinal bacteria derived extracellular vesicles in inflammatory bowel disease. Zhonghua Nei Ke Za Zhi. 2021;60:932–6. https://doi.org/10.3760/cma.j.cn112138-20201013-00862.
Li M, et al. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release Off J Control Release Soc. 2020;323:253–68. https://doi.org/10.1016/j.jconrel.2020.04.031.
Zhou Y, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018. https://doi.org/10.1128/mSystems.00188-17.
Nah G, et al. Type-2 diabetics reduces spatial variation of microbiome based on extracellur vesicles from gut microbes across human body. Sci Rep. 2019;9:20136. https://doi.org/10.1038/s41598-019-56662-x.
Yang J, et al. Lung disease diagnostic model through IgG sensitization to microbial extracellular vesicles. Allergy Asthma Immunol Res. 2020;12:669–83. https://doi.org/10.4168/aair.2020.12.4.669.
Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–52. https://doi.org/10.1080/19490976.2017.1290757.
Schwab A, et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol. 2015;6:1132. https://doi.org/10.3389/fmicb.2015.01132.
Fredriksen JH, et al. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–79.
Sierra GV, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207.
Thornton V, et al. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine. 2006;24:1395–400. https://doi.org/10.1016/j.vaccine.2005.09.043.
Claassen I, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–8. https://doi.org/10.1016/0264-410x(96)00020-5.
Micoli F, MacLennan CA. Outer membrane vesicle vaccines. Semin Immunol. 2020;50:101433. https://doi.org/10.1016/j.smim.2020.101433.
Masforrol Y, et al. A deeper mining on the protein composition of VA-MENGOC-BC®: an OMV-based vaccine against N. meningitidis serogroup B and C. Hum Vaccin Immunother. 2017;13:2548–60. https://doi.org/10.1080/21645515.2017.1356961.
Beernink PT, Vianzon V, Lewis LA, Moe GR, Granoff DM. A meningococcal outer membrane vesicle vaccine with overexpressed mutant FHbp elicits higher protective antibody responses in infant rhesus macaques than a licensed serogroup B vaccine. MBio. 2019. https://doi.org/10.1128/mBio.01231-19.
Vanaja SK, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell. 2016;165:1106–19. https://doi.org/10.1016/j.cell.2016.04.015.
Simpson BW, Trent MS. Pushing the envelope: LPS modifications and their consequences. Nat Rev Microbiol. 2019;17:403–16. https://doi.org/10.1038/s41579-019-0201-x.
Takada K, Ohno N, Yadomae T. Binding of lysozyme to lipopolysaccharide suppresses tumor necrosis factor production in vivo. Infect Immun. 1994;62:1171–5. https://doi.org/10.1128/iai.62.4.1171-1175.1994.
Peng LH, et al. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma. Sci Adv. 2020;6:eaba2735. https://doi.org/10.1126/sciadv.aba2735.
Christodoulides M, Heckels J. Novel approaches to Neisseria meningitidis vaccine design. Pathog Dis. 2017. https://doi.org/10.1093/femspd/ftx033.
Keiser PB, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011;29:1413–20. https://doi.org/10.1016/j.vaccine.2010.12.039.
van der Ley P, et al. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69:5981–90. https://doi.org/10.1128/iai.69.10.5981-5990.2001.
Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. 2018;9:1379. https://doi.org/10.1038/s41467-018-03847-z.
Prados-Rosales R, et al. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. MBio. 2014;5:e01921-e11914. https://doi.org/10.1128/mBio.01921-14.
Choi SJ, et al. Active immunization with extracellular vesicles derived from staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via th1 cell-mediated immunity. PLoS ONE. 2015;10:e0136021. https://doi.org/10.1371/journal.pone.0136021.
Lee WH, et al. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp Mol Med. 2015;47:e183. https://doi.org/10.1038/emm.2015.59.
Song Z, et al. Outer membrane vesicles of Helicobacter pylori 7.13 as adjuvants promote protective efficacy against Helicobacter pylori infection. Front Microbiol. 2020;11:1340. https://doi.org/10.3389/fmicb.2020.01340.
Gasperini G, et al. Outer membrane vesicles (OMV)-based and proteomics-driven antigen selection identifies novel factors contributing to Bordetella pertussis adhesion to epithelial cells. Mol Cell Proteomics MCP. 2018;17:205–15. https://doi.org/10.1074/mcp.RA117.000045.
Kunjantarachot A, Phanaksri T. Effective platform for the production of recombinant outer membrane vesicles in gram-negative bacteria. J Microbiol Biotechnol. 2022;32:621–9. https://doi.org/10.4014/jmb.2003.03023.
Ojima Y, Sawabe T, Konami K, Azuma M. Construction of hypervesiculation Escherichia coli strains and application for secretory protein production. Biotechnol Bioeng. 2020;117:701–9. https://doi.org/10.1002/bit.27239.
Price NL, et al. Glycoengineered outer membrane vesicles: a novel platform for bacterial vaccines. Sci Rep. 2016;6:24931. https://doi.org/10.1038/srep24931.
Wu G, et al. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomed Nanotechnol Biol Med. 2020;24:102148. https://doi.org/10.1016/j.nano.2019.102148.
Alves NJ, Turner KB, Medintz IL, Walper SA. Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci Rep. 2016;6:24866. https://doi.org/10.1038/srep24866.
Arigita C, et al. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine. 2004;22:629–42. https://doi.org/10.1016/j.vaccine.2003.08.027.
Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1523. https://doi.org/10.1002/wnan.1523.
Badi SA, et al. Extraction and evaluation of outer membrane vesicles from two important gut microbiota members, bacteroides fragilis and bacteroides thetaiotaomicron. Cell J. 2020;22:344–9. https://doi.org/10.22074/cellj.2020.6499.
Rubio APD, et al. Transcytosis of Bacillus subtilis extracellular vesicles through an in vitro intestinal epithelial cell model. Sci Rep. 2020;10:3120. https://doi.org/10.1038/s41598-020-60077-4.
Tran AX, et al. Periplasmic cleavage and modification of the 1-phosphate group of Helicobacter pylori lipid A. J Biol Chem. 2004;279:55780–91. https://doi.org/10.1074/jbc.M406480200.
Shi J, et al. Biofilm-encapsulated nano drug delivery system for the treatment of colon cancer. J Microencapsul. 2020;37:481–91. https://doi.org/10.1080/02652048.2020.1797914.
Huang W, et al. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J Control Release Off J Control Release Soc. 2020;317:1–22. https://doi.org/10.1016/j.jconrel.2019.11.017.
Meng W, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Delivery. 2020;27:585–98. https://doi.org/10.1080/10717544.2020.1748758.
Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95. https://doi.org/10.7150/thno.52570.
Xu X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. https://doi.org/10.1016/j.biomaterials.2020.120539.
Liang Y, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938–47. https://doi.org/10.1021/acsami.0c10458.
Liang Y, et al. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12:4866–78. https://doi.org/10.7150/thno.69368.
Liang Y, et al. Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading. Biomater Sci. 2022;10:4095–106. https://doi.org/10.1039/d2bm00480a.
Liang Y, et al. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Mol Ther. 2022. https://doi.org/10.1016/j.ymthe.2022.10.008.
Li DF, et al. Nanoparticles for oral delivery: targeted therapy for inflammatory bowel disease. J Mater Chem B. 2022;10:5853–72. https://doi.org/10.1039/d2tb01190e.
Duan L, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387–97. https://doi.org/10.1039/d0nr07622h.
Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. https://doi.org/10.1016/j.semcdb.2015.02.006.
Lee JH, et al. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS ONE. 2013;8:e73196. https://doi.org/10.1371/journal.pone.0073196.
Cuesta CM, Guerri C, Ureña J, Pascual M. Role of microbiota-derived extracellular vesicles in gut-brain communication. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22084235.
Schulz E, Karagianni A, Koch M, Fuhrmann G. Hot EVs—How temperature affects extracellular vesicles. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2020;146:55–63. https://doi.org/10.1016/j.ejpb.2019.11.010.
Valguarnera E, Scott NE, Azimzadeh P, Feldman MF. Surface exposure and packing of lipoproteins into outer membrane vesicles are coupled processes in Bacteroides. mSphere. 2018. https://doi.org/10.1128/mSphere.00559-18.
Bryant WA, et al. In silico analysis of the small molecule content of outer membrane vesicles produced by Bacteroides thetaiotaomicron indicates an extensive metabolic link between microbe and host. Front Microbiol. 2017;8:2440. https://doi.org/10.3389/fmicb.2017.02440.
Zakharzhevskaya NB, et al. Interaction of Bacteroides fragilis toxin with outer membrane vesicles reveals new mechanism of its secretion and delivery. Front Cell Infect Microbiol. 2017;7:2. https://doi.org/10.3389/fcimb.2017.00002.
Stentz R, et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014;6:646–56. https://doi.org/10.1016/j.celrep.2014.01.021.
Hickey CA, et al. Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe. 2015;17:672–80. https://doi.org/10.1016/j.chom.2015.04.002.
Alvarez CS, et al. Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction. BMC Microbiol. 2019;19:166. https://doi.org/10.1186/s12866-019-1534-3.
Chelakkot C, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. https://doi.org/10.1038/emm.2017.282.
Ashrafian F, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155. https://doi.org/10.3389/fmicb.2019.02155.
Ashrafian F, et al. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol Hepatol Bed Bench. 2019;12:163–8.
Fábrega MJ, et al. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 2016;7:705. https://doi.org/10.3389/fmicb.2016.00705.
Canas MA, Fabrega MJ, Gimenez R, Badia J, Baldoma L. Outer membrane vesicles from probiotic and commensal Escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Front Microbiol. 2018;9:498. https://doi.org/10.3389/fmicb.2018.00498.
Seo MK, Park EJ, Ko SY, Choi EW, Kim S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J Dairy Sci. 2018;101:8662–71. https://doi.org/10.3168/jds.2018-15014.
Maerz JK, et al. Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing. Gut Microbes. 2018;9:1–12. https://doi.org/10.1080/19490976.2017.1344810.
Al-Nedawi K, et al. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J Off Publ Fed Am Soc Exp Biol. 2015;29:684–95. https://doi.org/10.1096/fj.14-259721.
Yamasaki-Yashiki S, Miyoshi Y, Nakayama T, Kunisawa J, Katakura Y. IgA-enhancing effects of membrane vesicles derived from Lactobacillus sakei subsp. sakei NBRC15893. Biosci Microbiota Food Health. 2019;38:23–9. https://doi.org/10.12938/bmfh.18-015.
López P, et al. Treg-inducing membrane vesicles from Bifidobacterium bifidum LMG13195 as potential adjuvants in immunotherapy. Vaccine. 2012;30:825–9. https://doi.org/10.1016/j.vaccine.2011.11.115.
Kim JH, et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J Allergy Clin Immunol. 2016;137:507-516.e508. https://doi.org/10.1016/j.jaci.2015.08.016.
Sacks D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke Off J Int Stroke Soc. 2018;13:612–32. https://doi.org/10.1177/1747493018778713.
Díaz-Garrido N, et al. Transcriptomic microRNA Profiling of dendritic cells in response to gut microbiota-secreted vesicles. Cells. 2020. https://doi.org/10.3390/cells9061534.
Acknowledgements
Thanks prof. Yu-qiang Nie for editing the manuscript.
Funding
This work was supported by Science and Technology Innovation Committee of Shenzhen (JCYJ20190807145617113 and JCYJ20210324113802006), Guangdong Basic and Applied Basic Research Foundation (Grant 2021A1515010985, 2023A1515011936).
Author information
Authors and Affiliations
Contributions
YL contributed towards the conceiving and illustration of this manuscript. CT, DL, MY was responsible for writing of this review. YLQT, HX, JF and MZ were responsible for assisting in collecting the literature and revising this review. YZ, YN and JW provided some suggestions. JY and LW contributed to discussion. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
No applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tian, Cm., Yang, Mf., Xu, Hm. et al. Emerging role of bacterial outer membrane vesicle in gastrointestinal tract. Gut Pathog 15, 20 (2023). https://doi.org/10.1186/s13099-023-00543-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-023-00543-2